Quantitative and qualitative estimation of amino acidsNinhydrin test


























- Slides: 37

Quantitative and qualitative estimation of amino acids-Ninhydrin test Amino acid determination is required for the quantitative and qualitative determination of the specific amino acid or all the amino acid. Specific reagents have been used for such experimental analysis and this amino acid reaction forms the basics behind the protein sequencing studies. Related LOs: Amino acid properties > Prior Viewing – IDD-1. Extraction of bacterial protein, IDD-6. Extraction of serum protein > Future Viewing – IDD-17. SDS-PAGE, IDD-33. Western blot assay Course Name: Quantitative and qualitative analysis of amino acids-Ninhydrin test Level(UG/PG): UG Author(s): Dinesh Raghu, Vinayak Pachapur Mentor: Dr. Sanjeeva Srivastava *The contents in this ppt are licensed under Creative Commons Attribution-Non. Commercial-Share. Alike 2. 5 India license

1 2 3 4 5 Learning objectives After interacting with this learning object, the learner will be able to: 1. Define the presence of amino acid in the sample 2. Identify the mechanism involved in the detection 3. Operate the steps used in colorimeter 4. Infer the law governing the colorimetric analysis 5. Assess the troubleshooting steps involved in the experiments.

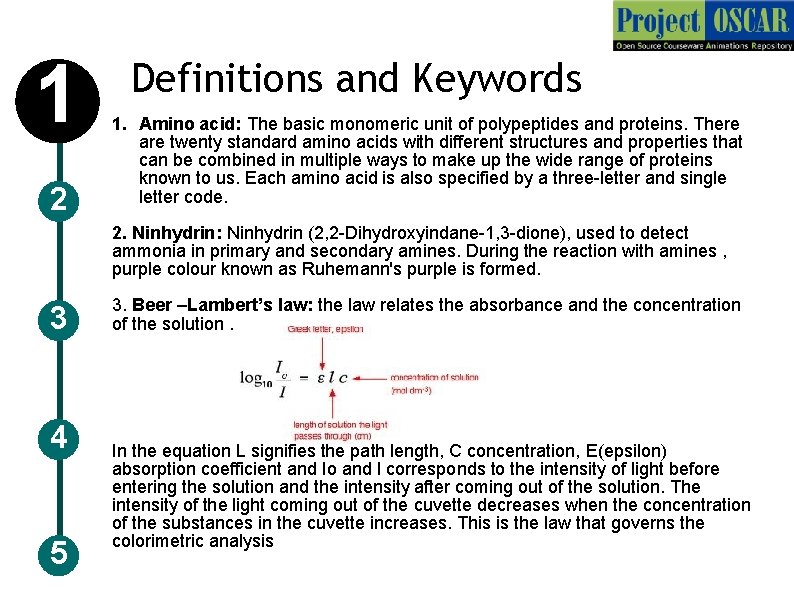
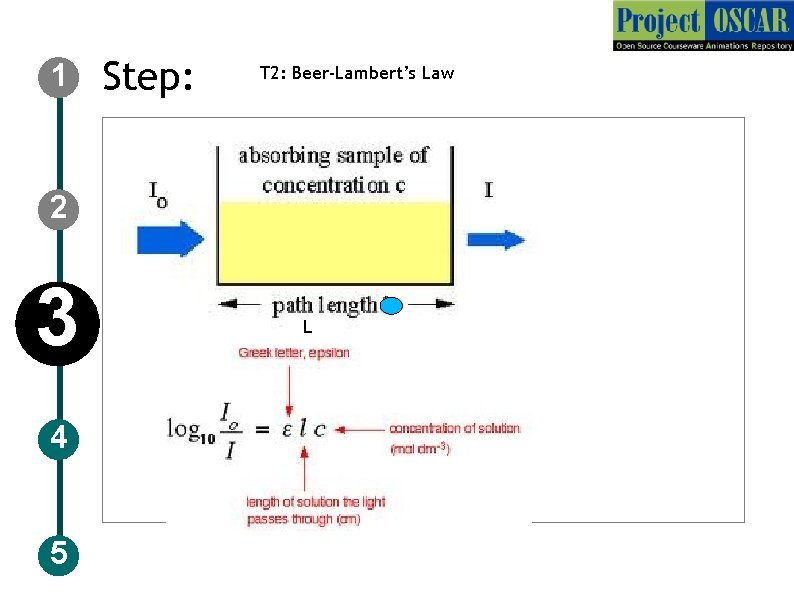
1 2 Definitions and Keywords 1. Amino acid: The basic monomeric unit of polypeptides and proteins. There are twenty standard amino acids with different structures and properties that can be combined in multiple ways to make up the wide range of proteins known to us. Each amino acid is also specified by a three-letter and single letter code. 2. Ninhydrin: Ninhydrin (2, 2 -Dihydroxyindane-1, 3 -dione), used to detect ammonia in primary and secondary amines. During the reaction with amines , purple colour known as Ruhemann's purple is formed. 3 4 5 3. Beer –Lambert’s law: the law relates the absorbance and the concentration of the solution. In the equation L signifies the path length, C concentration, E(epsilon) absorption coefficient and Io and I corresponds to the intensity of light before entering the solution and the intensity after coming out of the solution. The intensity of the light coming out of the cuvette decreases when the concentration of the substances in the cuvette increases. This is the law that governs the colorimetric analysis

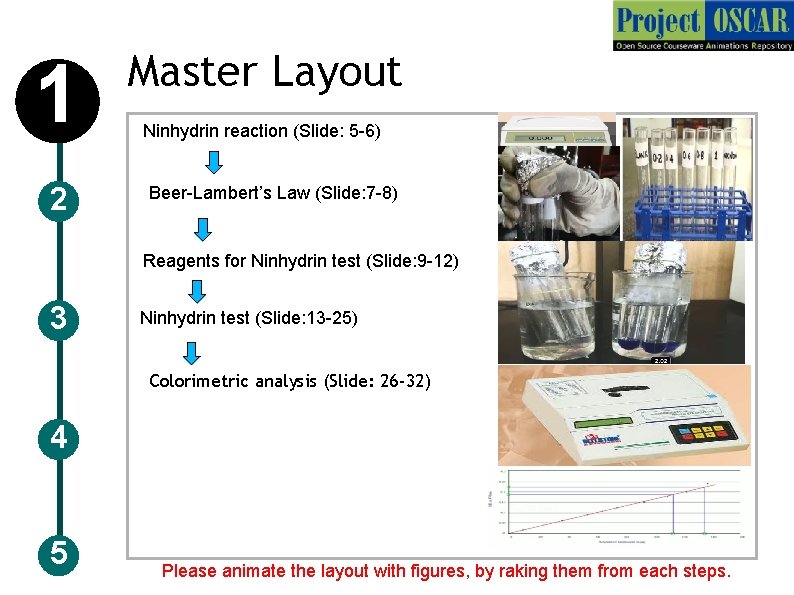
1 2 Master Layout Ninhydrin reaction (Slide: 5 -6) Beer-Lambert’s Law (Slide: 7 -8) Reagents for Ninhydrin test (Slide: 9 -12) 3 Ninhydrin test (Slide: 13 -25) Colorimetric analysis (Slide: 26 -32) 4 5 Please animate the layout with figures, by raking them from each steps.

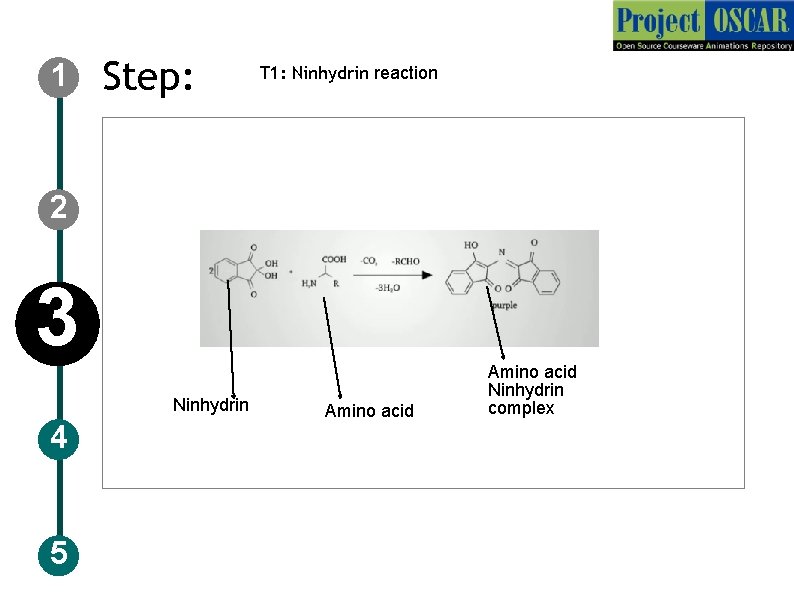
1 Step: T 1: Ninhydrin reaction 2 3 Ninhydrin 4 5 Amino acid Ninhydrin complex

1 Step: T 1: Ninhydrin reaction Description of the action 2 3 4 5 Show a tab labeled as theory behind the ninhydrin’s test and colorimeteric absorbance. Now first show the ninhydrin structure followed by amino acid and the product as in the slide. Audio Narration Alpha amino acid reacts with the excess of the ninhydrin reagent to give the purple color, the intensity of the color signifies the concentration of the amino acid, more the color intensity signifies the presence of excess amino acid.

1 Step: T 2: Beer-Lambert’s Law 2 3 4 5 L

1 Step : T 2: Beer-Lambert’s Law Description of the action 2 3 4 5 Now show the figure as in slide above (redraw) followed by the formula which is given in the slide , audio narration should take place simultaneously Audio Narration Colorimeter works on the basis of Beer-Lambert’s law, the law relates the absorbance and the concentration of the solution. In the equation L: signifies the path length, C: concentration, E(epsilon) : absorption coefficient and Io and I corresponds to the intensity of light before entering the solution and the intensity after coming out of the solution. The intensity of the light coming out of the cuvette decreases when the concentration of the substances in the cuvette increases.

1 Step 1: T 3: Reagents for Ninhydrin test 2 3 4 5 Description of the action Audio Narration (if any) Show a measuring balance, with display, ON, OFF and TARE/0 buttons on it. let When measuing user ON it, display reading as 0. 000 g, let with paper, the user picks up the paper from the rack, weight of the makes 1/10 of folding on the sides and paper need to be places it on the balance. Now the display tared from actual reading changes to 0. 003 g. Instruct user reading. to TARE the reading. And animate to click the tare button. Once user clicks it, reading must show ” 0”

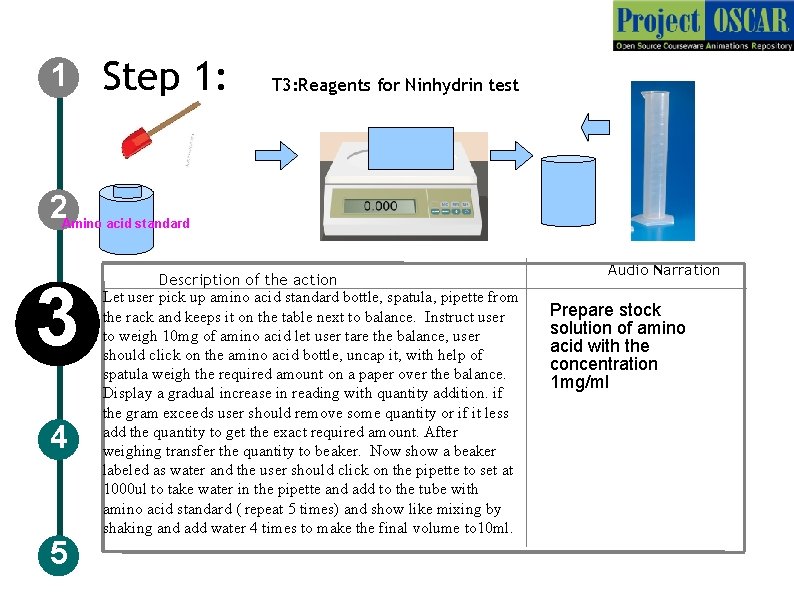
1 Step 1: T 3: Reagents for Ninhydrin test 2 Amino acid standard 3 4 5 Description of the action Let user pick up amino acid standard bottle, spatula, pipette from the rack and keeps it on the table next to balance. Instruct user to weigh 10 mg of amino acid let user tare the balance, user should click on the amino acid bottle, uncap it, with help of spatula weigh the required amount on a paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Now show a beaker labeled as water and the user should click on the pipette to set at 1000 ul to take water in the pipette and add to the tube with amino acid standard ( repeat 5 times) and show like mixing by shaking and add water 4 times to make the final volume to 10 ml. Audio Narration Prepare stock solution of amino acid with the concentration 1 mg/ml

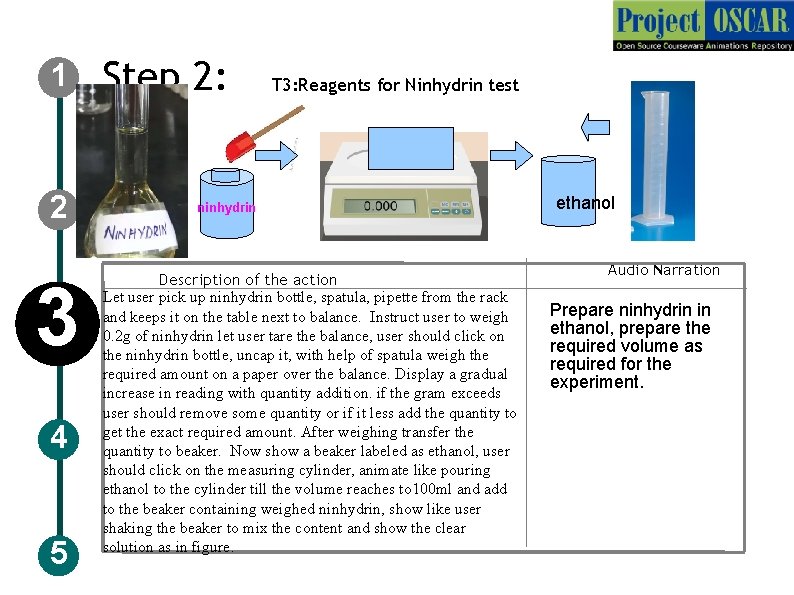
1 2 3 4 5 Step 2: T 3: Reagents for Ninhydrin test ninhydrin Description of the action Let user pick up ninhydrin bottle, spatula, pipette from the rack and keeps it on the table next to balance. Instruct user to weigh 0. 2 g of ninhydrin let user tare the balance, user should click on the ninhydrin bottle, uncap it, with help of spatula weigh the required amount on a paper over the balance. Display a gradual increase in reading with quantity addition. if the gram exceeds user should remove some quantity or if it less add the quantity to get the exact required amount. After weighing transfer the quantity to beaker. Now show a beaker labeled as ethanol, user should click on the measuring cylinder, animate like pouring ethanol to the cylinder till the volume reaches to 100 ml and add to the beaker containing weighed ninhydrin, show like user shaking the beaker to mix the content and show the clear solution as in figure. ethanol Audio Narration Prepare ninhydrin in ethanol, prepare the required volume as required for the experiment.

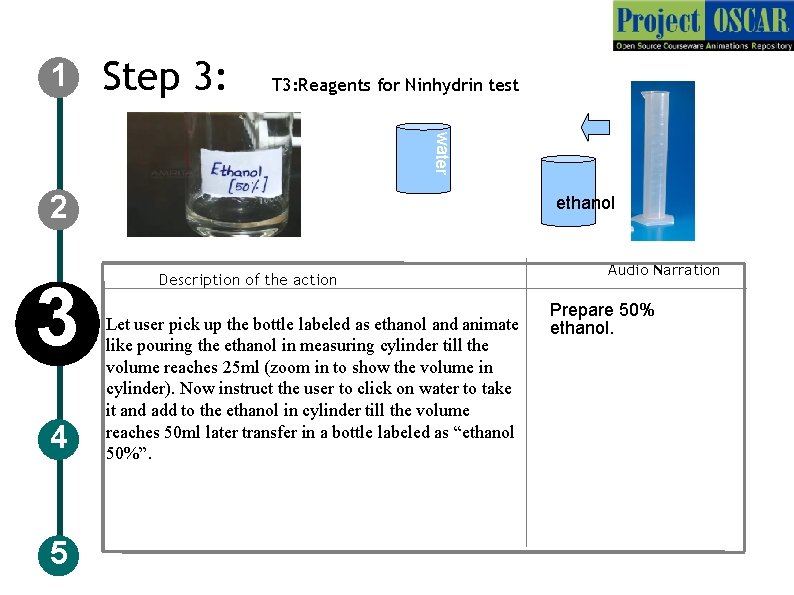
1 Step 3: T 3: Reagents for Ninhydrin test water 2 3 4 5 ethanol Description of the action Let user pick up the bottle labeled as ethanol and animate like pouring the ethanol in measuring cylinder till the volume reaches 25 ml (zoom in to show the volume in cylinder). Now instruct the user to click on water to take it and add to the ethanol in cylinder till the volume reaches 50 ml later transfer in a bottle labeled as “ethanol 50%”. Audio Narration Prepare 50% ethanol.

1 2 3 4 5 Step 4: T 4: Ninhydrin test

1 Step 4: T 4: Ninhydrin test Description of the action 2 3 4 5 Now instruct the user to take the boiling tubes as shown in figure and animate like user washing and drying the tubes. Now instruct the user to take marker and label the tubes as shown in previous slide and place it on the stand as shown. But animate 3 more tubes labeled as unknown 1, 2, 3. Audio Narration Wash the boiling tubes, label it accordingly for blank, 0. 2 -1 ml and then unknown.

1 2 3 4 5 Step 5: T 4: Ninhydrin test

1 Step 5: T 4: Ninhydrin test Description of the action 2 3 4 5 Animate like user taking the pipette setting the value to 200 ul to take out amino acid to pipette out 200 ul into the tube labeled as “ 0. 2”, again instruct the user to set the pipette to 400 ul and pipette out the amino acid in to the tube labeled as” 0. 4” follow the same instruction by setting the pipette to 600 ul, 800 ul and 1000 ul , pipette out the amino acid standard and adding to the tube labeled as 0. 6, 0. 8, 1. 0. Now show a tube labeled as unknown (sample) and instruct user to take the pipette set to 100 ul to take the unknown add to the tube labelled as unknown 1, follow the same by setting pipette to 200, 300 ul and adding to tubes unknown 2, 3. events must happen when the user clicks on pipette. Audio Narration Prepare the working standard in the concentration of 0. 2 mg 1 mg and unknown samples taking in different volumes.

1 Step 6: T 4: Ninhydrin test Description of the action 2 3 4 5 Animate like the user taking the glass pipette or 5 ml pipette and taking the distilled water beaker and pipette out 4 ml when user clicks on it and adding to the tube labeled as “blank”, again instruct the user to take 3. 8 ml of water in pipette and add to the tube labeled as” 0. 2” follow the same instruction by taking the water in the pipette to 3. 6 ml, 3. 4 ml, 3. 2 ml and 3 ml adding to the tube labeled as 0. 4, 0. 6, 0. 8, 1. 0 to make final volume 4 ml. Now show a tube labeled as unknown and instruct the user to take water in the pipette to 3. 9 ml and add to unknown: 1 , 3. 8 ml to unknown: 2 and 3. 7 ml to unknown: 3 to make final volume to 4 ml in all the tubes. Audio Narration Make the volume to 4 ml using the distilled water in all the tubes.

1 Step 7: T 4: Ninhydrin test Description of the action 2 3 4 5 Animate like the user taking the pipette setting the value to 1000 ul to take the ninhydrin solution and pipetting out 1000 ul in each tubes. Animate each time user taking 1000 ul using pipette and adding to the tubes. animate like the user taking the tubes and mixing by gently shaking it. Audio Narration Add 1000 ul of ninhydrin reagent in all the tubes and mix well for the reaction to take place.

1 2 3 4 5 Step 8: T 4: Ninhydrin test Aluminum foil

1 Step 8: T 4: Ninhydrin test Description of the action 2 3 4 5 Show aluminum foil, user must click on it, show like user tearing it in small pieces and covering all the tubes as shown in the figure. Audio Narration Cover the tubes with aluminum foil before a hot water treatment.

1 Step 9: T 4: Ninhydrin test 2 3 4 5 Water bath tubes

1 Step 9: T 4: Ninhydrin test Description of the action 2 3 4 5 Show instrument labeled as water bath. Redraw the image, show the buttons like start, set and stop with red light up and down arrow button. Instruct user to click on bottle labeled as distilled water and animate like pouring in the water bath, now instruct the user to click “start” and then set the temperature by pressing the up arrows to 100’C. After 15 mins (animate a clock) show like the water boiling. Now instruct user to take the tubes covered with foil to keep it in the water bath as shown in the figure animate a clock for 15 minutes. Audio Narration Place the tubes in the boiling water for 15 minutes for the reaction to occur.

1 2 3 4 5 Step 10: T 4: Ninhydrin test

1 2 3 4 5 Step 11: T 4: Ninhydrin test

1 Step 10 and 11: T 4: Ninhydrin test Description of the action 2 3 4 5 After 15 minutes show like taking the tubes to keep in the cold water in the beaker as shown in the figure. Animate the colors as in figure. Animate like the user taking the pipette setting the value to 1000 ul and taking the 50% ethanol bottle and pipette out 1000 ul when user clicks on it and adding to the tube labeled as “blank”, again animate like pipetting 1000 ul and adding to the tubes” 0. 2, 0. 4, 0. 6, 0. 8, 1 unknown “. Animate each time the user taking 1000 ul using pipette and adding to the tubes. animate like the user taking the tubes and mixing by gently shaking it. After sometime show the color intensity from blank (left to right) should increase as in the tubes like shown in figure. Audio Narration Place the tubes in cold water and allow it to cool down. Add 1 ml of 50% ethanol in all the tubes.

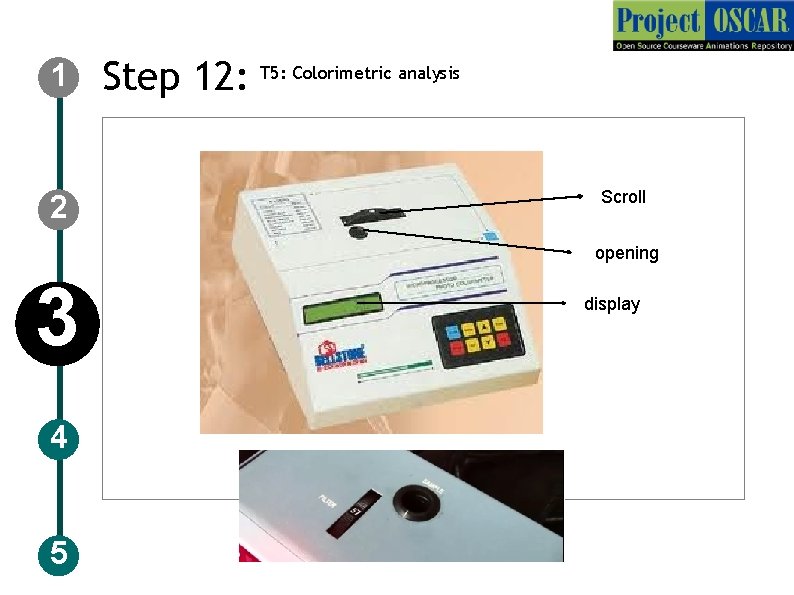
1 2 Step 12: T 5: Colorimetric analysis Scroll opening 3 4 5 display

1 Step 12: T 5: Colorimetric analysis Description of the action 2 3 4 5 Show a instrument labeled as “colorimeter” and draw it as shown in the figure. Animate a scroll, a opening and a display screen, auto zero and absorbance Instruct the user to click on start in the instrument and the user should move the scroll so that the wavelength is set to 570 nm as shown in the image and allow it to stand for 30 minutes. Audio Narration Switch on the colorimeter and set the wavelength to 570 nm to take the absorbance.

1 Step 13: T 5: Colorimetric analysis 1 2 2 3 4 4 5 3

1 Step 13: T 5: Colorimetric analysis Description of the action 2 3 4 5 After 30 minutes instruct user to take the cuvette (as in figure), set the pipette to 1000 ul to draw the blank solution into the cuvette and show like the user inverting the cuvette and pouring out the solution in a beaker, again instruct user to take 1000 ul of the blank and add to the cuvette, (repeat it once more to have the volume of 2000 ul in the cuvette) clean the cunette with tissue on sides and place the cuvette in the opening, and click on “absorbance” the reading in the instrument should show ” 0. 00”. now ask the user to click “auto zero” and the display should show “ 0. 00” and then animate like removing the cuvette and pouring the solution out. Follow the same steps for other tubes also. Show the values as in next slides. Audio Narration Rinse the cuvette with the blank and discard it. Fill the cuvette with the blank solution and take the OD, auto zero the instrument and take the readings for all the other tubes.

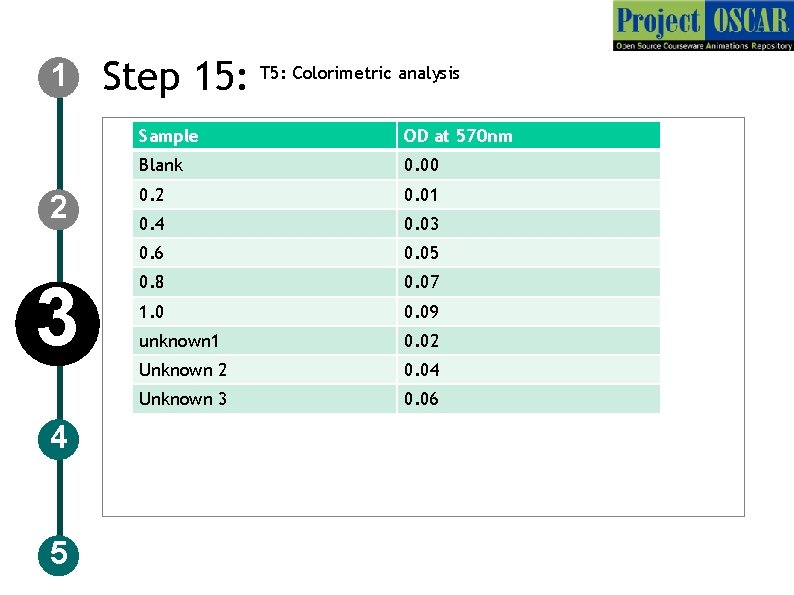
1 2 3 4 5 Step 15: T 5: Colorimetric analysis Sample OD at 570 nm Blank 0. 00 0. 2 0. 01 0. 4 0. 03 0. 6 0. 05 0. 8 0. 07 1. 0 0. 09 unknown 1 0. 02 Unknown 2 0. 04 Unknown 3 0. 06

1 2 3 4 5 Step 16: T 5: Colorimetric analysis

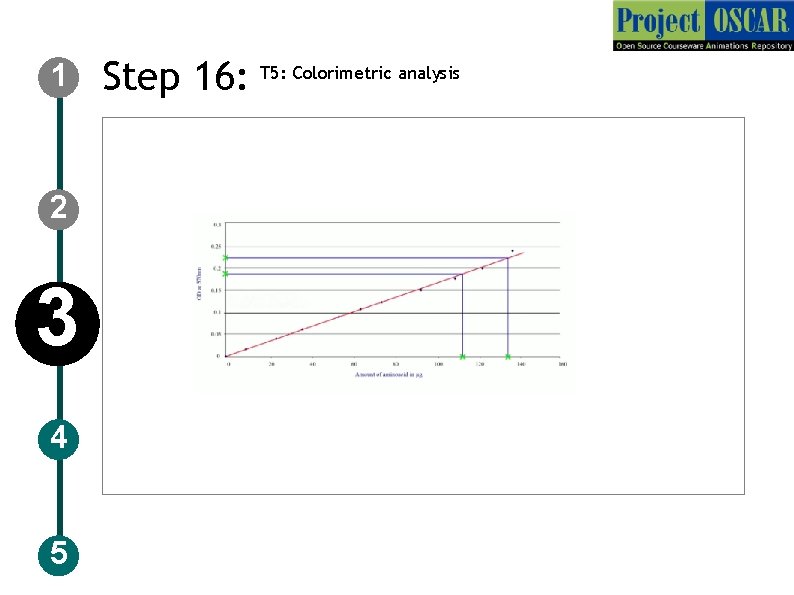
1 Step 16: T 5: Colorimetric analysis Description of the action 2 3 4 5 Instruct the user to plot the graph as OD in y axis and the concentration in x axis to show a straight line drawn (red line) meeting the standard points with absorbance. animate like the user locating the point on y axis for “unknown 1 (0. 02) and drawing a line towards the red line and when the line touches the red line drag the line down to find the concentration as 0. 3 mg follow the same for other two unknowns and show the concentration as 0. 5 mg, 0. 7 mg Audio Narration Plot the graph between OD at 570 nm and the concentration of the sample and extrapolate the unknown OD value to find the concentration.

Slide 56 Tab 01 Slide 7 - Slide 98 12 Tab 03 Slide 13 -25 Tab 04 Slide 26 -33 Tab 05 Tab 06 Tab 07 Name of the section/stage Interactivity area Animation area Slide 28 - 31 Show like the user touching the sides of the cuvette and doing the experiment and taking the reading , show the blank absorbance value as “ 0. 10” Button 01 Button 02 Instruction: Now instruct the user to clean the sides of the cuvette using the tissue and taking the reading , show the blank absorbance as “ 0. 01” Button 03 Instructions/ Working area Credits

APPENDIX 1 Questionnaire: Question 1 Amino acid can be detected using a) b) c) d) Chloroform Ethanol Acetone Ninhydrin Question 2 Amines when react with Ninhydrin gives a) Red color b) Blue clor c) Orange color d) Purple color Question 3 Absorbance can be taken using a) Calorimetry b) Colorimetry c) Spectrometry d) refractometry

APPENDIX 1 Questionnaire: Question 4 Colorimetry works based on a) b) c) d) Beers law Lamberts Law Beer-Lamberts Law Raman spectrum Question 5: As the absorbance increases , the intensity of the outgoing light a) b) c) d) Decreases Increases Remains same zero

APPENDIX 2 Links for further reading Reference websites: http: //www. youtube. com/watch? v=Jd. Xb. TWf. Oc 18 &feature=related Book Biochemistry by Voet & Voet, 3 rd edition

APPENDIX 3 Summary The method mostly involves the quantitative and qualitative estimation of amino acid using the Ninhydrin test. Steps involved are preparation of standards, ninhydrin and ethanol, preparing working standards, addition of water, ninhydrin followed by incubation and addition of ethanol to see the color, followed by colorimetric analysis. Depending on the color intensity developed, one can make out the presence and absence of the amino acid.
 Quantitative estimation of amino acids by ninhydrin
Quantitative estimation of amino acids by ninhydrin Qualitative and quantitative test for lipids
Qualitative and quantitative test for lipids Quantitative estimation of protein by lowry method
Quantitative estimation of protein by lowry method Enzymatic method of blood glucose estimation
Enzymatic method of blood glucose estimation Lowry method
Lowry method Qualitative tests for amino acids
Qualitative tests for amino acids Findings of qualitative research
Findings of qualitative research Role of quantitative research
Role of quantitative research Difference between qualitative and quantitative data
Difference between qualitative and quantitative data Qualitative vs quantitative observations
Qualitative vs quantitative observations Quantitative and qualitative difference
Quantitative and qualitative difference A qualitative variable
A qualitative variable Qualitative and quantitative data analysis
Qualitative and quantitative data analysis Quantitative and qualitative variables examples
Quantitative and qualitative variables examples Observations quantitative
Observations quantitative Similarities between qualitative and quantitative research
Similarities between qualitative and quantitative research Similarities between qualitative and quantitative research
Similarities between qualitative and quantitative research Qualitative physical properties
Qualitative physical properties Qualitative and quantitative difference
Qualitative and quantitative difference Qualitative and quantitative difference
Qualitative and quantitative difference What is the sample size in qualitative research?
What is the sample size in qualitative research? Research content example
Research content example Quantitative and qualitative traits
Quantitative and qualitative traits Limitations of qualitative research
Limitations of qualitative research Disadvantage of qualitative research
Disadvantage of qualitative research Qualitative and quantitative
Qualitative and quantitative Data analysis and interpretation examples
Data analysis and interpretation examples Quantitative and qualitative in chemistry
Quantitative and qualitative in chemistry Integrating qualitative and quantitative methods
Integrating qualitative and quantitative methods Quantitative and qualitative traits
Quantitative and qualitative traits Is a bipolar survey qualitative or quantitative
Is a bipolar survey qualitative or quantitative Qualitative analysis
Qualitative analysis Longitudinal study qualitative or quantitative
Longitudinal study qualitative or quantitative Qualitative vs quantitative measurements
Qualitative vs quantitative measurements Qualitative vs quantitative
Qualitative vs quantitative Qualitative data biology definition
Qualitative data biology definition Telephone number quantitative or qualitative
Telephone number quantitative or qualitative Qualitative und quantitative differenzierung
Qualitative und quantitative differenzierung