MEASUREMENTS Measurements are fundamental to science Measurements may
















- Slides: 19

MEASUREMENTS • Measurements are fundamental to science • Measurements may be: • a. Qualitative • b. Quantitative

MEASUREMENTS • Qualitative Measurement: is a nonnumerical measurement • Example: The solution turned brown when ammonia was added to iron (III) chloride

QUANTITATIVE MEASUREMENTS • Quantitative Measurements: consist of two parts • a. A number • b. A scale (or unit) • Example: • The rock has a mass of 9. 0 kg

SI UNITS AND PREFIXES • • • The SI system used prefixes “Kilo-” means 1000; symbol is k “Hecto” means 100; symbol is h “Deca” mean 10; symbol is da “Base” means 1; meter/liter/gram “Deci-” means 0. 1; symbol is d “Centi-” means 0. 01; symbol is c “Milli-” means 0. 001; symbol is m King Henry Died By Drinking Chocolate Milk

LENGTH • Length- is the distance between two points • The SI unit of length is the meter (m) • Devices used to measure length: • Rulers, Tape Measurers, and Meter Sticks

VOLUME (V) • Volume- is the amount of space an object occupies • Volume of a cube is length * width * height • Volume of a cylinder is V=πr 2 h • The SI unit for volume is m 3(meter cubed) • Other units for V: m. L, cm 3, dm 3 • The Liter (L) is also used for volume of liquids

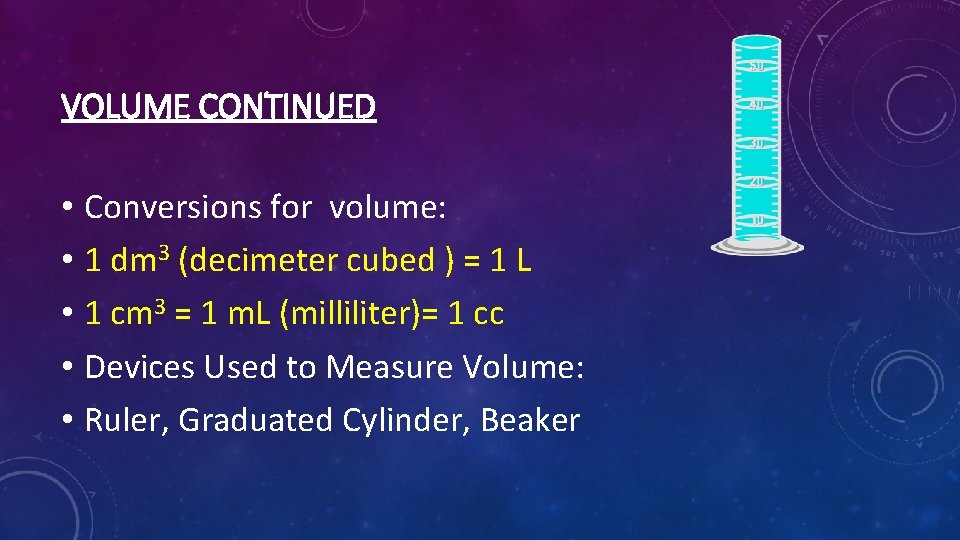
VOLUME CONTINUED • Conversions for volume: • 1 dm 3 (decimeter cubed ) = 1 L • 1 cm 3 = 1 m. L (milliliter)= 1 cc • Devices Used to Measure Volume: • Ruler, Graduated Cylinder, Beaker

MASS • Mass-is the amount of matter that an object contains • SI Unit for Mass is measured in kilograms (kg) • Grams (g) are also used but are very small

TIME • Time- is the interval between two events • Unit of time is the second

TEMPERATURE • Temperature- is the measure of the average kinetic energy of particles • Temperature is measured in Kelvin (K) in the SI system • K = 273 + °C • ˚C= K- 273 • Temperature is usually given in °C

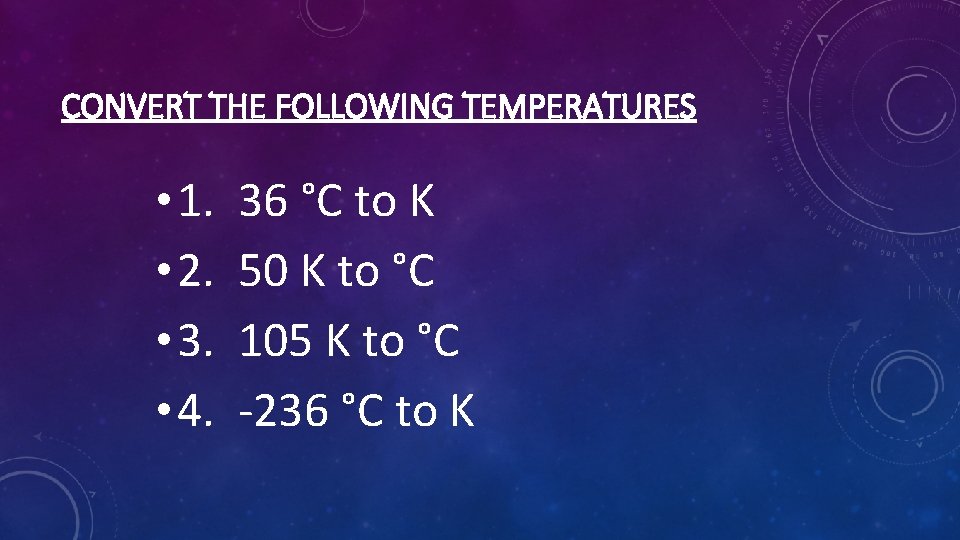
CONVERT THE FOLLOWING TEMPERATURES • 1. • 2. • 3. • 4. 36 °C to K 50 K to °C 105 K to °C -236 °C to K

TERMS USED FOR MEASUREMENTS • In comparing scientific results, the following terms are applied: • a. accuracy • b. precision

ACCURACY • Accuracy refers to the agreement of one experimental result to the true or accepted value • The closer the value is to the true value, the more accurate one is

ACCURACY • Examples of accuracy: • kicking a soccer goal • hitting a three point shot • determining the value of to be 3. 13

PRACTICE: TELL IF THE ACCURACY IS GOOD OR POOR • a. Finding the percent H 2 O 2 to be 2. 9% (the bottle says it contains 3%) • b. Finding that a 2 Liter bottle only contains 1. 5 Liters of soda • c. Filling a 1000 m. L volumetric flask with 1050 m. L of water

PRECISION • Precision refers to how close several different experimental values are to each other

PRECISION • Examples: • scoring three goals in a soccer game • Shooting an air ball five times • finding the value for to be 3. 12, 3. 15, 3. 13

PRECISION AND ACCURACY PROBLEMS • Precision problems usually arise from the skill of the person doing the experiment or the division of the measuring instruments • Accuracy problems usually related to the quality of the equipment used to make measurements

DESCRIBE THE ACCURACY AND PRECISION OF THE FOLLOWING AS EITHER GOOD OR POOR • A student makes the following grades: • a. 99, 100, 98, 100 • b. 45, 43, 44, 42 • c. 100, 23, 60, 89
 Antigentest åre
Antigentest åre My favorite subject is art because
My favorite subject is art because Random errors may be detected by repeating the measurements
Random errors may be detected by repeating the measurements First principle of fingerprint
First principle of fingerprint Hci patterns may or may not include code for implementation
Hci patterns may or may not include code for implementation Social science vs natural science
Social science vs natural science Brances of science
Brances of science Natural and physical science
Natural and physical science Applied science vs pure science
Applied science vs pure science Natural science and social science similarities
Natural science and social science similarities Think central k5
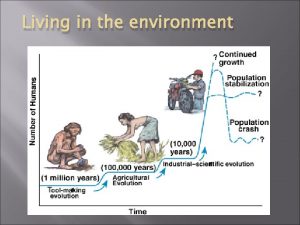
Think central k5 Rule of 70 in population growth
Rule of 70 in population growth Julie lundquist
Julie lundquist Hard science and soft science
Hard science and soft science What two measurements are necessary for calculating speed
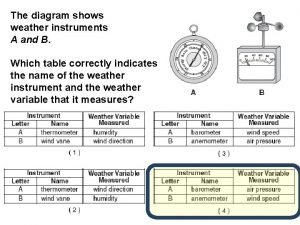
What two measurements are necessary for calculating speed The diagram below shows weather instruments a and b
The diagram below shows weather instruments a and b God genesis
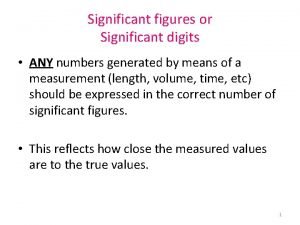
God genesis Significant figures definition
Significant figures definition Ruler sig figs
Ruler sig figs It consists of numbers representing counts or measurements
It consists of numbers representing counts or measurements