Ch 3 Scientific Measurement Measurement Measurement A quantity

















- Slides: 42

Ch. 3, Scientific Measurement

Measurement • Measurement: A quantity that has a number and a unit. Like 52 meters.

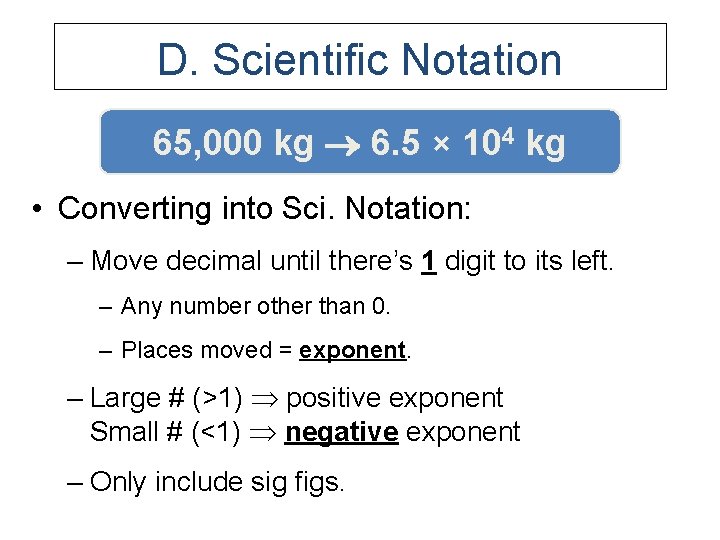
D. Scientific Notation 65, 000 kg 6. 5 × 104 kg • Converting into Sci. Notation: – Move decimal until there’s 1 digit to its left. – Any number other than 0. – Places moved = exponent. – Large # (>1) positive exponent Small # (<1) negative exponent – Only include sig figs.

D. Scientific Notation Practice Problems 7. 2, 400, 000 g 2. 4 8. 0. 00256 kg 2. 56 6 10 g -3 10 kg 7 10 -5 km 0. 00007 km 10. 6. 2 104 mm 62, 000 mm 9.

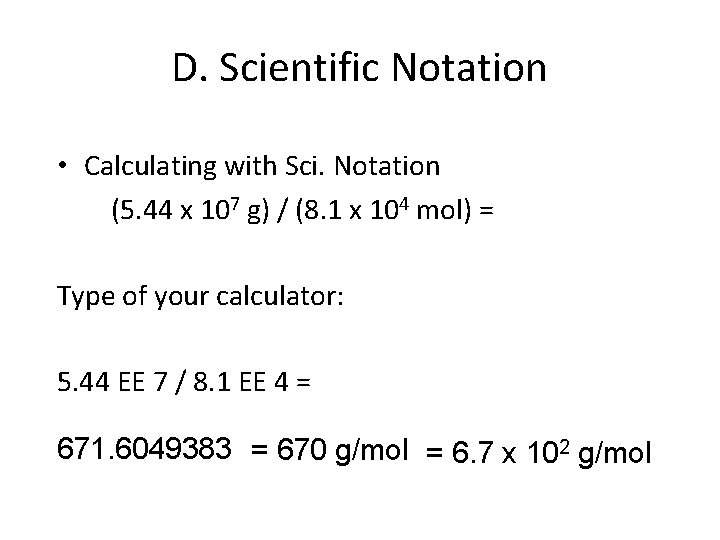
D. Scientific Notation • Calculating with Sci. Notation (5. 44 x 107 g) / (8. 1 x 104 mol) = Type of your calculator: 5. 44 EE 7 / 8. 1 EE 4 = 671. 6049383 = 670 g/mol = 6. 7 x 102 g/mol

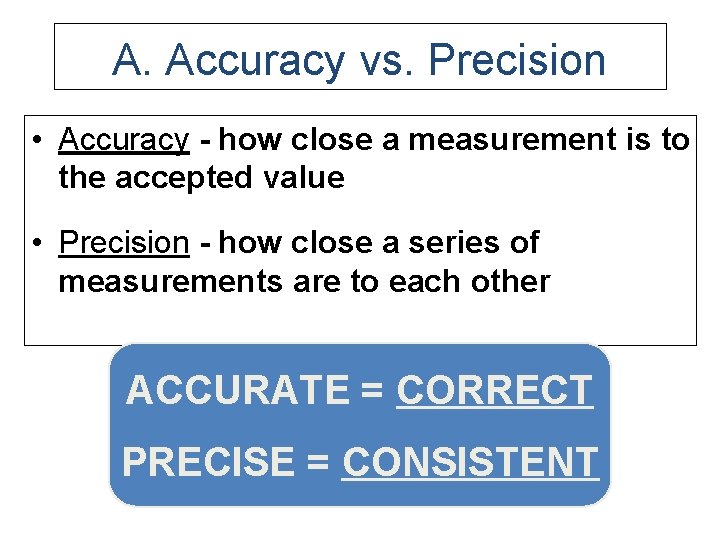
A. Accuracy vs. Precision • Accuracy - how close a measurement is to the accepted value • Precision - how close a series of measurements are to each other ACCURATE = CORRECT PRECISE = CONSISTENT

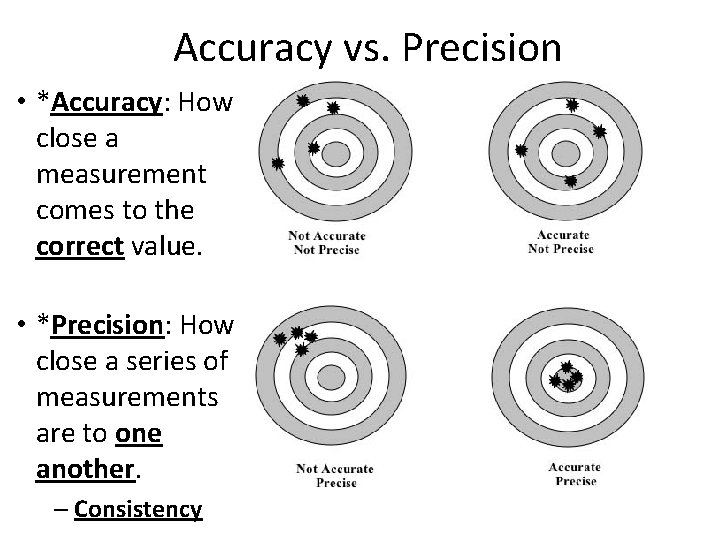
Accuracy vs. Precision • *Accuracy: How close a measurement comes to the correct value. • *Precision: How close a series of measurements are to one another. – Consistency

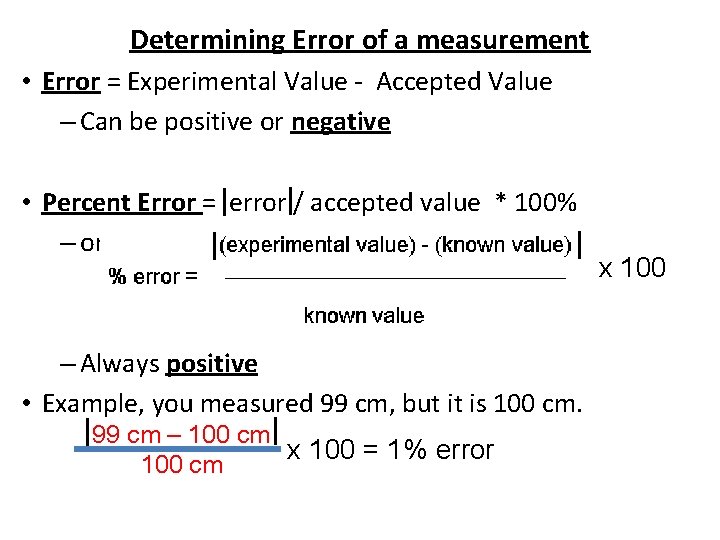
Determining Error of a measurement • Error = Experimental Value - Accepted Value – Can be positive or negative • Percent Error = error / accepted value * 100% – or – Always positive • Example, you measured 99 cm, but it is 100 cm. 99 cm – 100 cm x 100 = 1% error 100 cm x 100

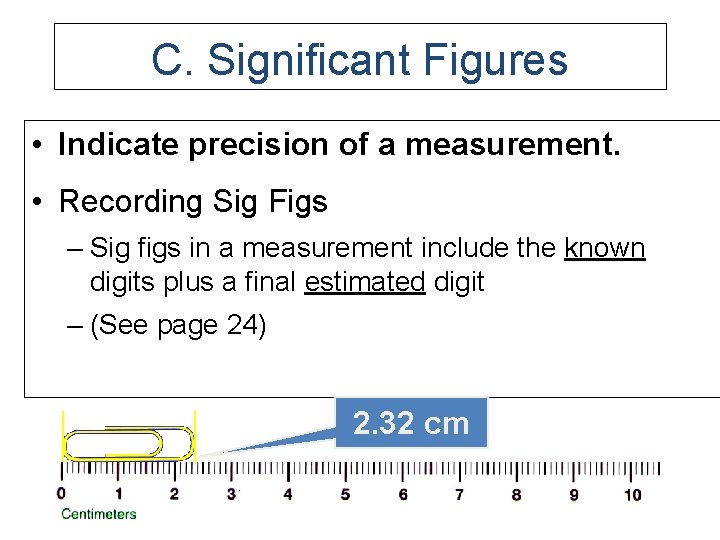
C. Significant Figures • Indicate precision of a measurement. • Recording Sig Figs – Sig figs in a measurement include the known digits plus a final estimated digit – (See page 24) 2. 32 cm

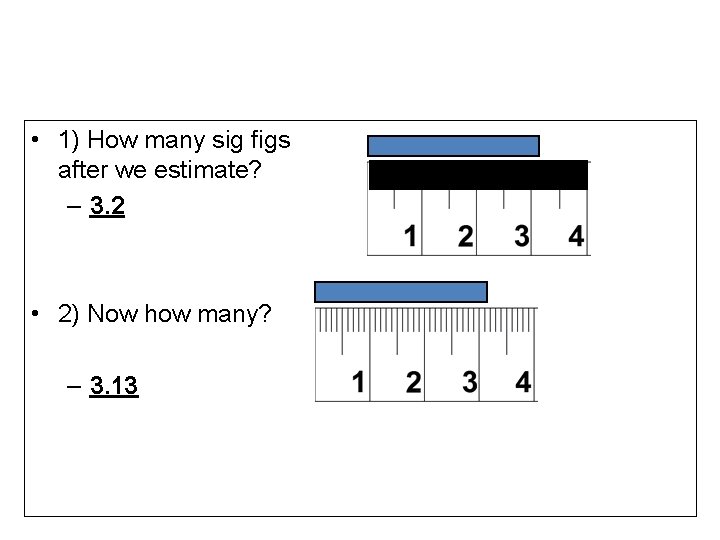
• 1) How many sig figs after we estimate? – 3. 2 • 2) Now how many? – 3. 13

Rules for Sig Figs • Non-Zero Numbers are always significant – Ex). 236 m has 3 sig figs. • Zeros between non-zero numbers are significant. – Ex) 7003 m and 40. 79 m each have 4 sig figs. • Leftmost zeros are not significant – Ex). 000099 m, 0. 42 m, and. 073 m each have 2 sig figs. • Rightmost zeros are significant IF they come before or after a decimal. – Ex) 9000. m, 90. 00 m, and 9. 090 m each have 4 sig figs. • Rightmost zeros with no decimal points are not significant. • Ex) 300 m has 1 sig fig. **The number of left handed people in the class is insignificant compared to the number of right handed people

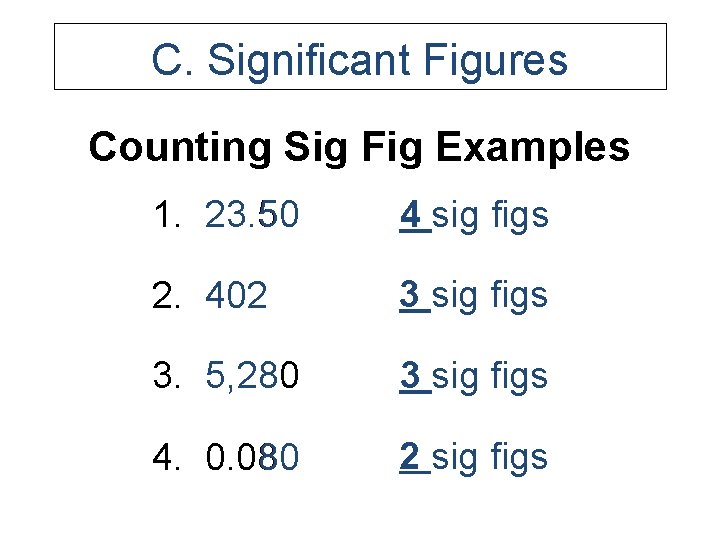
C. Significant Figures Counting Sig Fig Examples 1. 23. 50 4 sig figs 2. 402 3 sig figs 3. 5, 280 3 sig figs 4. 0. 080 2 sig figs

Is a Zero a Significant Number or Not? • 504 L YES – 3 sig figs • . 06 m. L NO – 1 sig figs • 50. 0 m YES – 3 sig figs • 7, 000 km NO – 1 sig fig

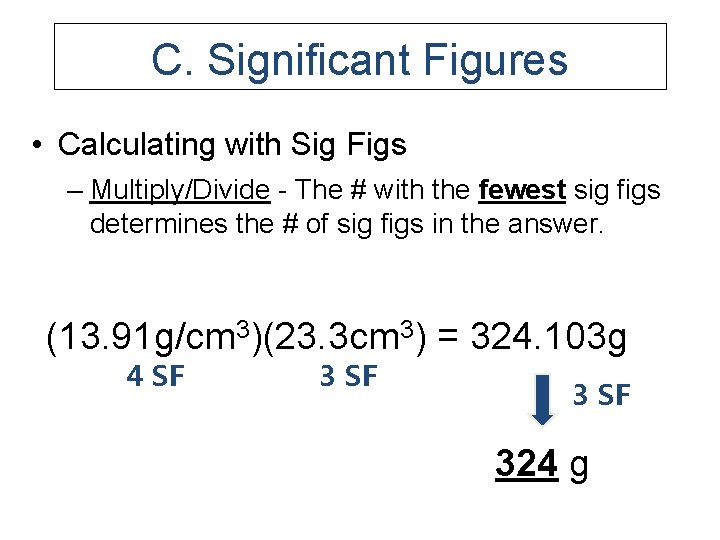
C. Significant Figures • Calculating with Sig Figs – Multiply/Divide - The # with the fewest sig figs determines the # of sig figs in the answer. (13. 91 g/cm 3)(23. 3 cm 3) = 324. 103 g 4 SF 324 g

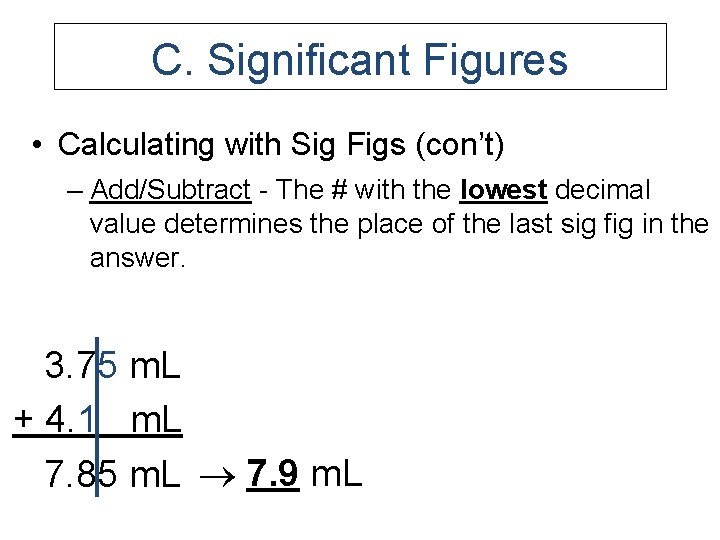
C. Significant Figures • Calculating with Sig Figs (con’t) – Add/Subtract - The # with the lowest decimal value determines the place of the last sig fig in the answer. 3. 75 m. L + 4. 1 m. L 7. 85 m. L 7. 9 m. L

C. Significant Figures • Calculating with Sig Figs (con’t) – Exact Numbers do not limit the # of sig figs in the answer. • Counting numbers: 12 students • Exact conversions: 1 m = 100 cm • “ 1” in any conversion: 1 in = 2. 54 cm

• In this class, delay rounding until the very end. • 1. 8 × 2. 10 × 1. 542 Rounding: 1. 8 x 2. 10 = 3. 8 x 1. 542 = 5. 9 Round at end: 1. 8 x 2. 10 x 1. 542 = 5. 82876 = 5. 8

C. Significant Figures Practice Problems 5. (15. 30 g) ÷ (6. 4 m. L) 4 SF 2 SF = 2. 390625 g/m. L 2. 4 g/m. L 6. 18. 9 g - 0. 84 g 18. 06 g 18. 1 g 2 SF

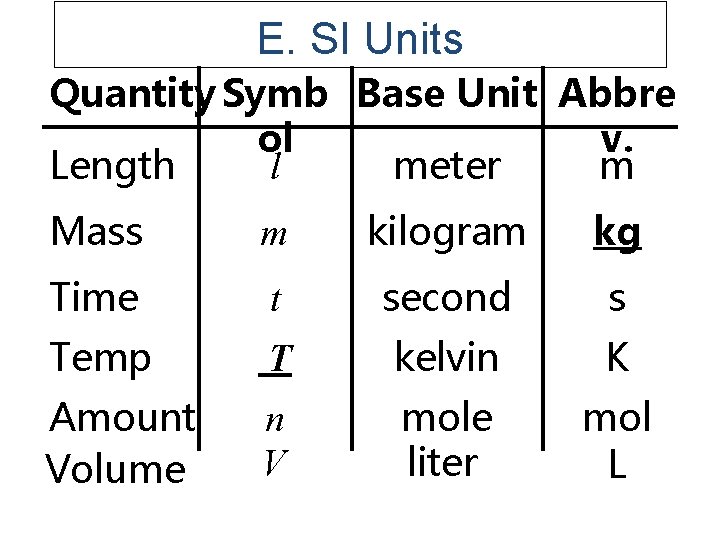
E. SI Units Quantity Symb Base Unit Abbre ol v. Length meter m l Mass m kilogram kg Time t second s Temp T kelvin K Amount Volume n V mole liter mol L

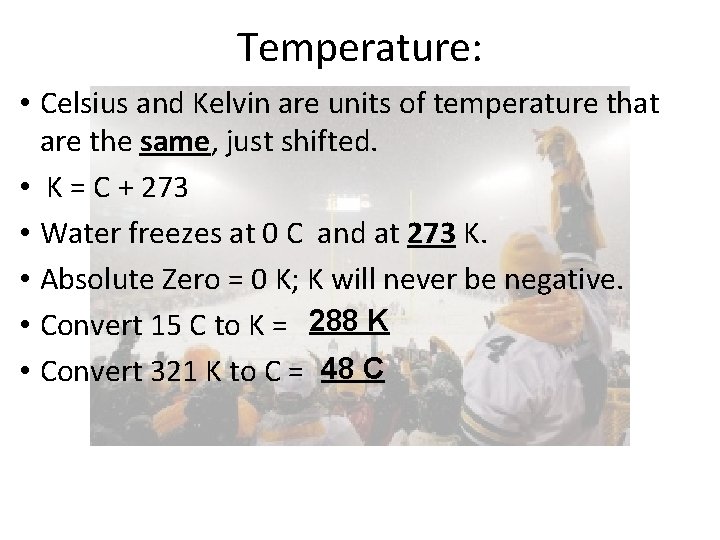
Temperature: • Celsius and Kelvin are units of temperature that are the same, just shifted. • K = C + 273 • Water freezes at 0 C and at 273 K. • Absolute Zero = 0 K; K will never be negative. • Convert 15 C to K = 288 K • Convert 321 K to C = 48 C

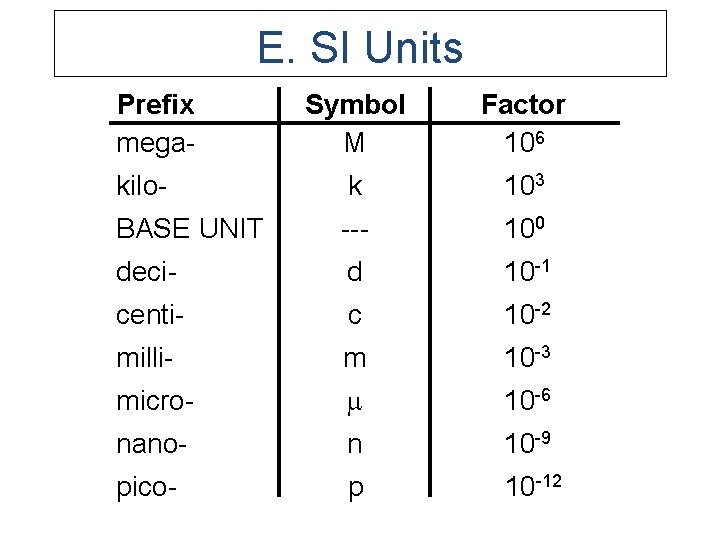
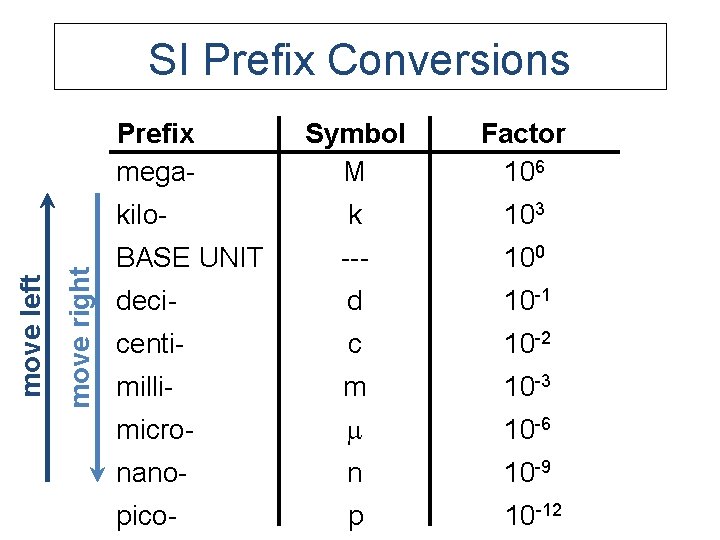
E. SI Units Prefix mega- Symbol M Factor 106 kilo- k 103 BASE UNIT --- 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12

Different way of looking at it… • 1 km = 1000 m • 1 m = 10 dm • 1 m = 100 cm • 1 m = 1000 mm • 1 mm = 1000 um

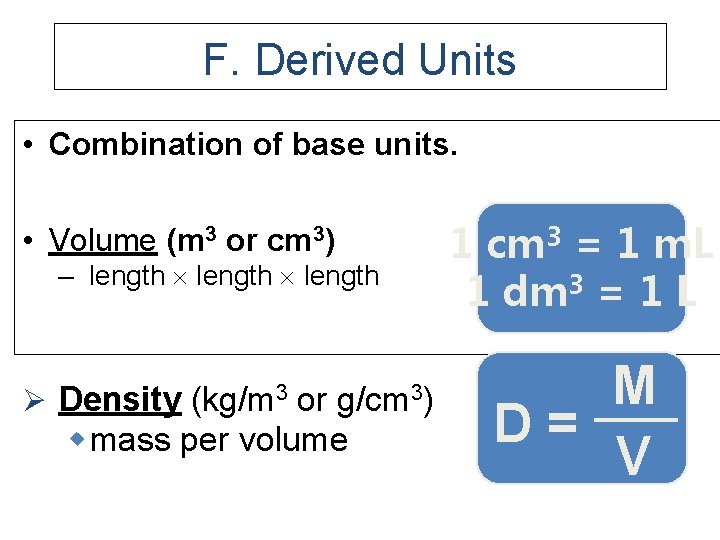
F. Derived Units • Combination of base units. • Volume (m 3 or cm 3) – length Ø Density (kg/m 3 or g/cm 3) w mass per volume 1 cm 3 = 1 m. L 1 dm 3 = 1 L M D= V

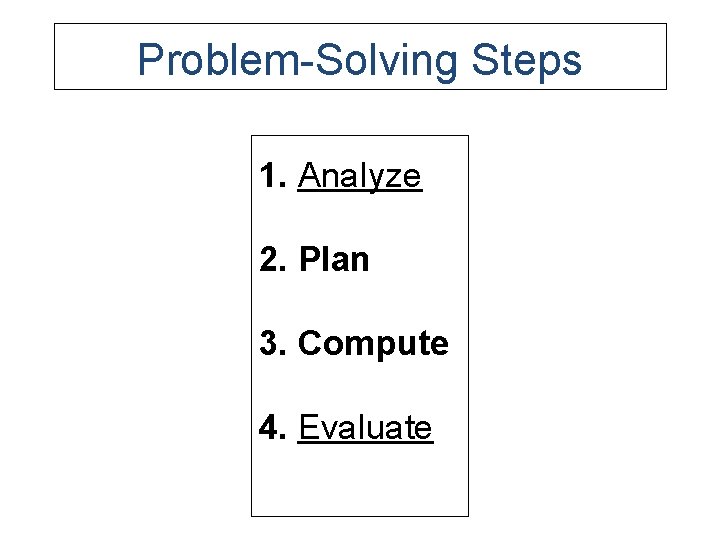
Problem-Solving Steps 1. Analyze 2. Plan 3. Compute 4. Evaluate

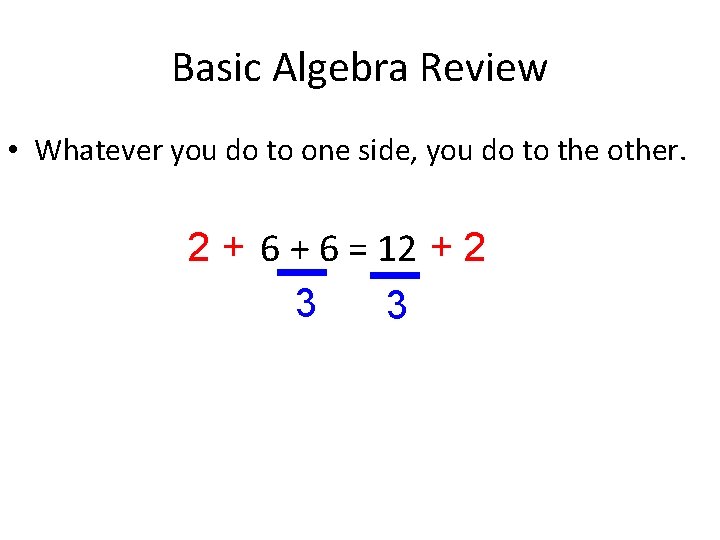
Basic Algebra Review • Whatever you do to one side, you do to the other. 2 + 6 = 12 + 2 3 3

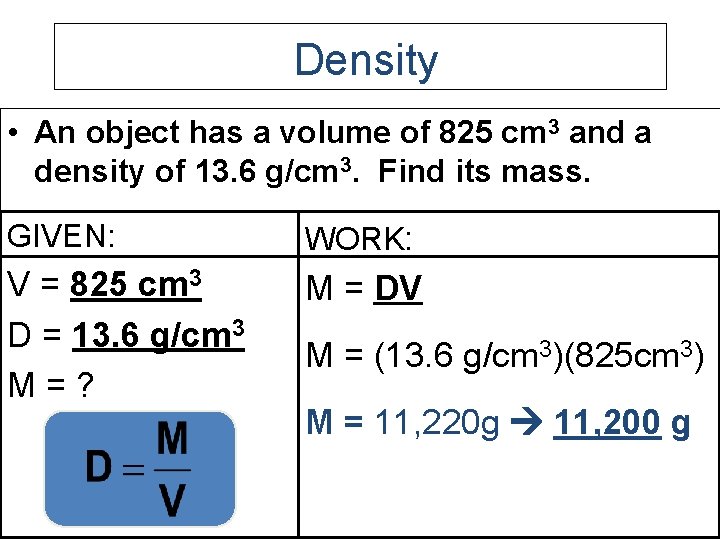
Density • An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M=? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 220 g 11, 200 g

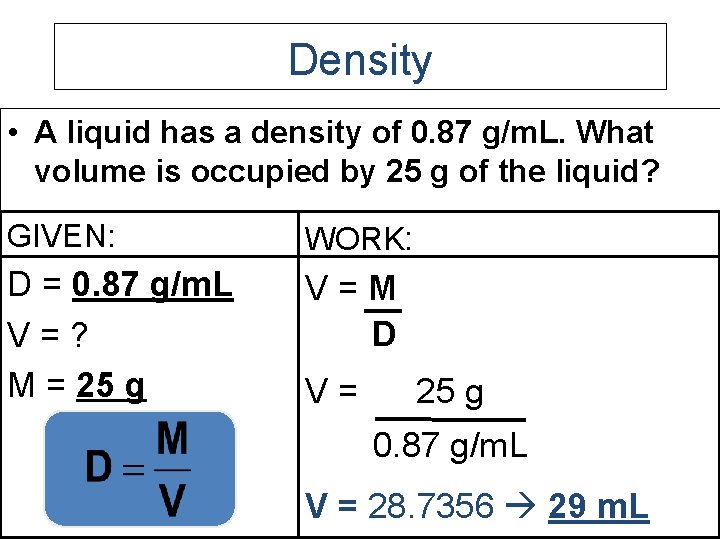
Density • A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V=? M = 25 g V=M D V= 25 g 0. 87 g/m. L V = 28. 7356 29 m. L

SI Prefix Conversions move right move left Prefix mega- Symbol M Factor 106 kilo- k 103 BASE UNIT --- 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12

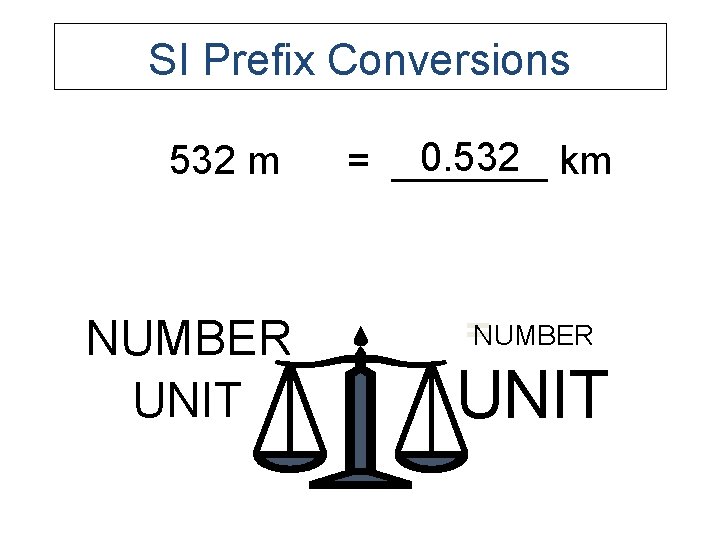
SI Prefix Conversions 532 m NUMBER UNIT 0. 532 km = _______ =NUMBER UNIT

SI Unit Conversions • King __doofus can milk many newts. • k__ d c m m n • kilo, deci, centi, milli, micro, nano • What’s yours?

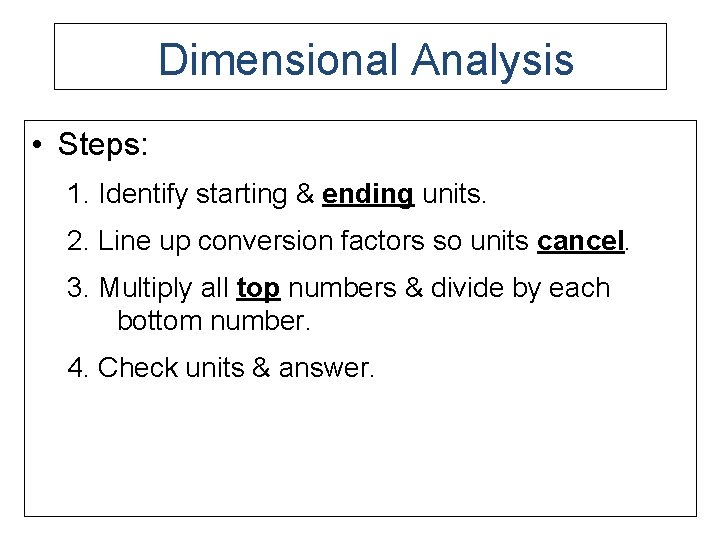
Dimensional Analysis • Steps: 1. Identify starting & ending units. 2. Line up conversion factors so units cancel. 3. Multiply all top numbers & divide by each bottom number. 4. Check units & answer.

Dimensional Analysis • Lining up conversion factors: 1 in = 2. 54 cm =1 2. 54 cm 1 in = 2. 54 cm 1= 1 in

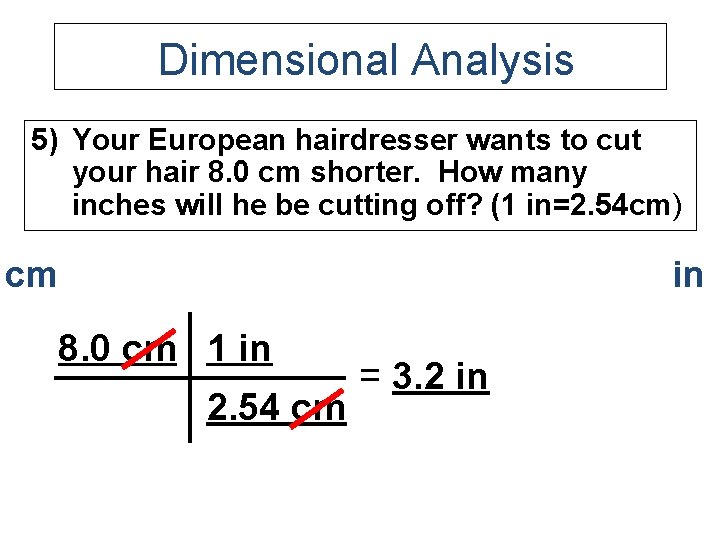
Dimensional Analysis 5) Your European hairdresser wants to cut your hair 8. 0 cm shorter. How many inches will he be cutting off? (1 in=2. 54 cm) cm in 8. 0 cm 1 in 2. 54 cm = 3. 2 in

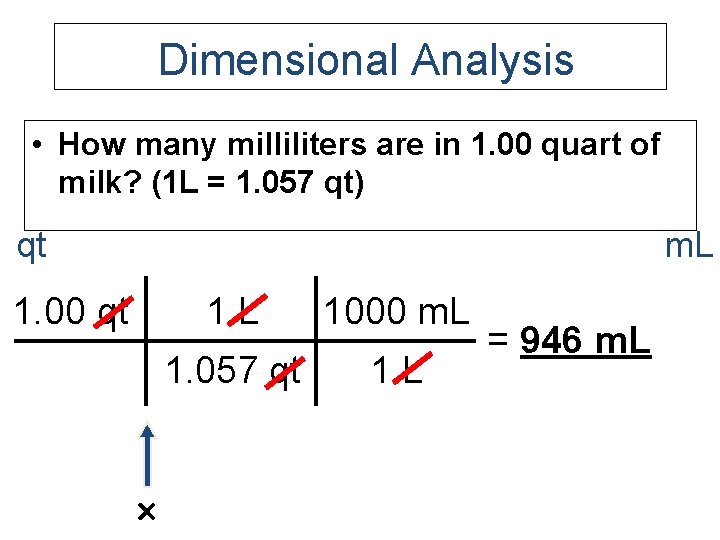
Dimensional Analysis • How many milliliters are in 1. 00 quart of milk? (1 L = 1. 057 qt) qt m. L 1. 00 qt 1 L 1000 m. L 1. 057 qt 1 L = 946 m. L

Dimensional Analysis • You have 1. 5 pounds of gold. Find its volume in cm 3 if the density of gold is 19. 3 g/cm 3. (1 kg = 2. 2 lbs) lb cm 3 1. 5 lb 1 kg 1000 g 1 cm 3 2. 2 lb 1 kg 19. 3 g = 35 cm 3

Dimensional Analysis • Convert 60. 0 km/hr into m/sec 60. 0 km 1000 1 hr m 1 hr 1 min 1 km 60 min 60 sec = 16. 7 m/sec

SI Prefix Conversions 1) 20 cm = 0. 2 _______ m 32 2) 0. 032 L = _______ m. L 3) 45 m = 45, 000 ____ mm 0. 0805 4) 805 dm = _______ km

Example Problems!!!

• 1. How many sig figs in: 1. 480*105. 00070 1. 000080 3. 00 E 3 • 2. Perform these calculations and come up with answers that have the correct number of sig figs: 5. 667 +. 34 5. 007 E 3 / 1. 20 4. 3 - 1. 3590 5. 309 E 3 * 3 • 3. Convert 3. 4 ug into kg. • 4. Convert 7 E 3 cm into Mm. • 5. What is the mass if a substance with a density of 4. 5 g/L has a volume of 3 L?

6. How many sig figs in: 1010 3000. 0 7. Convert 3 kg into g. Convert 34 cm into m. Convert 4 m into cm. 8. You have a 4 kg box. You measure it to be 5. 8 kg. What is the error? The % error? 9. What is the difference between accuracy and precision?

• 11. Put 5, 009, 000 into Scientific Notation • 12. Put 0. 00007890 into Scientific Notation. • 13. Put 8. 7 * 10 -4 into a regular number.

• 14. Which is the largest amount? • A. 2*102 g B. 30 kg C. 45 mg D. 190 g • 15. Convert 40 Kelvin into Celsius • 16. Convert 99 Celsius into Kelvin.
 Vector quantities measure
Vector quantities measure Scalar quantity
Scalar quantity What is vector quantity
What is vector quantity A value that only has magnitude
A value that only has magnitude Quantity y varies inversely as quantity x.
Quantity y varies inversely as quantity x. Scientific inquiry vs scientific method
Scientific inquiry vs scientific method How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Chapter 3 scientific measurement
Chapter 3 scientific measurement Measurement and scientific tools lesson 2
Measurement and scientific tools lesson 2 Understanding science lesson 1 answer key
Understanding science lesson 1 answer key Lesson 2 measurement and scientific tools
Lesson 2 measurement and scientific tools Chemistry chapter 3 scientific measurement
Chemistry chapter 3 scientific measurement Means want satisfying power of a product
Means want satisfying power of a product Kurba ng demand
Kurba ng demand Pivot confidence interval
Pivot confidence interval Velocity formula econ
Velocity formula econ Quantity theory of money equation
Quantity theory of money equation Force is a vector quantity because it has both
Force is a vector quantity because it has both Make verbs agree after prepositional phrases
Make verbs agree after prepositional phrases Quantity factor
Quantity factor Quantity theory of money slideshare
Quantity theory of money slideshare Work = force x distance
Work = force x distance How to calculate real gdp from price and quantity
How to calculate real gdp from price and quantity Strengthening or concentration preparations can be made by:
Strengthening or concentration preparations can be made by: Is distance a scalar quantity
Is distance a scalar quantity Inflation means taking on
Inflation means taking on L quantity
L quantity What is monetarism
What is monetarism Paasche index
Paasche index Equalibrium
Equalibrium Tatiana erukhimova
Tatiana erukhimova Physical quantity of microammeter
Physical quantity of microammeter Marginal unit quantity discount
Marginal unit quantity discount Whole quantity
Whole quantity Quantity
Quantity Quantity theory of money
Quantity theory of money Quantity of angular motion possessed by a body
Quantity of angular motion possessed by a body Eoq formula
Eoq formula Rol in inventory
Rol in inventory Quality over quantity
Quality over quantity Efficient scale quantity
Efficient scale quantity Divide a quantity in a given ratio
Divide a quantity in a given ratio 32000/240
32000/240