What is chemistry Chemistry The study of the








- Slides: 20

What is chemistry? Chemistry: The study of the composition, structure, and properties of matter and the changes it undergoes. Composition: Structure: Properties: Change:

Chemistry is the study of everything! Why is Chemistry Important?

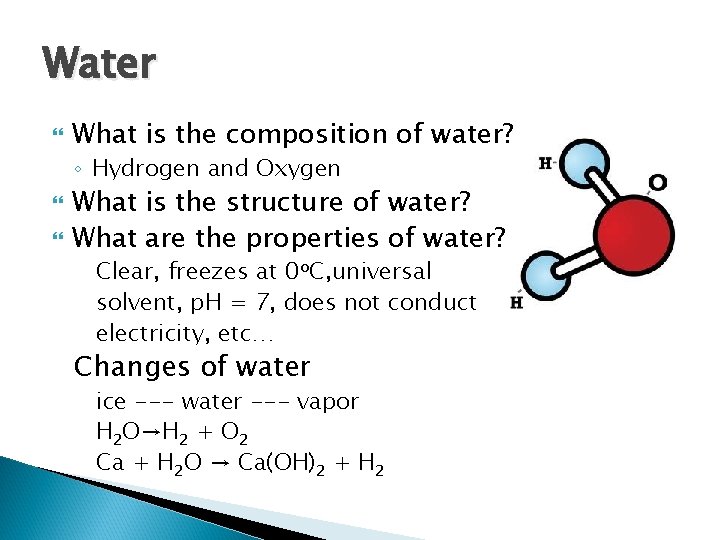
Water What is the composition of water? ◦ Hydrogen and Oxygen What is the structure of water? What are the properties of water? Clear, freezes at 0 o. C, universal solvent, p. H = 7, does not conduct electricity, etc… Changes of water ice --- water --- vapor H 2 O→H 2 + O 2 Ca + H 2 O → Ca(OH)2 + H 2

The Branches of Chemistry

Organic Chemistry The study of carbon containing compounds.

Inorganic Chemistry The study of compounds that do not contain carbon.

Physical Chemistry The study of the properties and changes in matter and their relation to energy.

Analytical Chemistry The identification of the components and composition of materials.

Biochemistry The study of substances and processes occurring in living things.

Theoretical Chemistry The use of mathematics and computers to understand the principles behind observed chemical behavior and to design and predict the properties of new compounds.

Questions In which of the branches of chemistry would a scientist be working if he or she were doing the following: ◦ Investigating energy relationships for various chemical reactions. ◦ Comparing properties of glucose (C 6 H 12 O 6) with sucrose (C 12 H 22 O 11). ◦ Studying reactions that occur during the digestion of food.

Matter and Its Properties Matter: Anything that has mass and takes up space (has a volume). How is matter quantified?

Mass vs. Weight Mass: A measure of the quantity of matter within an object. ◦ As long as the amount of matter does not change, neither should the mass. Weight: A measure of the earth’s gravitational pull on an object. ◦ Because the earth’s gravitational pull on an object can vary from place to place, so can an objects weight ◦ http: //www. nyu. edu/pages/mathmol/textbook/w eightvmass. html

Physical or Chemical Properties can help identify an unknown substance. A property can either be extensive or intensive. ◦ Extensive: properties that depend upon the amount of matter present ◦ Intensive: properties that do not depend upon the amount of matter present.

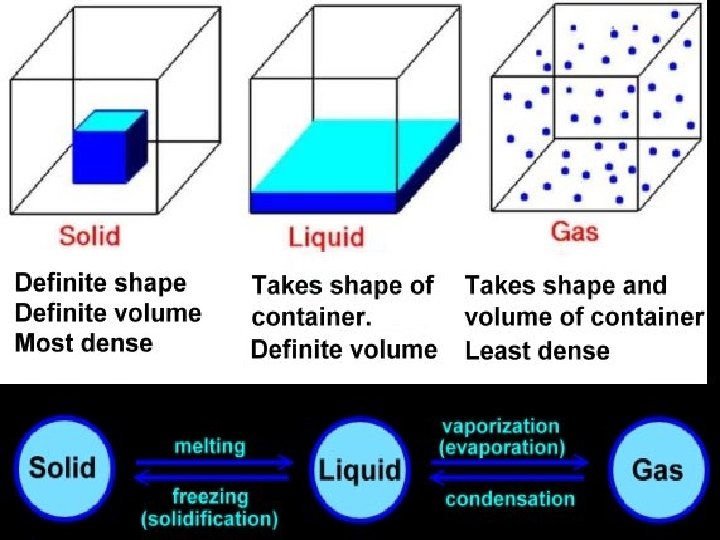
Physical or Chemical Properties and change can either be physical or chemical. Test question: Do I have the same substance that I started with? Physical NO change in the identity of the substance. Chemical The identity of the substance HAS changed.

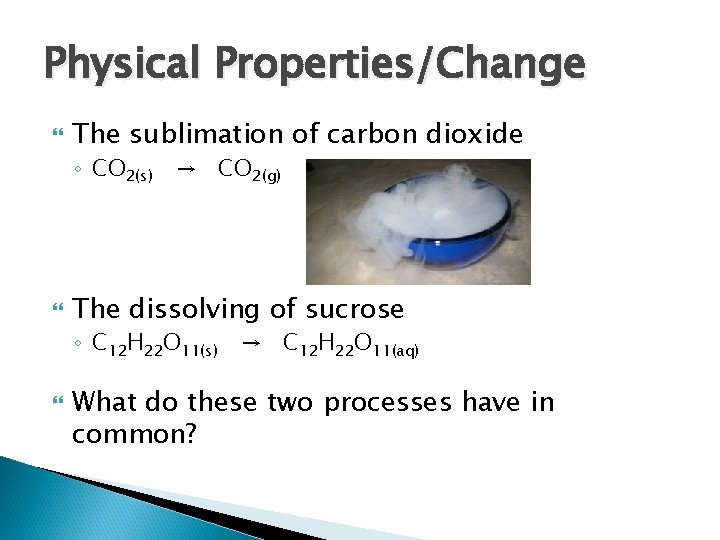
Physical Properties/Change The sublimation of carbon dioxide ◦ CO 2(s) → CO 2(g) The dissolving of sucrose ◦ C 12 H 22 O 11(s) → C 12 H 22 O 11(aq) What do these two processes have in common?


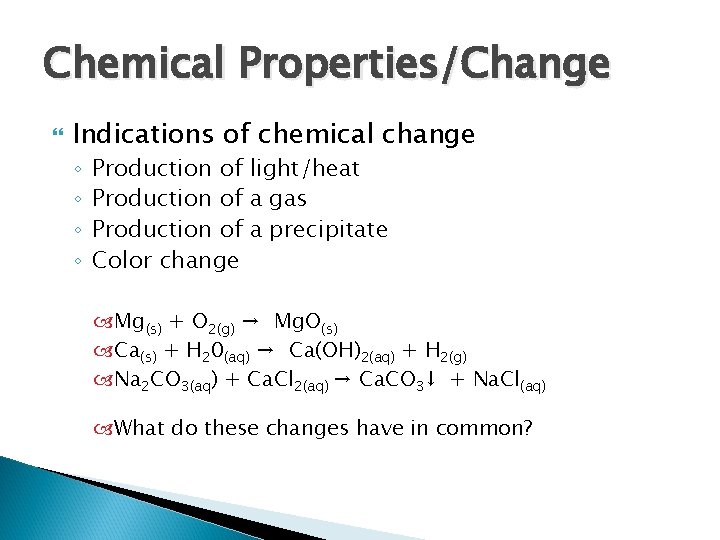
Chemical Properties/Change Indications of chemical change ◦ ◦ Production of light/heat Production of a gas Production of a precipitate Color change Mg(s) + O 2(g) → Mg. O(s) Ca(s) + H 20(aq) → Ca(OH)2(aq) + H 2(g) Na 2 CO 3(aq) + Ca. Cl 2(aq) → Ca. CO 3↓ + Na. Cl(aq) What do these changes have in common?

-Water decomposes through electrolysis to produce hydrogen and oxygen. -Calcium nitrate is heated to 561 o. C and liquefied. -An aqueous solution is prepared by dissolving potassium chromate in water. -Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen gas.

Questions In trying to identify a sample of a pure substance, we observe the following properties. Identify each as physical or chemical: ◦ ◦ ◦ Its mass is 124. 3 g. It melts when heated to 670 o. C. It is easily etched by nitric acid. It is a good heat conductor. It burns in the air.