Atomic Structure Review Which of the following is















- Slides: 21

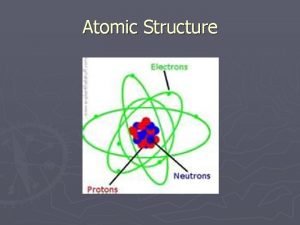
Atomic Structure Review Which of the following is NOT true about electrons? a) They have a negative charge b) They are the heaviest subatomic particle c) They are the lightest subatomic particle d) They are located outside the nucleus

Atomic Structure Review What is the relative mass of an electron? a) 1/1840 the mass of a hydrogen atom b) 1/1840 the mass of a proton + neutron c) 1/1840 the mass of a carbon-12 atom d) 1/1840 the mass of an alpha particle

Atomic Structure Review Which of the following is NOT true about protons? a) They are one of the heaviest subatomic particles b) They are located in the nucleus c) They have a positive charge d) They always equal the number of neutrons in an atom

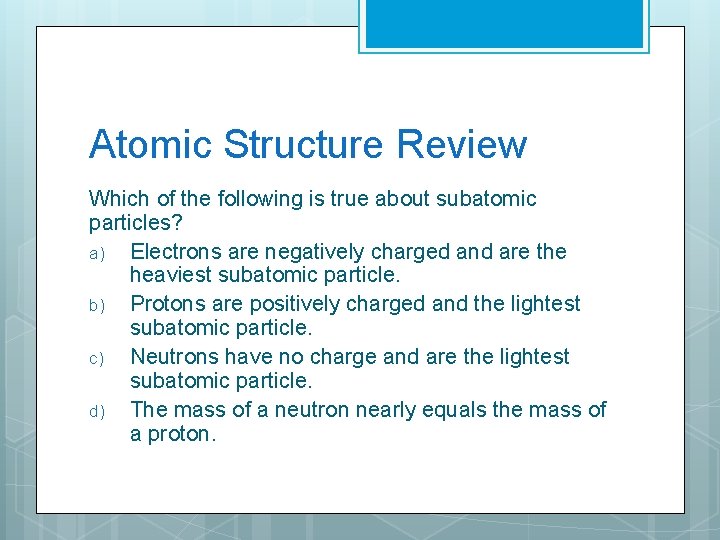
Atomic Structure Review Which of the following is true about subatomic particles? a) Electrons are negatively charged and are the heaviest subatomic particle. b) Protons are positively charged and the lightest subatomic particle. c) Neutrons have no charge and are the lightest subatomic particle. d) The mass of a neutron nearly equals the mass of a proton.

Atomic Structure Review The number of electrons in a neutral atom is equal to the number of protons and neutrons. a) True b) False

Atomic Structure Review The atomic number of an element is equal to the total number of which particles in the nucleus? a) Protons b) Electrons c) Neutrons d) Protons and neutrons

Atomic Structure Review Atoms are mostly empty space a) True b) False

Atomic Structure Review Atoms of the same element can have different masses. a) True b) False

Atomic Structure Review The number of neutrons in the nucleus of an atoms is found by subtracting the atomic number from the mass number of the isotope. a) True b) False

Atomic Structure Review In an isotope symbol, the atomic number is in the top left corner. a) True b) False

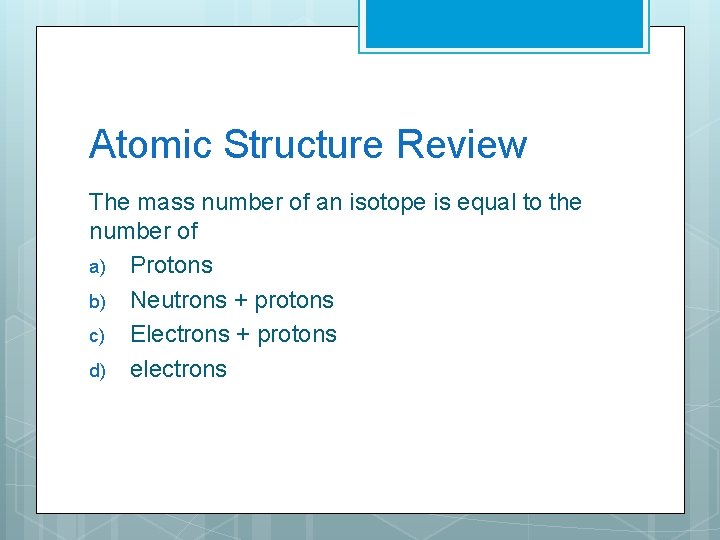
Atomic Structure Review The mass number of an isotope is equal to the number of a) Protons b) Neutrons + protons c) Electrons + protons d) electrons

Atomic Structure Review The nucleus of an atom is positively charged and has more protons than neutrons. a) True b) False

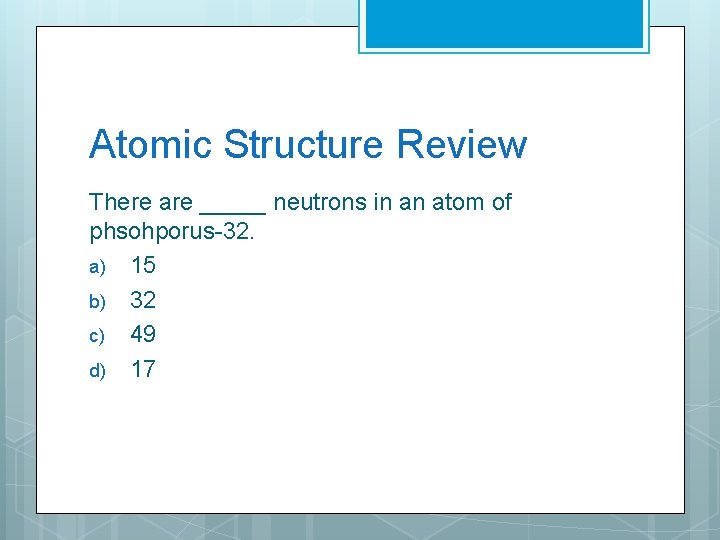
Atomic Structure Review There are _____ neutrons in an atom of phsohporus-32. a) 15 b) 32 c) 49 d) 17

Atomic Structure Review Isotopes of the same element have different numbers of protons. a) True b) False

Atomic Structure Review The top number in an isotope symbol is always the a) Atomic number b) Number of neutrons c) Number of protons d) Mass number

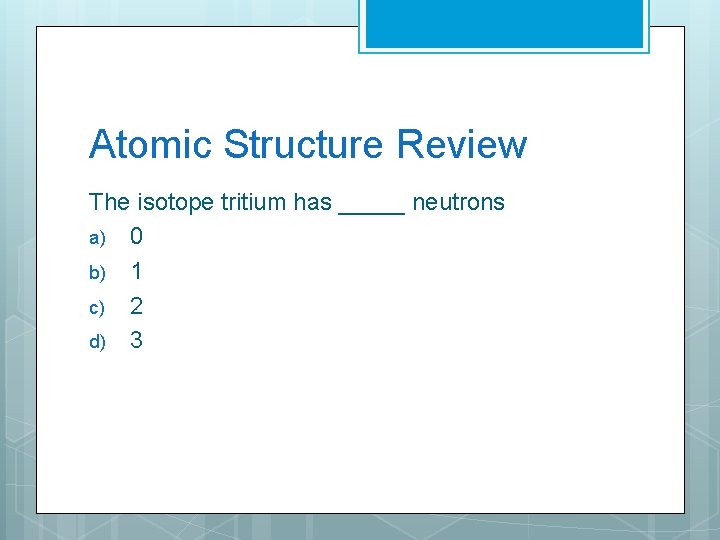
Atomic Structure Review The isotope tritium has _____ neutrons a) 0 b) 1 c) 2 d) 3

Atomic Structure Review In the name fluorine-19, the 19 represents a) The mass number b) The atomic number c) The number of protons and electrons d) The number of neutrons

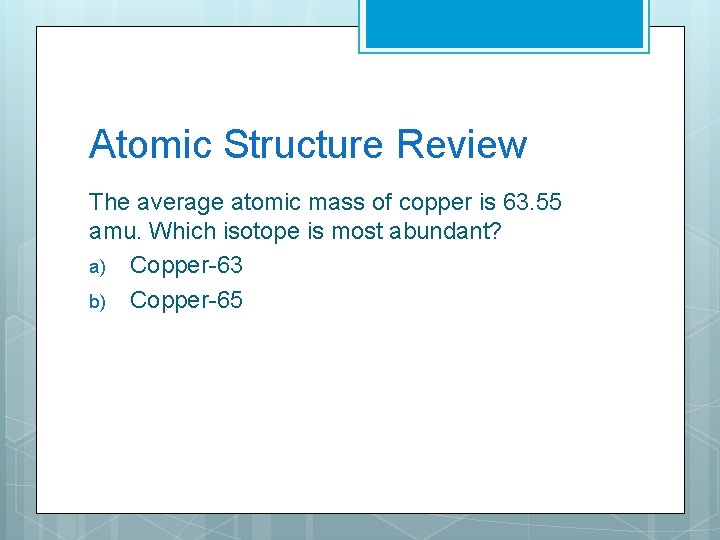
Atomic Structure Review The average atomic mass of copper is 63. 55 amu. Which isotope is most abundant? a) Copper-63 b) Copper-65

Atomic Structure Review One atomic mass unit is equal to which of the following a) The mass of a hydrogen-1 atom b) The mass of one electron c) The mass of one carbon-12 atom d) One-twelfth the mass of a carbon-12 atom

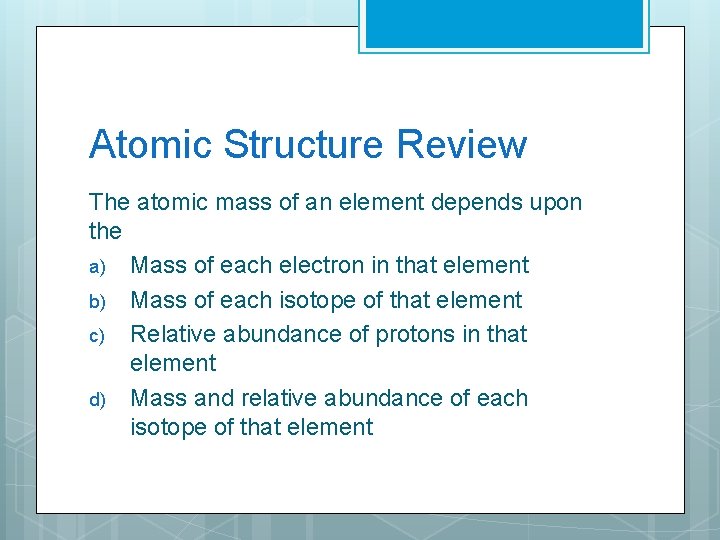
Atomic Structure Review The atomic mass of an element depends upon the a) Mass of each electron in that element b) Mass of each isotope of that element c) Relative abundance of protons in that element d) Mass and relative abundance of each isotope of that element

Atomic Structure Review What unit is used to measure average atomic mass? a) Angstrom b) Gram c) Nanogram d) Amu
 Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Period trends
Period trends The atomic radius in periodic table
The atomic radius in periodic table Difference between atomic mass and mass number
Difference between atomic mass and mass number Differentiate between atomic number and mass number
Differentiate between atomic number and mass number Atomic number vs atomic radius
Atomic number vs atomic radius Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry How electrons are arranged in an atom
How electrons are arranged in an atom 460+370
460+370 Full bridge rectifier animation
Full bridge rectifier animation What is ionisation
What is ionisation First ionization energy of calcium
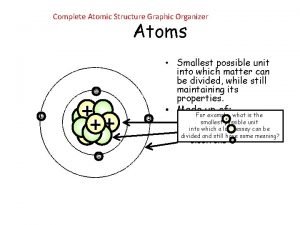
First ionization energy of calcium Atoms graphic organizer
Atoms graphic organizer Atomic structure timeline
Atomic structure timeline Z atomic symbol
Z atomic symbol Tungsten atomic structure
Tungsten atomic structure Nuclear symbol notation
Nuclear symbol notation Atomic structure poster project
Atomic structure poster project History of the atom graphic organizer
History of the atom graphic organizer Schrodinger atom model
Schrodinger atom model Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Dalton atom modeli
Dalton atom modeli