Making Measurements Two Types of Measurements Qualitative Quantitative





- Slides: 10

Making Measurements

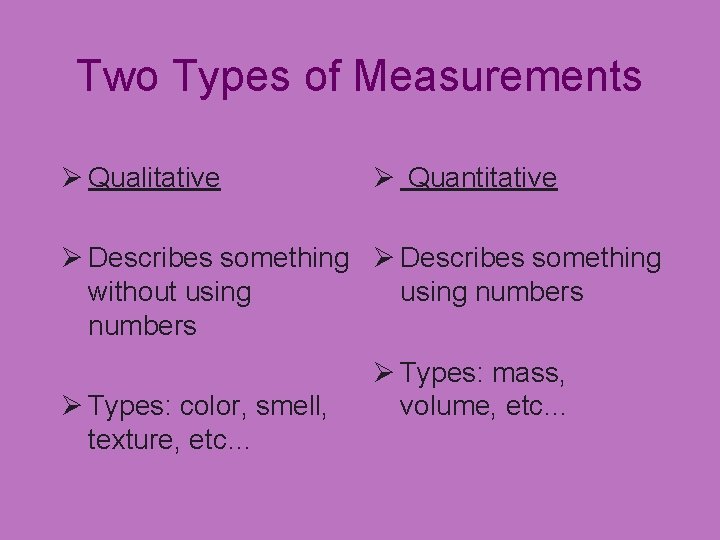
Two Types of Measurements Ø Qualitative Ø Quantitative Ø Describes something without using numbers Ø Types: color, smell, texture, etc… Ø Types: mass, volume, etc…

What are the parts of quantitative measurements? Ø Number Ø Unit Ø Tells you how much of something you have Ø Tells you in what capacity you have of that something In chemistry, numbers are meaningless without units! Example: 50 cents, 50 dimes, 50 dollars, 50 million

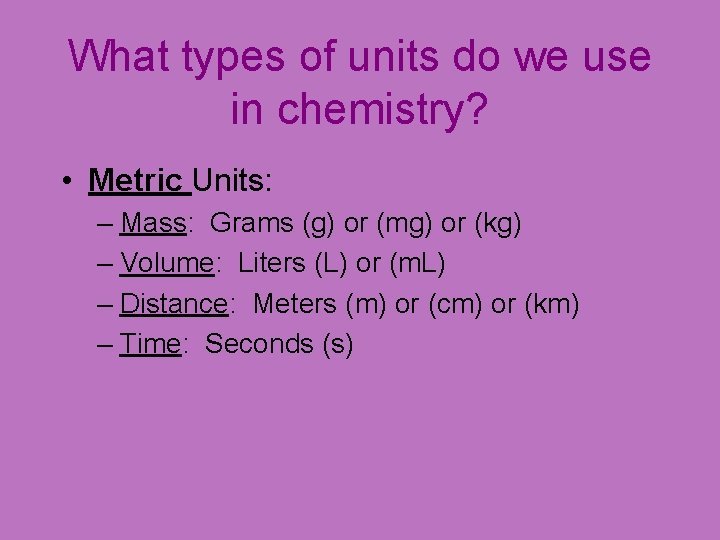
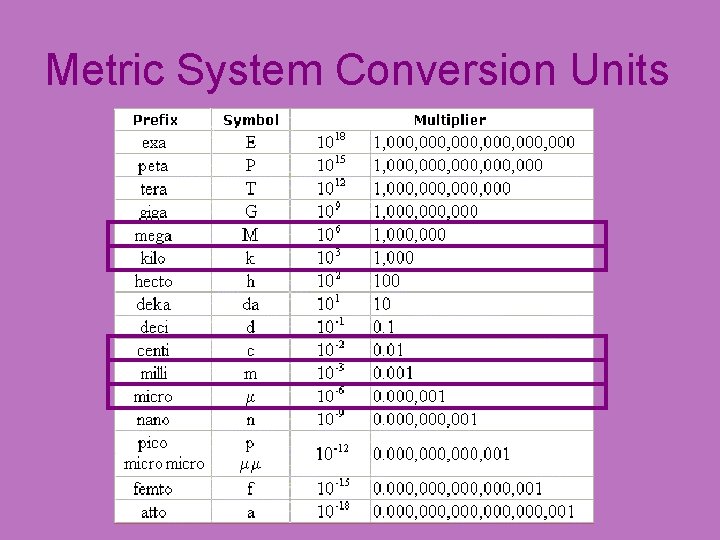
What types of units do we use in chemistry? • Metric Units: – Mass: Grams (g) or (mg) or (kg) – Volume: Liters (L) or (m. L) – Distance: Meters (m) or (cm) or (km) – Time: Seconds (s)

Metric System Conversion Units

Reporting Measurements Ø Purpose: To know how close we are to the “true” value. Ø When reporting a measurement, two things need to be considered… Ø Accuracy and Precision

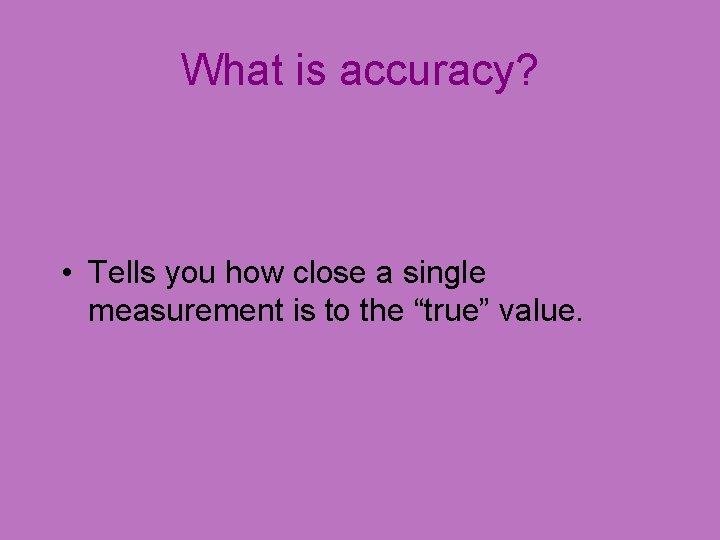
What is accuracy? • Tells you how close a single measurement is to the “true” value.

What is precision? • Tells you how close a series of measurements are to each other.

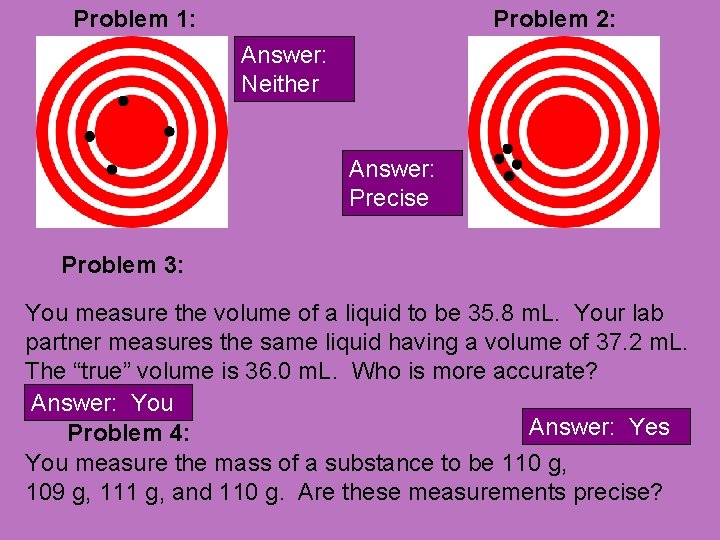
Left Side pg 14 • With the person next to you, decide whether the following examples are accurate, precise, both, or neither.

Problem 1: Problem 2: Answer: Neither Answer: Precise Problem 3: You measure the volume of a liquid to be 35. 8 m. L. Your lab partner measures the same liquid having a volume of 37. 2 m. L. The “true” volume is 36. 0 m. L. Who is more accurate? Answer: You Answer: Yes Problem 4: You measure the mass of a substance to be 110 g, 109 g, 111 g, and 110 g. Are these measurements precise?
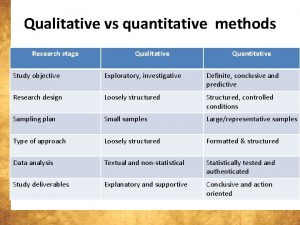
 Qualitative vs quantitative measurements
Qualitative vs quantitative measurements Two types of measurements
Two types of measurements What is qualitative data in geography
What is qualitative data in geography Qualitative research is viewed in a holistic perspective.
Qualitative research is viewed in a holistic perspective. Longitudinal study qualitative or quantitative
Longitudinal study qualitative or quantitative Words images objects qualitative or quantitative
Words images objects qualitative or quantitative Who is pictured above?
Who is pictured above? Qualitative data in biology
Qualitative data in biology Are telephone numbers qualitative or quantitative
Are telephone numbers qualitative or quantitative Group studied is smaller and not randomly selected
Group studied is smaller and not randomly selected Difference between qualitative and quantitative data
Difference between qualitative and quantitative data