Ch 3 Measurements and Their Uncertainty Scientific Notation










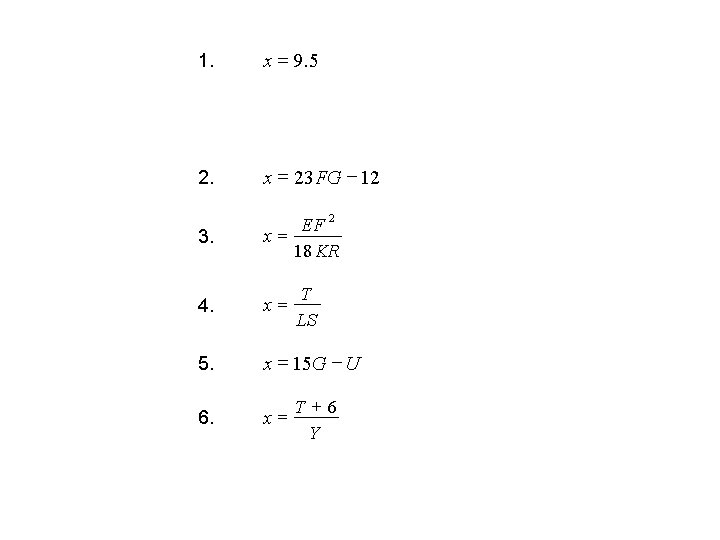
- Slides: 29

Ch. 3 Measurements and Their Uncertainty

Scientific Notation • *Why are units of measurement important? 70 vs. 7 feet 0 inches • Measurement = quantity that has both a number and a unit • Scientific notation = a given number is written as the product of 2 #’s: a coefficient and 10 raised to a power – Ex: 602, 000, 000, 000 is 6. 02 X 10 23 – The coefficient has to be = to or >1 and <10 – *RULE* If the number is larger than 1, the exponent is a positive number. If the number is smaller than 1, the exponent is a negative number • Ex: . 000045300 = 4. 5 X 10 -5 • 1, 700, 000 = 1. 7 X 109 • *a) 32, 700 b) 1, 024, 000 c) 0. 0047100 d) 0. 00003901 • e) 0. 0003412 f) 475, 500, 000 g) 0. 0000560 h) 18, 060, 000

Precision and Accuracy • Measurements are uncertain for 2 reasons: – 1. Measuring instruments are never completely free of flaws – 2. Measuring always involves some estimation • Digital – final digit is estimated • Scale - **Practice estimating water measurements in a graduated cylinder • *measure the width of an object twice and compare your value w/your partner’s value and come to a consensus and come up w/an uncertainty value • Accuracy = a measure of how close a measurement comes to the actual or true value of whatever is measured – Measured value must be compared to the correct value • Precision = a measure of how close a series of measurements are to one another – Must compare values of 2 or more repeated measurements • 1) 78⁰C, 76⁰C, 75⁰C 2) 77⁰C, 78⁰C 3) 80⁰C, 81⁰C, 82⁰C – If these were boiling point measurements of a liquid, which is the most precise? What would have to be known to determine which set is the most accurate? If 85⁰C is the actual BP, which set is the most accurate? • **Dartboard enhancer**

Error • Accepted value = the correct value based on reliable references • Experimental value = the value measured in the lab – can be positive or negative • Percent error = the absolute value of the error divided by the accepted value, multiplied by 100% • Percent error = [experimental – accepted]/accepted • *Ex: Measured value of the BP of water was 99. 1⁰C, what is the percent error?

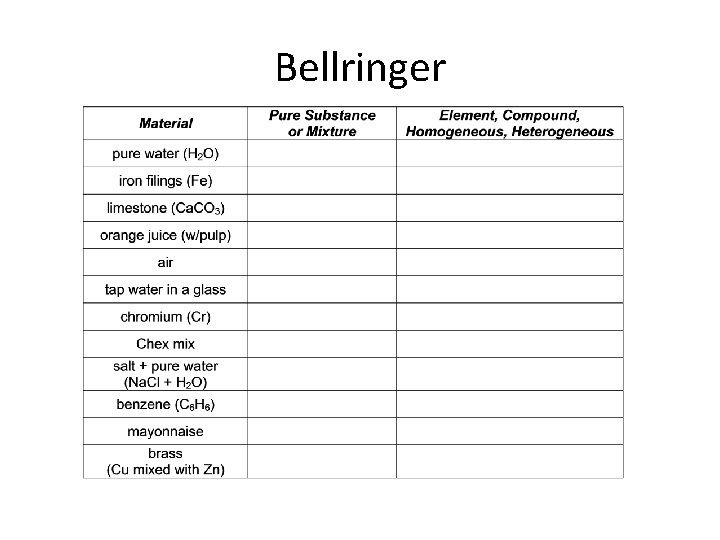
Bellringer

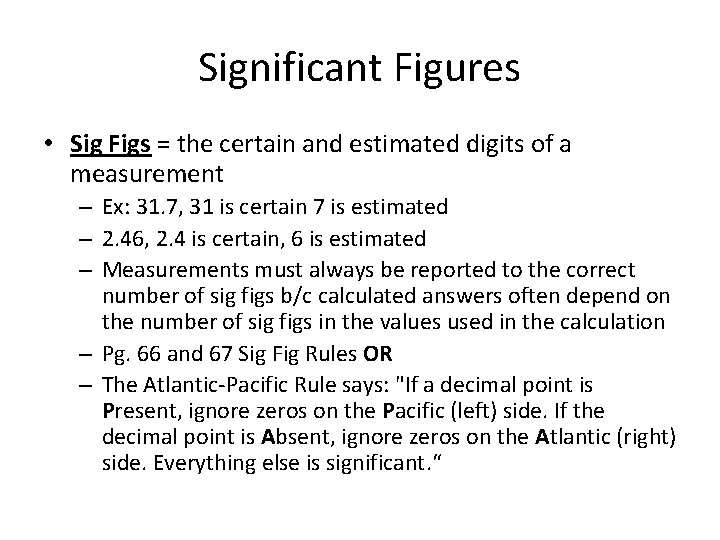
Significant Figures • Sig Figs = the certain and estimated digits of a measurement – Ex: 31. 7, 31 is certain 7 is estimated – 2. 46, 2. 4 is certain, 6 is estimated – Measurements must always be reported to the correct number of sig figs b/c calculated answers often depend on the number of sig figs in the values used in the calculation – Pg. 66 and 67 Sig Fig Rules OR – The Atlantic-Pacific Rule says: "If a decimal point is Present, ignore zeros on the Pacific (left) side. If the decimal point is Absent, ignore zeros on the Atlantic (right) side. Everything else is significant. “

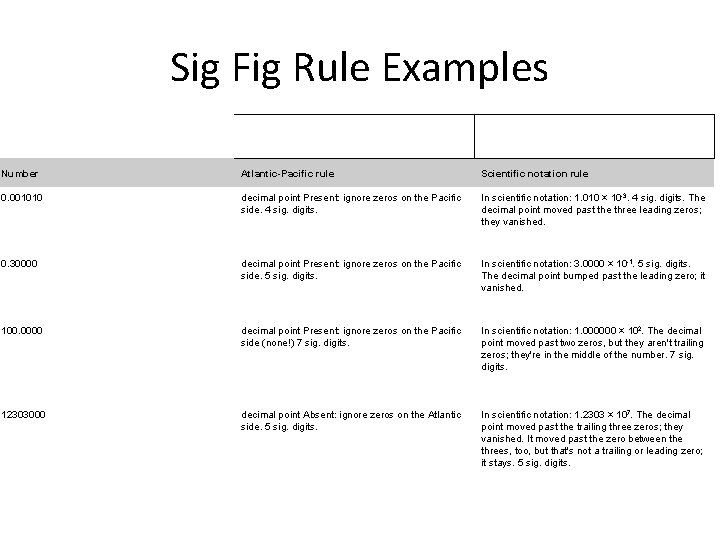
Sig Fig Rule Examples Number Atlantic-Pacific rule Scientific notation rule 0. 001010 decimal point Present: ignore zeros on the Pacific side. 4 sig. digits. In scientific notation: 1. 010 × 10 -3. 4 sig. digits. The decimal point moved past the three leading zeros; they vanished. 0. 30000 decimal point Present: ignore zeros on the Pacific side. 5 sig. digits. In scientific notation: 3. 0000 × 10 -1. 5 sig. digits. The decimal point bumped past the leading zero; it vanished. 100. 0000 decimal point Present: ignore zeros on the Pacific side (none!) 7 sig. digits. In scientific notation: 1. 000000 × 102. The decimal point moved past two zeros, but they aren't trailing zeros; they're in the middle of the number. 7 sig. digits. 12303000 decimal point Absent: ignore zeros on the Atlantic side. 5 sig. digits. In scientific notation: 1. 2303 × 107. The decimal point moved past the trailing three zeros; they vanished. It moved past the zero between the threes, too, but that's not a trailing or leading zero; it stays. 5 sig. digits.

• *Do Conceptual Problem 3. 1 Counting Sig Figs and Practice Problems 1 and 2 on pg. 68 • *Accuracy and Precision Quick Lab pg. 72

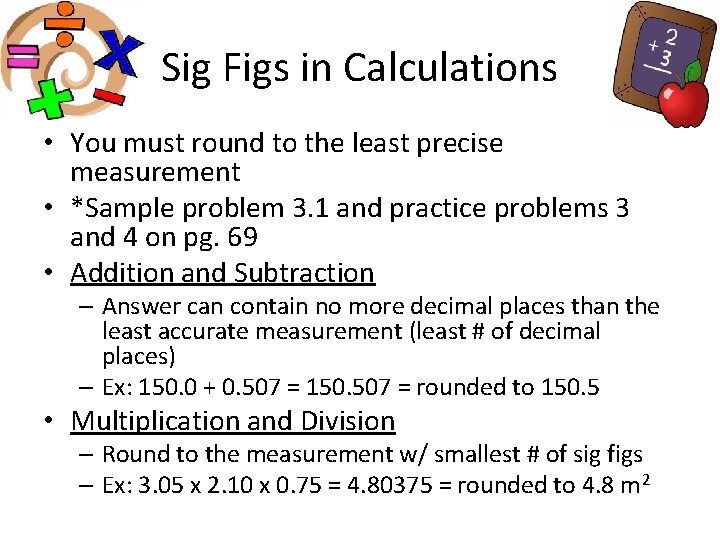
Sig Figs in Calculations • You must round to the least precise measurement • *Sample problem 3. 1 and practice problems 3 and 4 on pg. 69 • Addition and Subtraction – Answer can contain no more decimal places than the least accurate measurement (least # of decimal places) – Ex: 150. 0 + 0. 507 = 150. 507 = rounded to 150. 5 • Multiplication and Division – Round to the measurement w/ smallest # of sig figs – Ex: 3. 05 x 2. 10 x 0. 75 = 4. 80375 = rounded to 4. 8 m 2

• *Do sample problem 3. 2 and p. p. 5 & 6 on pg. 70 • *Do sample problem 3. 3 and p. p. 7 & 8

Significant Uncertainty If you were to weigh a small rock on a scale that could measure the mass of the rock to the nearest 0. 001 grams, then the mass of the rock would be, for example, 10. 871 + 0. 001 grams. The last digit is really just the best estimate of what the last digit should be. Perhaps it was rounded or perhaps not – there is no way to be certain 0 so the last digit is called uncertain. The first four digits were numbers about which no estimate Was made, so they are called significant figures. All nonzero numbers are significant; zeros between nonzeros are significant; place-holding zeros at the beginning and end of a number are not significant; and zeros at the end of a number after the decimal are significant. How many significant figures are in each of the following? a. 8. 01 b. 80. 1 c. 80 d. 8009 e. 0. 0083 f. 0. 1040900300 5

Bellringer • Solve each of the following expressions for x. (x = ? ? ) • • • • 1. 4 x – 2 z = 3 y + 8 (if y = 12 and z = – 3) 2. x + 12 = 23 FG 3. 18 KRx = E F 2 4. T = Lx. S 5. 15 G – x = U 6. Y = T + 6 x

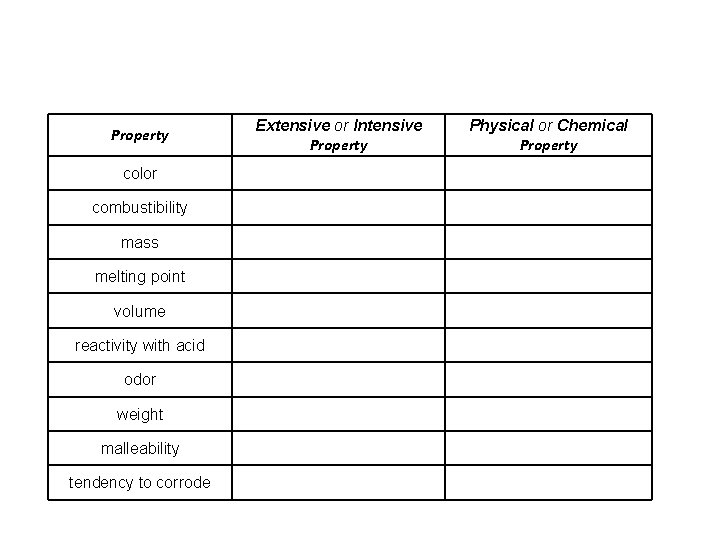
Property Extensive or Intensive Property Physical or Chemical Property color combustibility mass melting point volume reactivity with acid odor weight malleability tendency to corrode

• *Pencil Activity – Each group should measure the length and width of the table with their pencil and record the results on the board – Is there any problems using pencils as a unit of measurement?

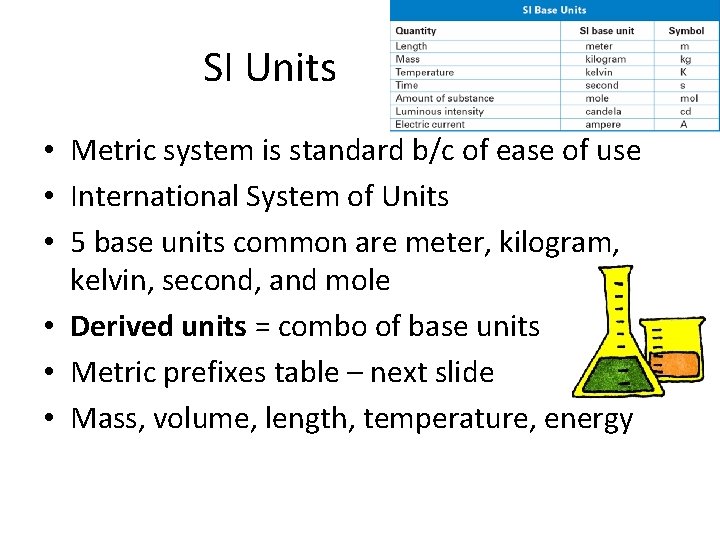
SI Units • Metric system is standard b/c of ease of use • International System of Units • 5 base units common are meter, kilogram, kelvin, second, and mole • Derived units = combo of base units • Metric prefixes table – next slide • Mass, volume, length, temperature, energy

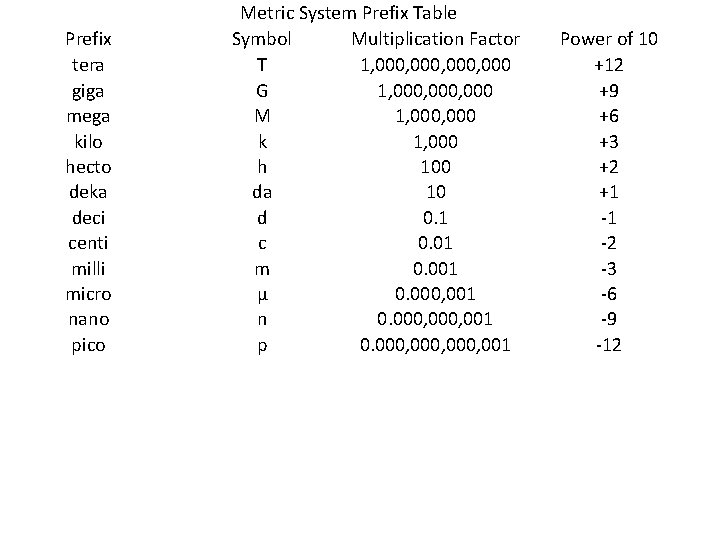
Prefix tera giga mega kilo hecto deka deci centi milli micro nano pico Metric System Prefix Table Symbol Multiplication Factor T 1, 000, 000 G 1, 000, 000 M 1, 000 k 1, 000 h 100 da 10 d 0. 1 c 0. 01 m 0. 001 µ 0. 000, 001 n 0. 000, 001 p 0. 000, 001 Power of 10 +12 +9 +6 +3 +2 +1 -1 -2 -3 -6 -9 -12

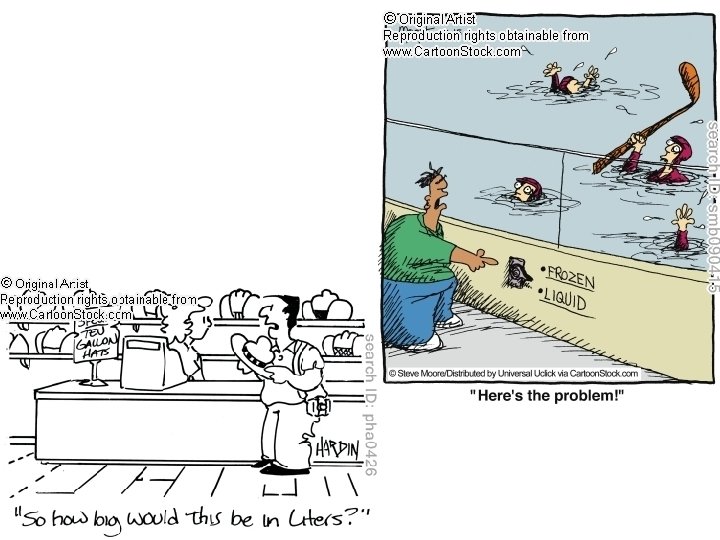
Quantities • Mass = amount of material in an object – Kilogram(kg) (mass of 1 L liquid water at 4⁰C), gram, milligram, microgram are most common – Balance to measure – Weight = force that measures the pull on a given mass by gravity – measure of force, mass measure of matter – wt. can change w/location, mass stays constant (never massless but can be weightless) • Volume = amt. of space an object occupies – Lx. Wx. H – A liter (L) is the volume of a cube with 10 cm along each edge (10 cm ((1 dm)) x 10 cm = 1000 cm 3) – 1 L = 1000 cm 3 = 1 dm 3 = 1000 m. L, 1 m. L = 1 cm 3

…cont • Length = distance b/w 2 pts. of reference – Meter (m), kilometer, centimeter most common • Temperature = measure of how hot or cold an object is – Transfers from H → L when 2 objects r in contact – Most substances expand w/ heat, contract w/ cold except water in solid form b/c of its structure – Celsius scale (⁰C) – freezing pt. of water is 0, boiling pt. is 100 – Kelvin scale (K) – fp of H 20 if 273, bp is 373 • Absolute zero = -273 ⁰C – K = ⁰C + 273, ⁰C = K – 273, ⁰F = C * 9/5 + 32, ⁰C =F – 32 *5/9 – *sample prob. 3. 4 and p. p. 16 & 17 pg. 78 – *Mass of a penny activity – pg. 76

Mr. Kirwan’s Steps to Converting Temperature • • • 1. What unit do they want 2. Write that unit = 3. What is known 4. Write down formula 5. Plug in #’s 6. Do math and label


…. cont • Energy = the capacity to do work (or produce heat) – Joule and calorie common units – calorie = the quantity of heat that raises the temp. of 1 g of pure water by 1 ⁰C – 1 J = 0. 2390 cal, 1 cal = 4. 184 J, 1 kilocalorie = 1 Calorie = 1000 calories

Conversion Factors • Conversion factors = a ratio of equivalent measurements 100 cm/1 m = 10 dm = 100 cm = 1000 mm 1 dollar = 4 quarters = 10 dimes = 20 nickels = 100 pennies When a measurement is multiplied by a conversion factor, the numerical value is generally changed, but the actual size of the quantity measured remains the same (1000 g/1 kg same) – Ex: a) 78. 5 cm = ? m b) 0. 056 L = ? cm 3 c) 77 kg = ? mg d) 0. 098 nm = ? dm e) 0. 96 cm = ? µm f) 0. 0067 mm = ? nm – –

Mr. Kirwan’s Steps to Solving Conversion Factor Problems • • 1. Which unit is bigger? 2. Do we need more or less to be equal? 3. How many spots from each other? 4. Move decimal left to make smaller, move right to make larger

Dimensional Analysis • Dimensional analysis = a way to analyze and solve problems using the units OR the technique of converting b/w units • *Expanding a recipe activity – pg. 81 – Problem solving: • 1. unit equality = equation that shows how diff. units are related • 2. conversion factors = Ex. 1 gal = 3. 785 L • 3. cancel units – Ex: You have 250 gallons, how many L? V = 250 gal x 3. 785 L/1 gallon V = 946 L

Mr. Kirwan’s Steps to Solve a Dimensional Analysis Problem • • • 1. Write down known 2. Find conversion factor 3. Make sure like units are opposite 4. Cancel units 5. Unit equality 6. Do math

D. A. Practice • Ex: How many inches are in 250 cm? • A chicken needs to be cooked 20 min. for each pound it weighs. How long should the chicken be cooked if it weighs 4. 5 pounds? • A football player is 6’ 4” 255 lbs. Convert to m and kg. • Problems: 1) gal in 39 L 2) cm in 16 in. Multistep: 3) sec in 5 hours 4) ft. in 86 cm 5) cm 3 in 2. 3 gal 6) m in 3. 5 miles - sample prob 3. 5 and 3. 6, pp 28 -31 pgs. 82 -83 - sample prob 3. 7 and 3. 8, pp 32 -35 pgs. 84 -85 - Derived units = sample prob 3. 9, pp 36 -37 pg. 86

Density • Density = ratio of the mass of an object to its volume – D = m/v, V = m/d, m = dv – Derived unit (g/cm 3) – Intensive property (depends on composition not size) – As T ↑ D↓ – *sample prob. 3. 10 & 3. 11, pp 46 -49 pgs. 91 -92

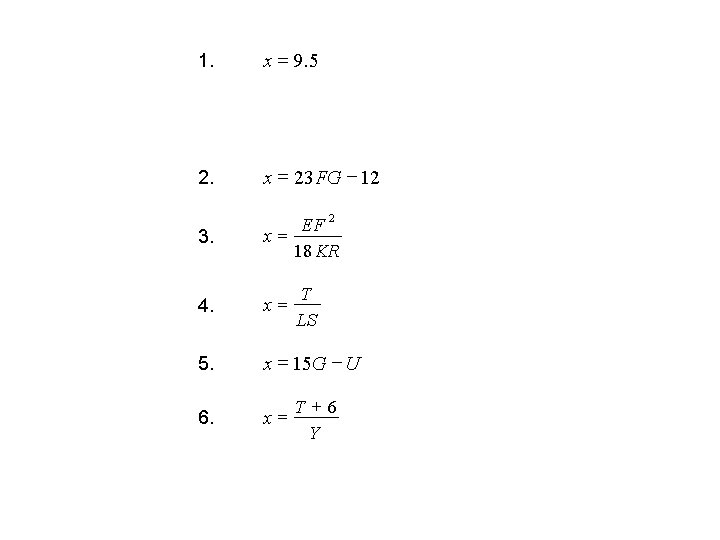
1. x = 9. 5 2. x = 23 FG - 12 3. EF 2 x= 18 KR T LS 4. x= 5. x = 15 G - U 6. x= T +6 Y