Introduction to Analytical Chemistry CHAPTER 18 CHEMICALS AND







- Slides: 24

Introduction to Analytical Chemistry CHAPTER 18 CHEMICALS AND APPARATUS: PUTTING THE TOOLS TO WORK

18 A-1 Classifying Chemicals Reagent Grade Reagent-grade chemicals conform to the minimum standards set forth by the Reagent Chemical Committee of the American Chemical Society. 18 -2 Copyright © 2011 Cengage Learning

18 A-1 Classifying Chemicals Primary-Standard Grade National Institute of Standards and Technology is an excellent source for primary standards. This agency also provides reference standards, which are complex substances that have been exhaustively analyzed. 18 -3 Copyright © 2011 Cengage Learning

18 A-1 Classifying Chemicals Special-Purpose Reagent Chemicals Included among these are solvents for spectrophotometry and high-performance liquid chromatography. 18 -4 Copyright © 2011 Cengage Learning

18 D-1 Types of Analytical Balances An analytical balance is a weighing instrument with a maximum capacity that ranges from 1 g to a few kilograms with a precision of at least 1 part in 10⁵ at maximum capacity. A typical microanalytical balance has a capacity of 1 to 3 g and a precision of ± 0. 001 mg. 18 -5 Copyright © 2011 Cengage Learning

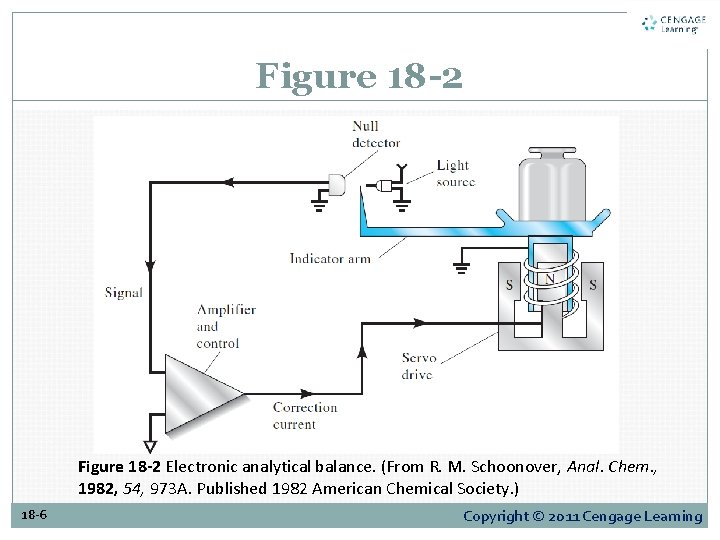
Figure 18 -2 Electronic analytical balance. (From R. M. Schoonover, Anal. Chem. , 1982, 54, 973 A. Published 1982 American Chemical Society. ) 18 -6 Copyright © 2011 Cengage Learning

18 D-3 The Single-Pan Mechanical Analytical Balance Figure 18 -4 is a diagram of a typical single-pan mechanical balance. 18 -7 Copyright © 2011 Cengage Learning

18 D-5 Sources of Error in Weighing Correction for Buoyancy A buoyancy error will affect data if the density of the object being weighed differs significantly from that of the standard weights. 18 -8 Copyright © 2011 Cengage Learning

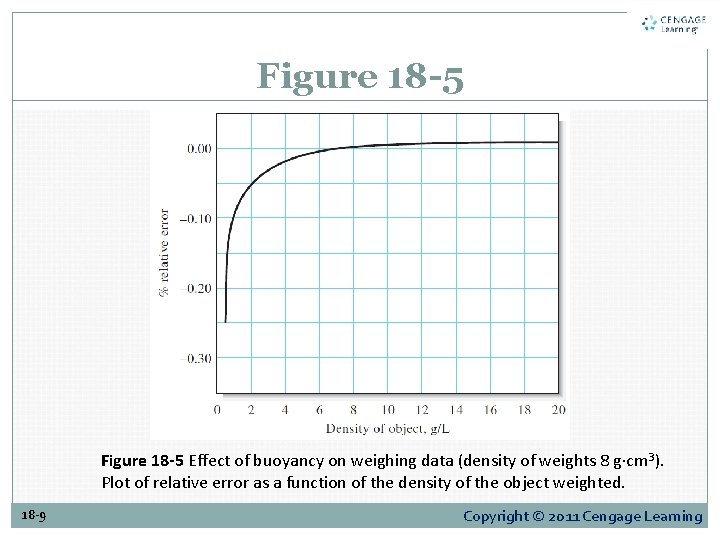
Figure 18 -5 Effect of buoyancy on weighing data (density of weights 8 g·cm 3). Plot of relative error as a function of the density of the object weighted. 18 -9 Copyright © 2011 Cengage Learning

18 D-5 Sources of Error in Weighing Temperature Effects Attempts to weigh an object whose temperature is different from that of its surroundings will result in a significant error. Errors due to a difference in temperature have two sources. First, convection currents within the balance case exert a buoyant effect on the pan and object. Second, warm air trapped in a closed container weighs less than the same volume at a lower temperature. 18 -10 Copyright © 2011 Cengage Learning

18 D-5 Sources of Error in Weighing Other Sources of Error A porcelain or glass object will occasionally acquire a static charge that is sufficient to cause a balance to perform erratically. 18 -11 Copyright © 2011 Cengage Learning

Figure 18 -7 Typical weighing bottles. 18 -12 Copyright © 2011 Cengage Learning

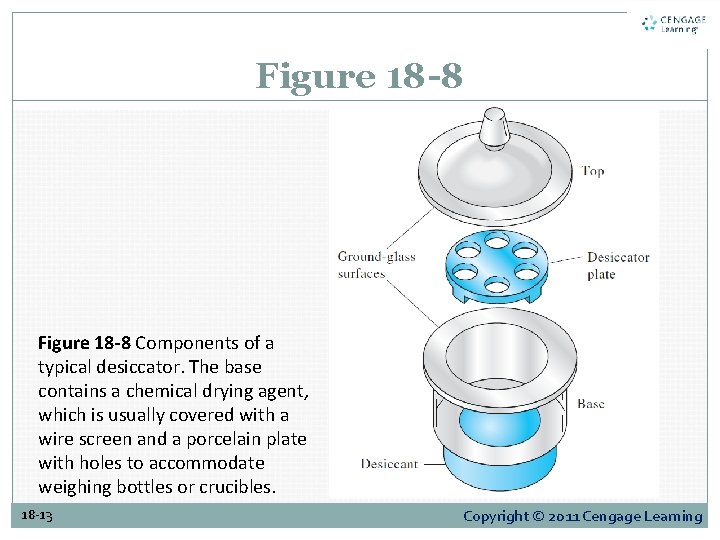
Figure 18 -8 Components of a typical desiccator. The base contains a chemical drying agent, which is usually covered with a wire screen and a porcelain plate with holes to accommodate weighing bottles or crucibles. 18 -13 Copyright © 2011 Cengage Learning

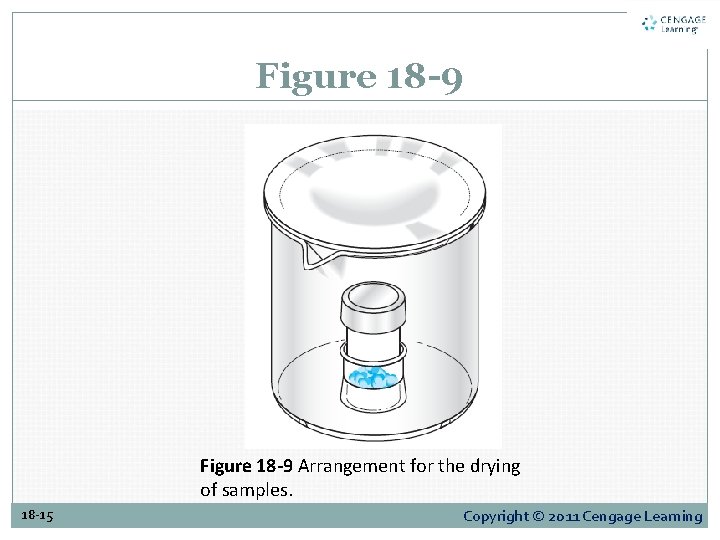
18 E-3 Manipulating Weighing Bottles Figure 18 -9 depicts the arrangement recommended for drying a sample. The weighing bottle is contained in a labeled beaker with a ribbed cover glass. 18 -14 Copyright © 2011 Cengage Learning

Figure 18 -9 Arrangement for the drying of samples. 18 -15 Copyright © 2011 Cengage Learning

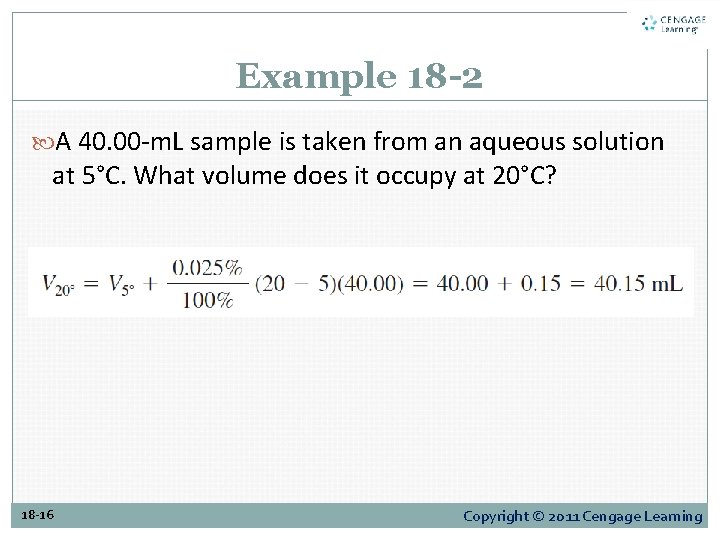
Example 18 -2 A 40. 00 -m. L sample is taken from an aqueous solution at 5°C. What volume does it occupy at 20°C? 18 -16 Copyright © 2011 Cengage Learning

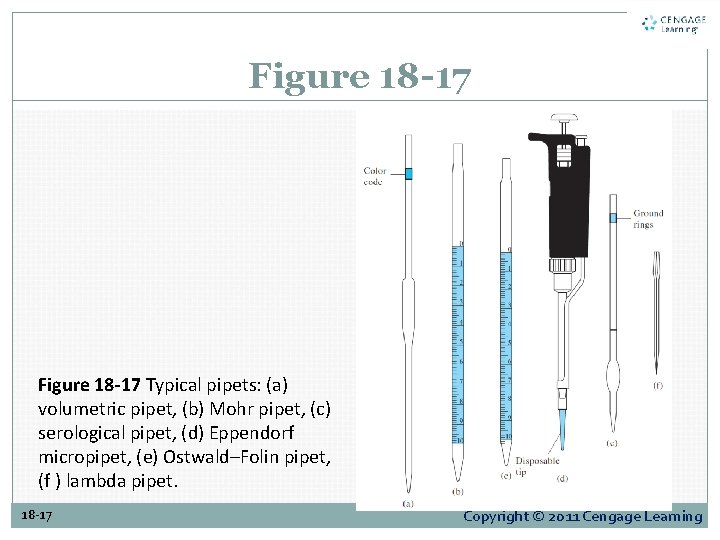
Figure 18 -17 Typical pipets: (a) volumetric pipet, (b) Mohr pipet, (c) serological pipet, (d) Eppendorf micropipet, (e) Ostwald–Folin pipet, (f ) lambda pipet. 18 -17 Copyright © 2011 Cengage Learning

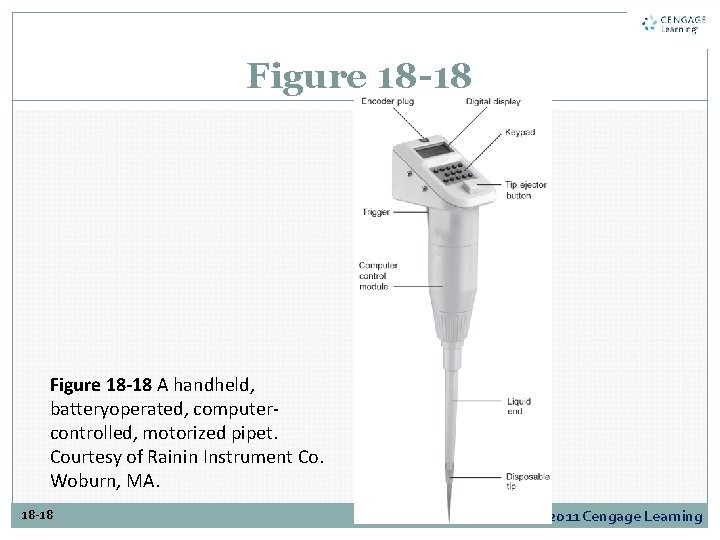
Figure 18 -18 A handheld, batteryoperated, computercontrolled, motorized pipet. Courtesy of Rainin Instrument Co. Woburn, MA. 18 -18 Copyright © 2011 Cengage Learning

18 H Calibrating Volumetric Ware Volumetric glassware is calibrated by measuring the mass of a liquid (usually distilled water) of known density and temperature that is contained in the volumetric ware. In carrying out a calibration, a buoyancy correction must be made (Section 18 D-4) since the density of water is quite different from that of the weights. 18 -19 Copyright © 2011 Cengage Learning

18 I The Laboratory Notebook The book should be permanently bound with consecutively numbered pages. 18 -20 Copyright © 2011 Cengage Learning

18 I-1 Maintaining the Laboratory Notebook 1. Record all data and observations directly into the notebook in ink. 2. Supply each entry or series of entries with a heading or label. 3. Date each page of the notebook as it is used. 4. Never attempt to erase or obliterate an incorrect entry. Instead, cross it out 5. Never remove a page from the notebook. 18 -21 Copyright © 2011 Cengage Learning

18 J Using Spreadsheets in Analytical Chemistry The spreadsheet is one of the most useful tools that has resulted from the personal computer revolution. Spreadsheets are used for record keeping, mathematical calculations, statistical analysis, curve fitting, data plotting, financial analysis, database management, and a variety of other tasks. 18 -22 Copyright © 2011 Cengage Learning

18 K Safety in The Laboratory Strict adherence to the rules will go far toward preventing (or minimizing the effect of ) accidents. 18 -23 Copyright © 2011 Cengage Learning

THE END 18 -24 Copyright © 2011 Cengage Learning
 Commodity vs specialty chemicals
Commodity vs specialty chemicals Introduction to analytical chemistry
Introduction to analytical chemistry Mechanical entrapment coprecipitation
Mechanical entrapment coprecipitation Analytical chemistry chapter 1
Analytical chemistry chapter 1 Analytical chemistry definition and examples
Analytical chemistry definition and examples Analysis
Analysis Errors in analytical chemistry
Errors in analytical chemistry Analytical chemistry statistics
Analytical chemistry statistics Chemistry apparatus
Chemistry apparatus Calibration verification and linearity
Calibration verification and linearity Normal error curve in analytical chemistry
Normal error curve in analytical chemistry What is equivalent weight in chemistry
What is equivalent weight in chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Q test in analytical chemistry
Q test in analytical chemistry Annual review of analytical chemistry
Annual review of analytical chemistry Correlation coefficient in analytical chemistry
Correlation coefficient in analytical chemistry Analytical chemistry
Analytical chemistry Excellence in analytical chemistry
Excellence in analytical chemistry Threshold limit value of ammonia
Threshold limit value of ammonia Unit 3 house and home
Unit 3 house and home светр
светр Chapter 1 introduction to chemistry
Chapter 1 introduction to chemistry Introduction to chemistry chapter 1
Introduction to chemistry chapter 1 Introduction to analytical separations
Introduction to analytical separations Immiscible
Immiscible