Developed in association with the European Thoracic Oncology


















- Slides: 112

Developed in association with the European Thoracic Oncology Platform European Cancer Congress (ECC) 2015 25– 29 September 2015 Vienna, Austria Supported by Eli Lilly and Company has not influenced the content of this publication

Letter from Prof Rolf Stahel Dear Colleagues It is my pleasure to present this ETOP slide set which has been designed to highlight and summarise key findings in thoracic cancers from the major congresses in 2015. This slide set specifically focuses on the 18 th ECCO – 40 th ESMO European Cancer Congress (ECC) and is available in 4 languages – English, French, Italian and Japanese. The area of clinical research in oncology is a challenging and ever changing environment. Within this environment we all value access to scientific data and research which helps to educate and inspire further advancements in our roles as scientists, clinicians and educators. I hope you find this review of the latest developments in thoracic cancers of benefit to you in your practice. If you would like to share your thoughts with us we would welcome your comments. Please send any correspondence to etop@etop. eu-org. I would like to thank our ETOP members Drs Solange Peters and Martin Reck for their roles as Editors – for prioritising abstracts and reviewing slide content – also Dr Serena Ricciardi for overseeing translation to Italian. The slide set you see before you would not be possible without their commitment and hard work. And finally, we are also very grateful to Lilly Oncology for their financial, administerial and logistical support in the realisation of this complex yet rewarding activity. Yours sincerely, Rolf Stahel President, ETOP Foundation Council

ETOP Medical Oncology Slide Deck Editors 2015 Focus: Advanced NSCLC (not radically treatable stage III & stage IV) and related biomarker data Dr Solange Peters Multidisciplinary Oncology Center, Lausanne Cancer Center, Lausanne, Switzerland Focus: Early and locally advanced NSCLC (stage I–III) and related biomarker data / other malignancies Dr Martin Reck Department of Thoracic Oncology, Hospital Grosshansdorf, Germany

Contents • Biomarkers and screening • Early stage and locally advanced NSCLC – Stages I, II and III • Advanced NSCLC – Not radically treatable stage III and stage IV – 1 st line – Later lines • Other malignancies – SCLC and mesothelioma – Rare tumours – Brain metastases

Biomarkers and screening

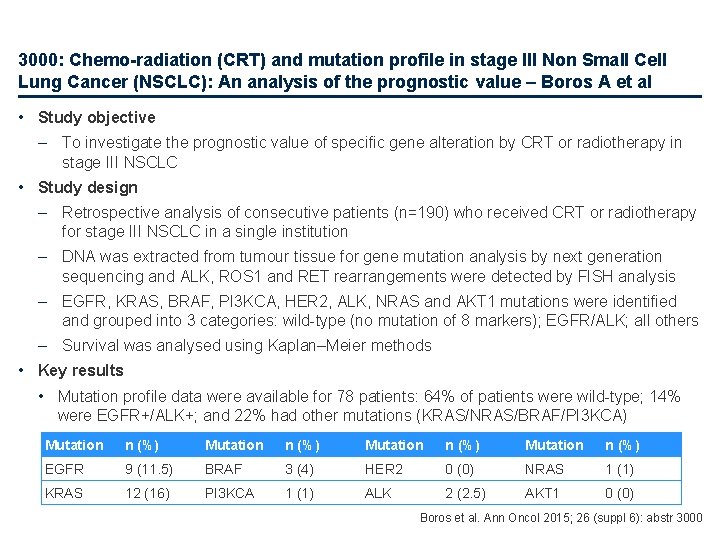
3000: Chemo-radiation (CRT) and mutation profile in stage III Non Small Cell Lung Cancer (NSCLC): An analysis of the prognostic value – Boros A et al • Study objective – To investigate the prognostic value of specific gene alteration by CRT or radiotherapy in stage III NSCLC • Study design – Retrospective analysis of consecutive patients (n=190) who received CRT or radiotherapy for stage III NSCLC in a single institution – DNA was extracted from tumour tissue for gene mutation analysis by next generation sequencing and ALK, ROS 1 and RET rearrangements were detected by FISH analysis – EGFR, KRAS, BRAF, PI 3 KCA, HER 2, ALK, NRAS and AKT 1 mutations were identified and grouped into 3 categories: wild-type (no mutation of 8 markers); EGFR/ALK; all others – Survival was analysed using Kaplan–Meier methods • Key results • Mutation profile data were available for 78 patients: 64% of patients were wild-type; 14% were EGFR+/ALK+; and 22% had other mutations (KRAS/NRAS/BRAF/PI 3 KCA) Mutation n (%) EGFR 9 (11. 5) BRAF 3 (4) HER 2 0 (0) NRAS 1 (1) KRAS 12 (16) PI 3 KCA 1 (1) ALK 2 (2. 5) AKT 1 0 (0) Boros et al. Ann Oncol 2015; 26 (suppl 6): abstr 3000

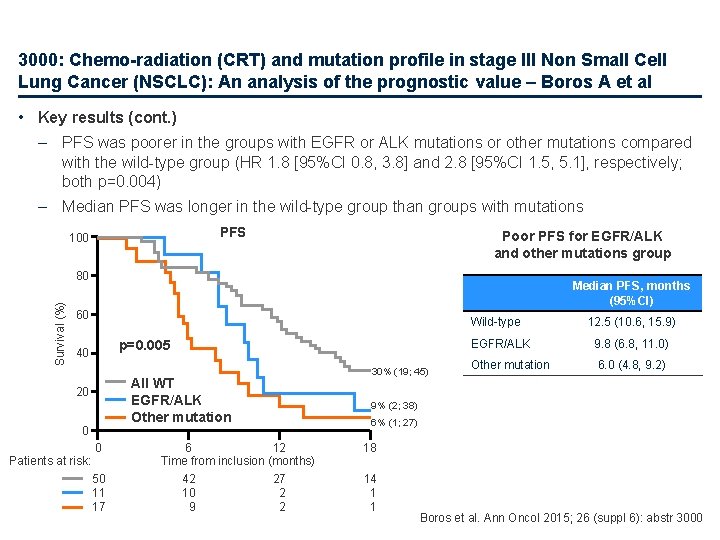
3000: Chemo-radiation (CRT) and mutation profile in stage III Non Small Cell Lung Cancer (NSCLC): An analysis of the prognostic value – Boros A et al • Key results (cont. ) – PFS was poorer in the groups with EGFR or ALK mutations or other mutations compared with the wild-type group (HR 1. 8 [95%CI 0. 8, 3. 8] and 2. 8 [95%CI 1. 5, 5. 1], respectively; both p=0. 004) – Median PFS was longer in the wild-type group than groups with mutations PFS 100 Poor PFS for EGFR/ALK and other mutations group Survival (%) 80 Median PFS, months (95%CI) 60 Wild-type p=0. 005 40 All WT EGFR/ALK Other mutation 20 0 Patients at risk: 0 50 11 17 6 12 Time from inclusion (months) 42 27 10 2 9 2 30% (19; 45) 12. 5 (10. 6, 15. 9) EGFR/ALK 9. 8 (6. 8, 11. 0) Other mutation 6. 0 (4. 8, 9. 2) 9% (2; 38) 6% (1; 27) 18 14 1 1 Boros et al. Ann Oncol 2015; 26 (suppl 6): abstr 3000

3000: Chemo-radiation (CRT) and mutation profile in stage III Non Small Cell Lung Cancer (NSCLC): An analysis of the prognostic value – Boros A et al • Key results (cont. ) – No significant difference was, however, observed for OS between the three groups – Median OS was 2. 4 years in the EGFR/ALK group compared with 1. 9 and 1. 1 years in the wild-type and other mutation groups, respectively • Conclusions – Prognostic and potential predictive role in patients with locally advanced NSCLC needs to be determined, a potential negative effect in PFS appeared in this exploratory analysis – Results must be interpreted with caution owing to the low number of patients and the fact that only a few patients with oncogenic alterations received adequate targeted therapies (retrospective analysis) – Further evaluation of a large prospective cohort is required Boros et al. Ann Oncol 2015; 26 (suppl 6): abstr 3000

3001: Prevalence and clinical association of gene mutations through Multiplex Mutation Testing in patients with NSCLC: Results from the ETOP Lungscape Project – Kerr K et al • Study objective – To describe the epidemiology of selected cancer-related mutations and investigate their association to clinicopathological features and patient outcomes • Study design – Multiplex Mutation Testing was performed on patients with resected stage I–III NSCLC with clinical data and FFPE tissue availability – Gene mutations were detected by Fluidigm technology, a microfluidics-based multiplex PCR platform with a sensitivity of >1% for most of the ∼ 150 (13 genes) mutations covered in the multiplex test – Mutant allele detection DNA was extracted at participating centres from FFPE tumour sections selected to maximise tumour content – Local quality assurance was verified in a central collaborating laboratory, where samples were standardised before genomic analysis • Key results – To date, multiplex testing has been evaluated in 1, 801 patients with a median follow-up of 4. 7 years; 65. 4% were male; 10. 3% never smokers; and median age was 66. 3 years Kerr et al. Ann Oncol 2015; 26 (suppl 6): abstr 3001

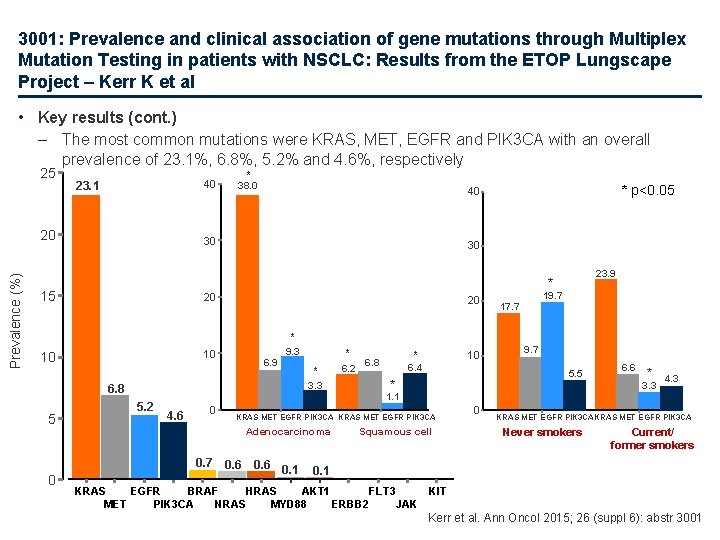
3001: Prevalence and clinical association of gene mutations through Multiplex Mutation Testing in patients with NSCLC: Results from the ETOP Lungscape Project – Kerr K et al • Key results (cont. ) – The most common mutations were KRAS, MET, EGFR and PIK 3 CA with an overall prevalence of 23. 1%, 6. 8%, 5. 2% and 4. 6%, respectively 25 40 23. 1 Prevalence (%) 20 * 38. 0 30 30 20 20 * 9. 3 10 10 6. 9 * * 3. 3 6. 8 5. 2 6. 2 10 * 6. 8 6. 4 19. 7 17. 7 9. 7 5. 5 * 4. 6 0 KRAS MET EGFR PIK 3 CA Adenocarcinoma 0. 6 0. 1 6. 6 * 3. 3 1. 1 0. 7 0 23. 9 * 15 5 * p<0. 05 40 Squamous cell 0 4. 3 KRAS MET EGFR PIK 3 CA Never smokers Current/ former smokers 0. 1 KRAS EGFR BRAF HRAS AKT 1 FLT 3 KIT MET PIK 3 CA NRAS MYD 88 ERBB 2 JAK Kerr et al. Ann Oncol 2015; 26 (suppl 6): abstr 3001

3001: Prevalence and clinical association of gene mutations through Multiplex Mutation Testing in patients with NSCLC: Results from the ETOP Lungscape Project – Kerr K et al • Key results (cont. ) – Prevalence of mutations were associated with the following: • KRAS: female gender (p<0. 001); adenocarcinoma (p<0. 001); lobectomy (p=0. 0052); younger age (p=0. 0017); and tumour size ≤ 4 cm (p=0. 0011) • EGFR: female gender (p<0. 001); non-smoker (p<0. 001); adenocarcinoma (p<0. 001); and tumour size ≤ 4 cm (p=0. 0047) • PIK 3 CA: tumour size >4 cm (p=0. 032); squamous cell (p=0. 010); stage III (p=0. 0026); and other surgery vs. lobectomy (p=0. 0184) – There were no signficant differences regarding MET mutation prevalence – KRAS was negatively associated with PIK 3 CA (p=0. 0038) and EGFR (p<0. 001) – Any associations between gene mutation status and outcomes (RFS, TTR and OS) were non-significant in all cases • Conclusions – In this predominantly European cohort, KRAS, MET, EGFR and PIK 3 CA were the most prevalent mutations identified – KRAS was negatively associated with PIK 3 CA and EGFR Kerr et al. Ann Oncol 2015; 26 (suppl 6): abstr 3001

3002: Predictors of benefits from adjuvant chemotherapy in stage IB lung adenocarcinoma – Jung-Jyh H et al • Study objective – To investigate the predictors of benefits from adjuvant therapy in resected stage IB lung adenocarcinoma • Study design – Retrospective analysis of patients (n=359) with resected pathological stage IB lung adenocarcinoma at Taipei Veterans General Hospital between 2004 and 2012 – Clinicopathological characteristics were analysed to investigate predictors of benefits from adjuvant therapy • Key results – 137 of 359 (38. 2%) patients studied had received adjuvant therapy – Micropapillary/solid predominant pattern (vs. lepidic/acinar/papillary predominant pattern) was a significant prognostic factor for worse OS (p=0. 027) and DFS (p=0. 001) for patients with surgery alone – Adjuvant platinum-based chemotherapy tended to be a significant prognostic factor for OS (p=0. 055) and was prognostic for better DFS (p=0. 011) in patients with micropapillary/ solid pattern, but not in those with lepidic/acinar/papillary predominant pattern • Conclusions – Classification of clinicopathological characteristics has significant predictive value – The micropapillary/solid predominant pattern appears to be a relevant predictor for benefits of adjuvant therapy for stage IB lung adenocarcinoma – Prospective validation of these findings is required Jung-Jyh et al. Ann Oncol 2015; 26 (suppl 6): abstr 3002

3014: Comprehensive analyses of oncogenic driver fusions using the Nano. String n. Counter in lung adenocarcinoma from Japanese never- and lightsmokers – Takamochi K et al • Study objective – To establish an efficient screening system for oncogenic driver fusions using the Nano. String n. Counter • Study design – – – 90 receptor tyrosine kinase (RTK) fusions were analysed using the Nano. String n. Counter in 233 lung adenocarcinoma specimens without any EGFR and KRAS mutations A Nano. String-based assay was designed to query the transcripts at two points: 5′ to the kinase domain (KD) and within the KD or 3′ to it All cases with standardized residuals (SRs) >2. 0 in known oncogenic driver fusions, including ALK, ROS 1, RET and NTRK 1, were followed by RT-PCT, FISH and IHC to confirm the fusion partners • Key results – – 18 candidate tumours of ALK fusions, 8 of ROS 1, 10 of RET, 0 of NTRK 1 were identified that showed an aberrant 5′ to 3′ expression with SRs >2. 0 based on the Nano. Sting-based assays • Of these, 11 were candidate tumours of ALK, 4 of ROS 1, 5 of RET that had SRs >3. 0 which were all confirmed by RT-PCR, FISH or IHC; of those tumours with SR values between 2. 0 and 3. 0, 57% of candidate tumours with ALK fusions could be confirmed for each fusion gene, but none for ROS 1 or RET could be confirmed ALK, ROS 1, RET and NTRK 1 fusions were identified in 6%, 1. 7%, 2. 1% and 0% of all lung specimens, respectively • Conclusion – The Nano. String n. Counter system allows screening for oncogenic driver fusions including ALK, ROS and RET Takamochi et al. Ann Oncol 2015; 26 (suppl 6): abstr 3014

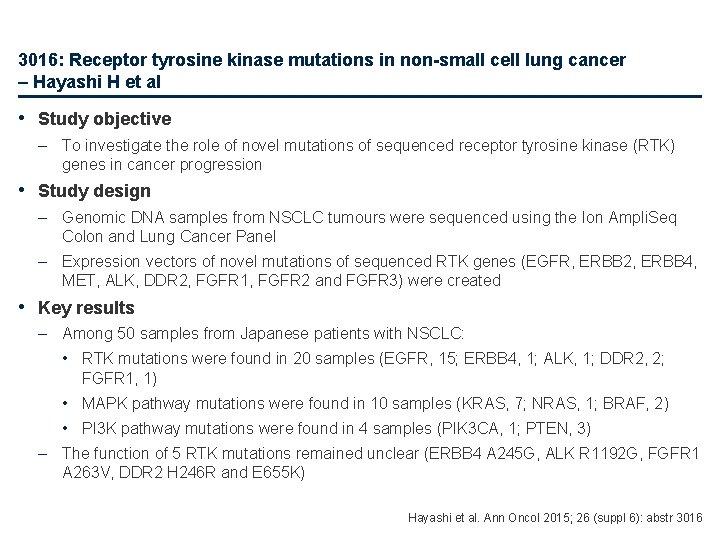
3016: Receptor tyrosine kinase mutations in non-small cell lung cancer – Hayashi H et al • Study objective – To investigate the role of novel mutations of sequenced receptor tyrosine kinase (RTK) genes in cancer progression • Study design – Genomic DNA samples from NSCLC tumours were sequenced using the Ion Ampli. Seq Colon and Lung Cancer Panel – Expression vectors of novel mutations of sequenced RTK genes (EGFR, ERBB 2, ERBB 4, MET, ALK, DDR 2, FGFR 1, FGFR 2 and FGFR 3) were created • Key results – Among 50 samples from Japanese patients with NSCLC: • RTK mutations were found in 20 samples (EGFR, 15; ERBB 4, 1; ALK, 1; DDR 2, 2; FGFR 1, 1) • MAPK pathway mutations were found in 10 samples (KRAS, 7; NRAS, 1; BRAF, 2) • PI 3 K pathway mutations were found in 4 samples (PIK 3 CA, 1; PTEN, 3) – The function of 5 RTK mutations remained unclear (ERBB 4 A 245 G, ALK R 1192 G, FGFR 1 A 263 V, DDR 2 H 246 R and E 655 K) Hayashi et al. Ann Oncol 2015; 26 (suppl 6): abstr 3016

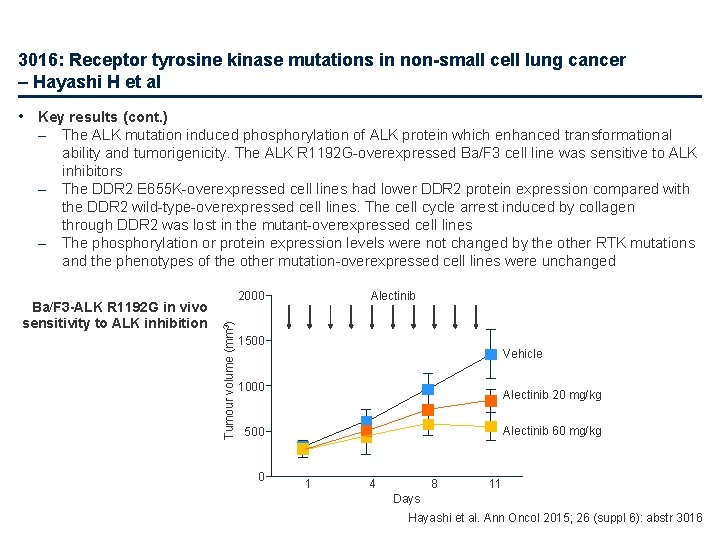
3016: Receptor tyrosine kinase mutations in non-small cell lung cancer – Hayashi H et al • Key results (cont. ) – – The ALK mutation induced phosphorylation of ALK protein which enhanced transformational ability and tumorigenicity. The ALK R 1192 G-overexpressed Ba/F 3 cell line was sensitive to ALK inhibitors The DDR 2 E 655 K-overexpressed cell lines had lower DDR 2 protein expression compared with the DDR 2 wild-type-overexpressed cell lines. The cell cycle arrest induced by collagen through DDR 2 was lost in the mutant-overexpressed cell lines The phosphorylation or protein expression levels were not changed by the other RTK mutations and the phenotypes of the other mutation-overexpressed cell lines were unchanged Ba/F 3 -ALK R 1192 G in vivo sensitivity to ALK inhibition Alectinib 2000 Tumour volume (mm 3) – 1500 Vehicle 1000 Alectinib 20 mg/kg Alectinib 60 mg/kg 500 0 1 4 8 11 Days Hayashi et al. Ann Oncol 2015; 26 (suppl 6): abstr 3016

3016: Receptor tyrosine kinase mutations in non-small cell lung cancer – Hayashi H et al • Conclusion – Of five novel RTK mutations identified, one was associated with cancer progression and one activating ALK mutation was sensitive to ALK inhibitors – The remainder had no role in cancer progression, suggesting that not all RTK mutations play a role in cancer progression and that molecular-targeted drugs may not always be effective for such cancers Hayashi et al. Ann Oncol 2015; 26 (suppl 6): abstr 3016

3017: NSCLC with high PD-L 1 expression on tumor cells or tumor-infiltrating immune cells represents distinct cancer subtypes – Schmid P et al • Study objective – To evaluate PD-L 1 expression on tumour cells (TC) and tumour-infiltrating immune cells (IC) and to examine the correlation with clinical efficacy of atezolizumab in patients with NSCLC • Study design – – – Samples were taken from pre-treatment NSCLC specimens from atezolizumab trials (n=498) and a non -trial cohort (n=706) The SP 142 IHC assay was used to assess PD-L 1 expression in TC and IC A subset of samples also underwent histopathological review, gene expression, mutational load and epigenetic analysis • Key results – TC 3 and IC 3 tumours represent two distinct populations with <1% overlap in NSCLC: • • – – IC 3 tumours have an immune-rich microenvironment with a high frequency of immune infiltrates (localised to the stroma, tumour and tumour/stroma interface), higher expression of effector T cell (Teff) signatures than TC 3 tumours, suggesting that PD-L 1 on IC reflects pre-existing active T-cell immunity TC 3 tumours had a dense desmoplastic and sclerotic tumour microenvironment, lower frequency of immune infiltrates than IC 3 tumours (primarily located in surrounding stroma) and had increased expression of genes associated with epithelial-mesenchymal transition (Snail 1, ZEB 1 and vimentin) PD-L 1 expression appears to be regulated via different mechanisms in TC and IC Despite differences in tumour microenvironment, PD-L 1 expression on both TC and IC was found to be independent predictors of response and OS improvement to atezolizumab • Conclusion – TC/IC expression of PD-L 1 is associated with sensitivity to atezolizumab; PD-L 1 expression by IC and TC possibly plays a non-redundant role in the regulation of cancer immunity and response to therapy Schmid et al. Ann Oncol 2015; 26 (suppl 6): abstr 3017

3025: Prognostic value of PDL 1 expression in stage III Non-Small Cell Lung Cancer (NSCLC) treated by chemo-radiotherapy (CRT) – Boros A et al • Study objective – To investigate the prognostic and predictive values of PD-L 1 expression in patients with stage III NSCLC treated by CRT or radiotherapy • Study design – – – Immunohistochemistry was performed on a Ventana Benchmark Ultra platform using the E 1 L 3 N clone Kaplan–Meier methods, log-rank test and Cox proportional hazards models were used for survival analysis, adjusting for performance status (0, ≥ 1), stage (IIIA, IIIB) and thoracic surgery (yes, no) Median follow-up was estimated by the Schemper method • Key results – – Data were available for 190 patients, 50 with NSCLC were evaluable for PD-L 1 expression No clinical or pathological features were related to PD-L 1 status, nor was global follow-up There was no association between PD-L 1 status and the rate of oesophagitis and pneumonitis following chemoradiation Median PFS was significantly longer in PD-L 1 negative patients than PD-L 1 positive patients (1. 0 vs. 0. 7 years, HR 2. 1 [95%CI 1. 1, 4. 0]; p=0. 03), similarly median OS was longer in PD-L 1 negative patients than PD-L 1 positive patients (2. 0 vs 1. 1 years, HR 2. 3 [95%CI 1. 2, 4. 5]; p=0. 01) • Conclusions – – The presence of PD-L 1 in stage III NSCLC is associated with poorer survival in patients who receive combined chemoradiation The prognostic and/or predictive value of PD-L 1 should be evaluated in a larger cohort Boros et al. Ann Oncol 2015; 26 (suppl 6): abstr 3025

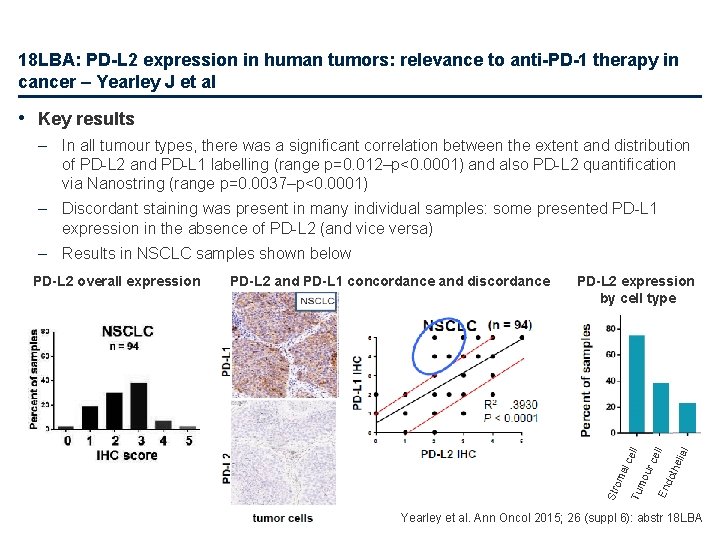
18 LBA: PD-L 2 expression in human tumors: relevance to anti-PD-1 therapy in cancer – Yearley J et al • Study objective – To examine the prevalence and distributional properties of PD-L 2, a ligand of PD-1, in multiple cohorts of archival human tumours to determine the potential relevance of PD-L 2 in patient responsiveness to anti-PD-1 therapies • Study design – After developing a novel IHC assay for PD-L 2 it was applied to archival groups of tissue from seven human tumour types: • renal cell carcinoma (n=71) • bladder carcinoma (n=34) • melanoma (n=83) • non-small cell lung cancer (NSCLC; n=94) • head and neck squamous carcinoma (n=40) • triple-negative breast cancer (TNBC; n=22) • gastric carcinoma (n=73) – Results of PD-L 2 IHC staining were compared to results of PD-L 1 IHC staining (Merck clone 22 C 3) and to PD-L 2 m. RNA levels as determined using the Nanostring platform Yearley et al. Ann Oncol 2015; 26 (suppl 6): abstr 18 LBA

18 LBA: PD-L 2 expression in human tumors: relevance to anti-PD-1 therapy in cancer – Yearley J et al • Key results – In all tumour types, there was a significant correlation between the extent and distribution of PD-L 2 and PD-L 1 labelling (range p=0. 012–p<0. 0001) and also PD-L 2 quantification via Nanostring (range p=0. 0037–p<0. 0001) – Discordant staining was present in many individual samples: some presented PD-L 1 expression in the absence of PD-L 2 (and vice versa) – Results in NSCLC samples shown below hel ial En dot mo ur al c Tu om cel l PD-L 2 expression by cell type ell PD-L 2 and PD-L 1 concordance and discordance Str PD-L 2 overall expression Yearley et al. Ann Oncol 2015; 26 (suppl 6): abstr 18 LBA

18 LBA: PD-L 2 expression in human tumors: relevance to anti-PD-1 therapy in cancer – Yearley J et al • Key results (cont. ) – To examine the clinical relevance of PD-L 2 status, logistical regression was applied to data from 144 patients with head and neck squamous cell carcinoma who had received pembrolizumab 200 mg q 3 w • PD-L 2 expression was associated with higher ORR after adjusting for PD-L 1 expression (p=0. 072) • PD-L 2 was significantly associated with longer PFS after adjusting for PD-L 1 status (p=0. 031) • Conclusions – PD-L 2 may be expressed within human tumours in the absence of PD-L 1 • This may partially account for the observation of positive clinical response to anti-PD-1 targeted therapies in PD-L 1 -negative patients – This suggests that there might be patient populations who may experience benefit from anti-PD-1 targeted therapies, i. e. those that block PD-1 interaction with both PD-L 1 and PD-L 2, but who might not experience benefit from specific anti-PD-L 1 targeted therapies – PD-L 2 status is associated with clinical outcome in patients treated with pembrolizumab Yearley et al. Ann Oncol 2015; 26 (suppl 6): abstr 18 LBA

3007: Comprehensive genomic profiling characterizes the spectrum of non. V 600 E activating BRAF alterations Including BRAF fusions in lung cancer – Ali S et al • Study objective – To investigate BRAF alterations in advanced lung carcinomas • Study design – Comprehensive genomic profiling of lung carcinoma tissue to detect 4 classes of genomic alterations for 315 genes: • Base substitutions • Short insertions/deletions • Copy number alterations • Fusions – The genomic profiles were analysed by: • Histological type • Alterations within BRAF • Other co-segregating alterations Ali et al. Ann Oncol 2015; 26 (suppl 6): abstr 3007

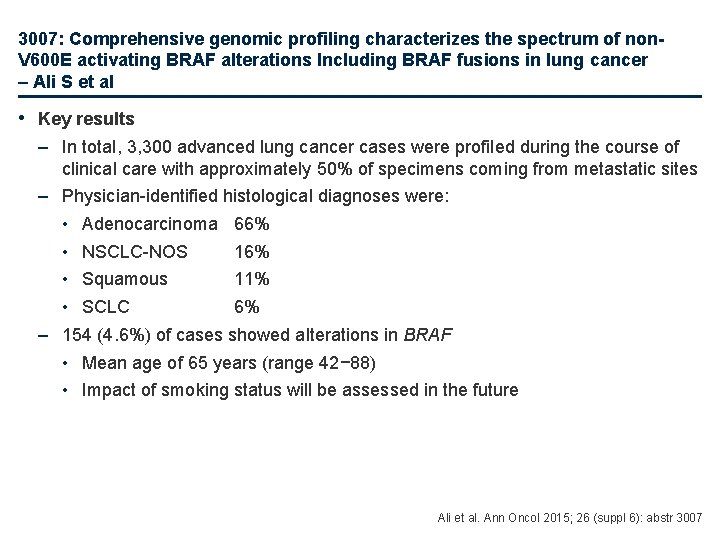
3007: Comprehensive genomic profiling characterizes the spectrum of non. V 600 E activating BRAF alterations Including BRAF fusions in lung cancer – Ali S et al • Key results – In total, 3, 300 advanced lung cancer cases were profiled during the course of clinical care with approximately 50% of specimens coming from metastatic sites – Physician-identified histological diagnoses were: • Adenocarcinoma 66% • NSCLC-NOS 16% • Squamous 11% • SCLC 6% – 154 (4. 6%) of cases showed alterations in BRAF • Mean age of 65 years (range 42− 88) • Impact of smoking status will be assessed in the future Ali et al. Ann Oncol 2015; 26 (suppl 6): abstr 3007

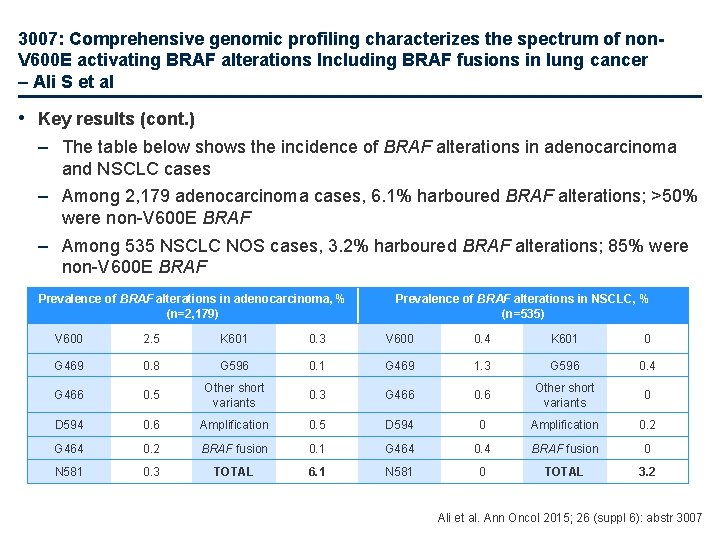
3007: Comprehensive genomic profiling characterizes the spectrum of non. V 600 E activating BRAF alterations Including BRAF fusions in lung cancer – Ali S et al • Key results (cont. ) – The table below shows the incidence of BRAF alterations in adenocarcinoma and NSCLC cases – Among 2, 179 adenocarcinoma cases, 6. 1% harboured BRAF alterations; >50% were non-V 600 E BRAF – Among 535 NSCLC NOS cases, 3. 2% harboured BRAF alterations; 85% were non-V 600 E BRAF Prevalence of BRAF alterations in adenocarcinoma, % (n=2, 179) Prevalence of BRAF alterations in NSCLC, % (n=535) V 600 2. 5 K 601 0. 3 V 600 0. 4 K 601 0 G 469 0. 8 G 596 0. 1 G 469 1. 3 G 596 0. 4 G 466 0. 5 Other short variants 0. 3 G 466 0. 6 Other short variants 0 D 594 0. 6 Amplification 0. 5 D 594 0 Amplification 0. 2 G 464 0. 2 BRAF fusion 0. 1 G 464 0. 4 BRAF fusion 0 N 581 0. 3 TOTAL 6. 1 N 581 0 TOTAL 3. 2 Ali et al. Ann Oncol 2015; 26 (suppl 6): abstr 3007

3007: Comprehensive genomic profiling characterizes the spectrum of non. V 600 E activating BRAF alterations Including BRAF fusions in lung cancer – Ali S et al • Key results (cont. ) – The table below shows the incidence of BRAF alterations in SCC and SCLC cases – None of the 201 SCLC cases harboured any BRAF alterations – Among 385 cases of SCC, 0. 8% harboured BRAF alterations; although none were BRAF V 600 E Prevalence of BRAF alterations in SCC, % (n=385) Prevalence of BRAF alterations in SCLC, % (n=201) V 600 0 K 601 0 G 469 0. 3 G 596 0 G 469 0 G 596 0 G 466 0 Other short variants 0 D 594 0 Amplification 0 G 464 0. 3 BRAF fusion 0 G 464 0 BRAF fusion 0 N 581 0 TOTAL 0. 8 N 581 0 TOTAL 0 Ali et al. Ann Oncol 2015; 26 (suppl 6): abstr 3007

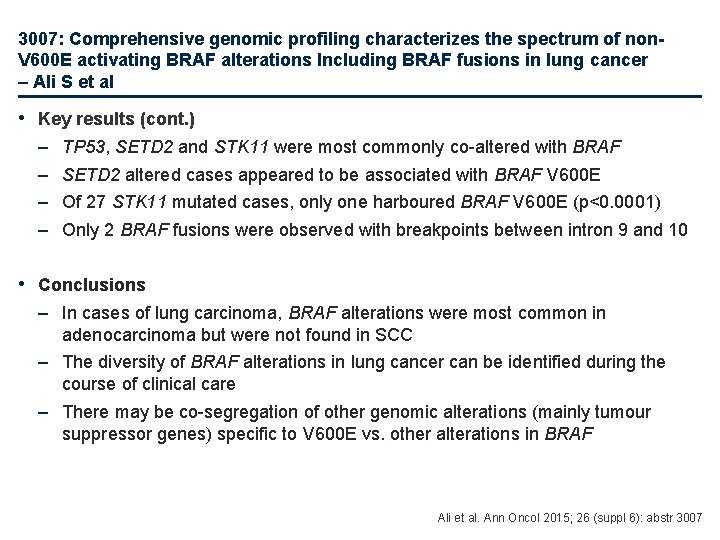
3007: Comprehensive genomic profiling characterizes the spectrum of non. V 600 E activating BRAF alterations Including BRAF fusions in lung cancer – Ali S et al • Key results (cont. ) – – TP 53, SETD 2 and STK 11 were most commonly co-altered with BRAF SETD 2 altered cases appeared to be associated with BRAF V 600 E Of 27 STK 11 mutated cases, only one harboured BRAF V 600 E (p<0. 0001) Only 2 BRAF fusions were observed with breakpoints between intron 9 and 10 • Conclusions – In cases of lung carcinoma, BRAF alterations were most common in adenocarcinoma but were not found in SCC – The diversity of BRAF alterations in lung cancer can be identified during the course of clinical care – There may be co-segregation of other genomic alterations (mainly tumour suppressor genes) specific to V 600 E vs. other alterations in BRAF Ali et al. Ann Oncol 2015; 26 (suppl 6): abstr 3007

Early and locally advanced NSCLC Stages I, II and III

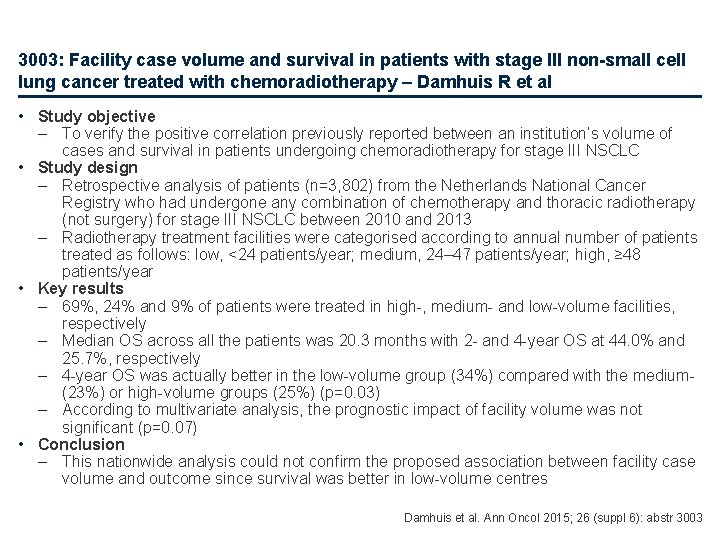
3003: Facility case volume and survival in patients with stage III non-small cell lung cancer treated with chemoradiotherapy – Damhuis R et al • Study objective – To verify the positive correlation previously reported between an institution’s volume of cases and survival in patients undergoing chemoradiotherapy for stage III NSCLC • Study design – Retrospective analysis of patients (n=3, 802) from the Netherlands National Cancer Registry who had undergone any combination of chemotherapy and thoracic radiotherapy (not surgery) for stage III NSCLC between 2010 and 2013 – Radiotherapy treatment facilities were categorised according to annual number of patients treated as follows: low, <24 patients/year; medium, 24– 47 patients/year; high, ≥ 48 patients/year • Key results – 69%, 24% and 9% of patients were treated in high-, medium- and low-volume facilities, respectively – Median OS across all the patients was 20. 3 months with 2 - and 4 -year OS at 44. 0% and 25. 7%, respectively – 4 -year OS was actually better in the low-volume group (34%) compared with the medium- (23%) or high-volume groups (25%) (p=0. 03) – According to multivariate analysis, the prognostic impact of facility volume was not significant (p=0. 07) • Conclusion – This nationwide analysis could not confirm the proposed association between facility case volume and outcome since survival was better in low-volume centres Damhuis et al. Ann Oncol 2015; 26 (suppl 6): abstr 3003

3004: Sublobar resection choice based on HRCT and FDG-PET/CT findings for clinical stage IA non-small cell lung cancer – Tsutani Y et al • Study objective – To identify the predictors of nodal-negative clinical stage IA NSCLC to choose optimal candidates for sublobar resection without systematic lymph node dissection • Study design – Retrospective analysis of data from completely resected clinical stage IA lung adenocarcinoma (n=502) or squamous cell carcinoma (n=100) – Data were collected from 4 institutions – Data were analysed to investigate any relationship between lymph node status and preoperative factors, including tumour size on HRCT and SUVmax on FDG-PET/CT HRCT, high-resolution computed tomography; SUVmax, maximum standardized uptake value Tsutani et al. Ann Oncol 2015; 26 (suppl 6): abstr 3004

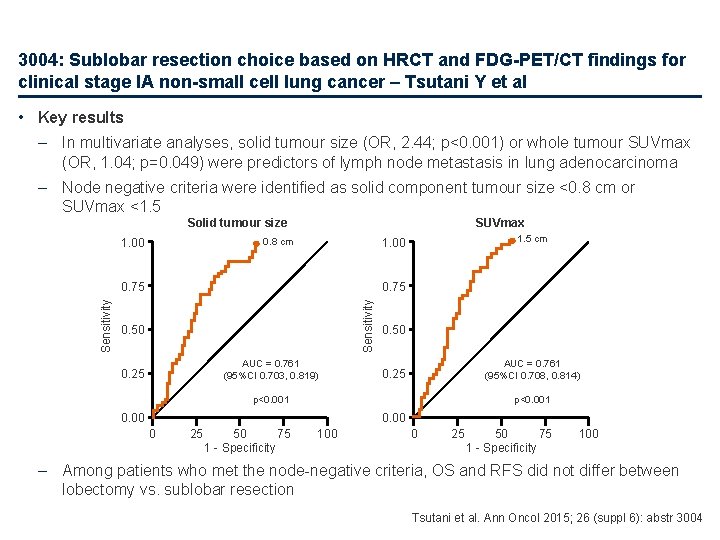
3004: Sublobar resection choice based on HRCT and FDG-PET/CT findings for clinical stage IA non-small cell lung cancer – Tsutani Y et al • Key results – In multivariate analyses, solid tumour size (OR, 2. 44; p<0. 001) or whole tumour SUVmax (OR, 1. 04; p=0. 049) were predictors of lymph node metastasis in lung adenocarcinoma – Node negative criteria were identified as solid component tumour size <0. 8 cm or SUVmax <1. 5 SUVmax Solid tumour size 1. 00 0. 75 Sensitivity 1. 5 cm 1. 00 0. 8 cm 0. 50 AUC = 0. 761 (95%CI 0. 703, 0. 819) 0. 25 0. 50 AUC = 0. 761 (95%CI 0. 708, 0. 814) 0. 25 p<0. 001 0. 00 0 25 50 75 1 - Specificity 100 – Among patients who met the node-negative criteria, OS and RFS did not differ between lobectomy vs. sublobar resection Tsutani et al. Ann Oncol 2015; 26 (suppl 6): abstr 3004

3004: Sublobar resection choice based on HRCT and FDG-PET/CT findings for clinical stage IA non-small cell lung cancer – Tsutani Y et al • Key results (cont. ) – No independent predictive factors for lymph node metastasis were identified in patients with lung squamous cell carcinoma • Conclusions – Solid tumour size on HRCT or SUVmax on FDG-PET/CT may be useful to predict node-negative stage IA lung adenocarcinoma – The node-negative criteria of solid tumour size <0. 8 cm or SUVmax <1. 5 may be helpful for choosing candidates for sublobar resection without systematic lymphadenectomy Tsutani et al. Ann Oncol 2015; 26 (suppl 6): abstr 3004

Advanced NSCLC Not radically treatable stage III and stage IV 1 st line

12 LBA: Biomarker-driven access to crizotinib in ALK, MET or ROS 1 positive (+) malignancies in adults and children: The French national Ac. Sé Program – Vassal G et al • Study objective – To evaluate the efficacy and safety of single agent crizotinib made available to patients with advanced malignancy through the Ac. Sé Program – The Ac. Sé Program provides equal access to tumour molecular diagnosis to generate high evidenced-based knowledge and to prevent off-label use • Study design – Biomarker program: Patients ≥ 1 years old with advanced disease (>15 malignancies) underwent single biomarker molecular testing for the crizotinib biomarkers, ROS 1, ALK and MET; biomarkers were identified by IHC and confirmed by FISH – Phase 2 trial: Patients were treated with crizotinib (adult dose 250 mg bid; child 280 mg/m 2 bid); tumour response was calculated every 2 months; primary endpoint: best response defined as CR or PR at 2 months Vassal et al. Ann Oncol 2015; 26 (suppl 6): abstr 12 LBA

12 LBA: Biomarker-driven access to crizotinib in ALK, MET or ROS 1 positive (+) malignancies in adults and children: The French national Ac. Sé Program – Vassal G et al • Key results – Biomarker program • 12, 586 tests have been performed in 5, 592 patients • MET amplification (threshold 6 copies) was identified in 196 of 4, 011 patients – 6. 6% NSCLC; 7. 3% glioblastoma; 10. 6% colon; 16% gastric; 1. 4% ovarian; and 1. 6% kidney • ROS 1 translocation was identified in 54 of 2, 072 patients – NSCLC, cholangiocarcinoma and inflammatory myofibroblastic tumour • ALK was identified in 6 out of 488 patients with breast cancer – 1 translocation and 5 amplifications – Phase 2 study • 131 patients included in the study; 53% were male; 51% current or ex-smokers; 6% were <18 years of age with a median age of 59 years Vassal et al. Ann Oncol 2015; 26 (suppl 6): abstr 12 LBA

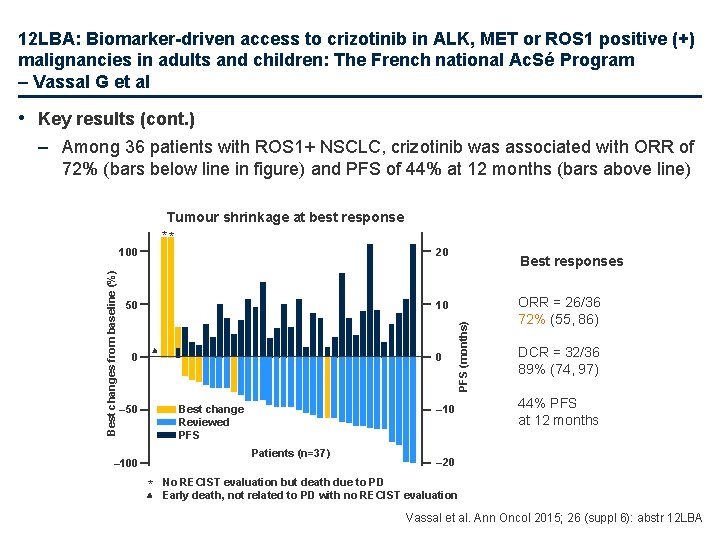
12 LBA: Biomarker-driven access to crizotinib in ALK, MET or ROS 1 positive (+) malignancies in adults and children: The French national Ac. Sé Program – Vassal G et al • Key results (cont. ) – Among 36 patients with ROS 1+ NSCLC, crizotinib was associated with ORR of 72% (bars below line in figure) and PFS of 44% at 12 months (bars above line) 100 20 50 10 0 0 Best change Reviewed PFS – 50 – 10 Patients (n=37) – 100 * Best responses PFS (months) Best changes from baseline (%) Tumour shrinkage at best response ** ORR = 26/36 72% (55, 86) DCR = 32/36 89% (74, 97) 44% PFS at 12 months – 20 No RECIST evaluation but death due to PD Early death, not related to PD with no RECIST evaluation Vassal et al. Ann Oncol 2015; 26 (suppl 6): abstr 12 LBA

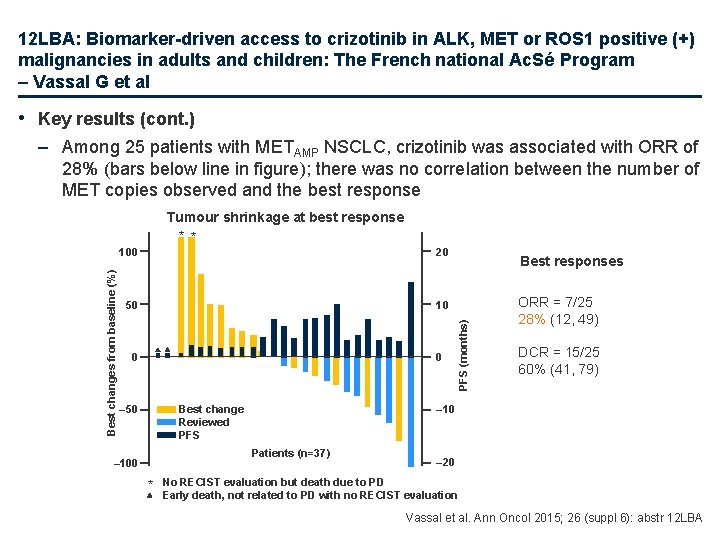
12 LBA: Biomarker-driven access to crizotinib in ALK, MET or ROS 1 positive (+) malignancies in adults and children: The French national Ac. Sé Program – Vassal G et al • Key results (cont. ) – Among 25 patients with METAMP NSCLC, crizotinib was associated with ORR of 28% (bars below line in figure); there was no correlation between the number of MET copies observed and the best response 100 20 50 10 0 0 Best change Reviewed PFS – 50 * ORR = 7/25 28% (12, 49) DCR = 15/25 60% (41, 79) – 10 Patients (n=37) – 100 Best responses PFS (months) Best changes from baseline (%) Tumour shrinkage at best response * * – 20 No RECIST evaluation but death due to PD Early death, not related to PD with no RECIST evaluation Vassal et al. Ann Oncol 2015; 26 (suppl 6): abstr 12 LBA

12 LBA: Biomarker-driven access to crizotinib in ALK, MET or ROS 1 positive (+) malignancies in adults and children: The French national Ac. Sé Program – Vassal G et al • Key results (cont. ) – No benefit of treatment was observed in 13 patients with METAMP colon cancer – Tolerability of crizotinib was no different to the expected • 55 grade ≥ 3 AEs or SAEs were reported in 40 of 122 evaluable patients • Most common AEs, mainly grade 1, were: visual disturbance (50%), nausea (47%), ALT/AST increased (both 40%), diarrhoea (36%) and fatigue (34%) • Conclusions – Equal and safe access to ALK, ROS and MET testing and to crizotinib outside its marketing indication is feasible – Crizotinib was active in ROS 1+ and METAMP NSCLC, but not in METAMP colon cancer – Accrual is continuing in other rare conditions Vassal et al. Ann Oncol 2015; 26 (suppl 6): abstr 12 LBA

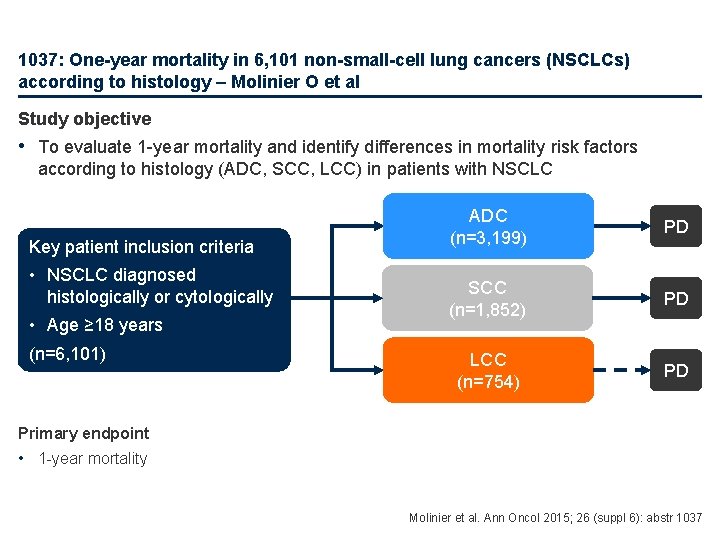
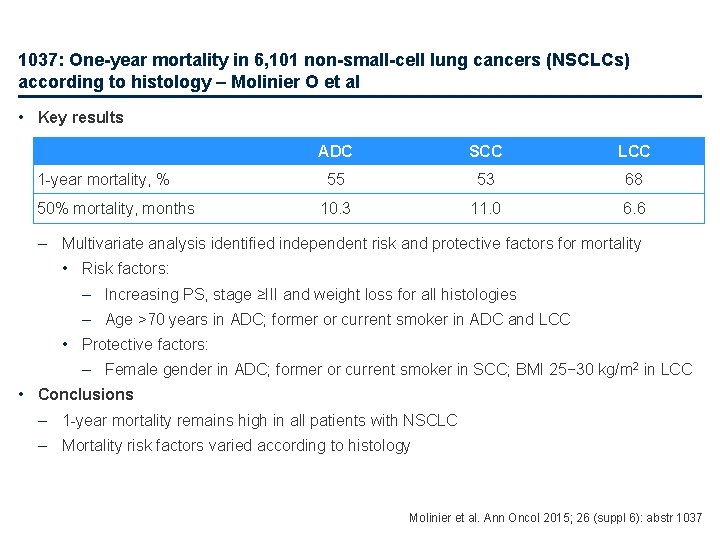
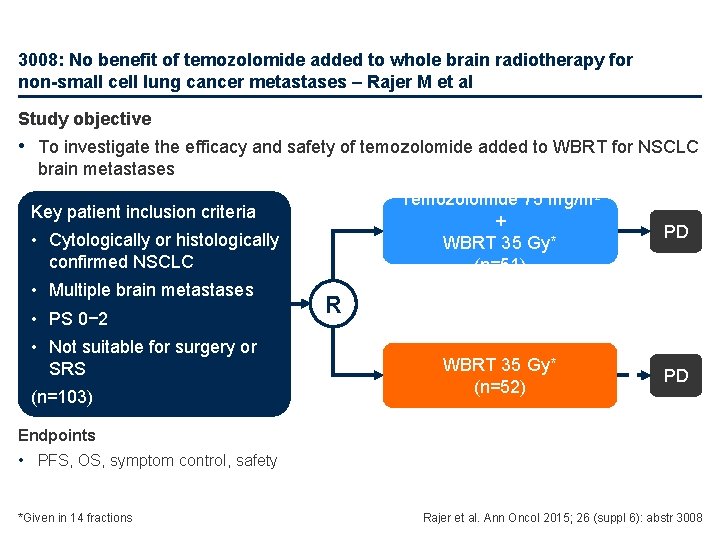
1037: One-year mortality in 6, 101 non-small-cell lung cancers (NSCLCs) according to histology – Molinier O et al Study objective • To evaluate 1 -year mortality and identify differences in mortality risk factors according to histology (ADC, SCC, LCC) in patients with NSCLC Key patient inclusion criteria • NSCLC diagnosed histologically or cytologically • Age ≥ 18 years (n=6, 101) ADC (n=3, 199) PD SCC (n=1, 852) PD LCC (n=754) PD Primary endpoint • 1 -year mortality Molinier et al. Ann Oncol 2015; 26 (suppl 6): abstr 1037

1037: One-year mortality in 6, 101 non-small-cell lung cancers (NSCLCs) according to histology – Molinier O et al • Key results 1 -year mortality, % 50% mortality, months ADC SCC LCC 55 53 68 10. 3 11. 0 6. 6 – Multivariate analysis identified independent risk and protective factors for mortality • Risk factors: – Increasing PS, stage ≥III and weight loss for all histologies – Age >70 years in ADC; former or current smoker in ADC and LCC • Protective factors: – Female gender in ADC; former or current smoker in SCC; BMI 25− 30 kg/m 2 in LCC • Conclusions – 1 -year mortality remains high in all patients with NSCLC – Mortality risk factors varied according to histology Molinier et al. Ann Oncol 2015; 26 (suppl 6): abstr 1037

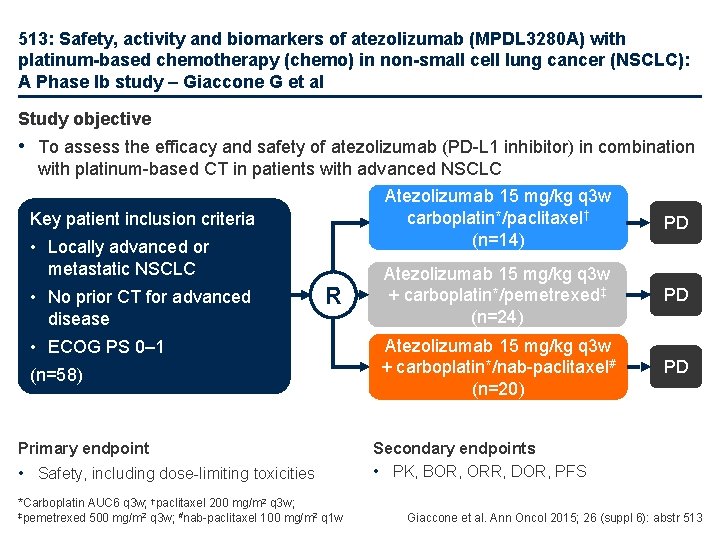
513: Safety, activity and biomarkers of atezolizumab (MPDL 3280 A) with platinum-based chemotherapy (chemo) in non-small cell lung cancer (NSCLC): A Phase Ib study – Giaccone G et al Study objective • To assess the efficacy and safety of atezolizumab (PD-L 1 inhibitor) in combination with platinum-based CT in patients with advanced NSCLC Key patient inclusion criteria • Locally advanced or metastatic NSCLC • No prior CT for advanced disease R • ECOG PS 0– 1 (n=58) Primary endpoint • Safety, including dose-limiting toxicities *Carboplatin AUC 6 q 3 w; †paclitaxel 200 mg/m 2 q 3 w; ‡pemetrexed 500 mg/m 2 q 3 w; #nab-paclitaxel 100 mg/m 2 q 1 w Atezolizumab 15 mg/kg q 3 w carboplatin*/paclitaxel† (n=14) PD Atezolizumab 15 mg/kg q 3 w + carboplatin*/pemetrexed‡ (n=24) PD Atezolizumab 15 mg/kg q 3 w + carboplatin*/nab-paclitaxel# (n=20) PD Secondary endpoints • PK, BOR, ORR, DOR, PFS Giaccone et al. Ann Oncol 2015; 26 (suppl 6): abstr 513

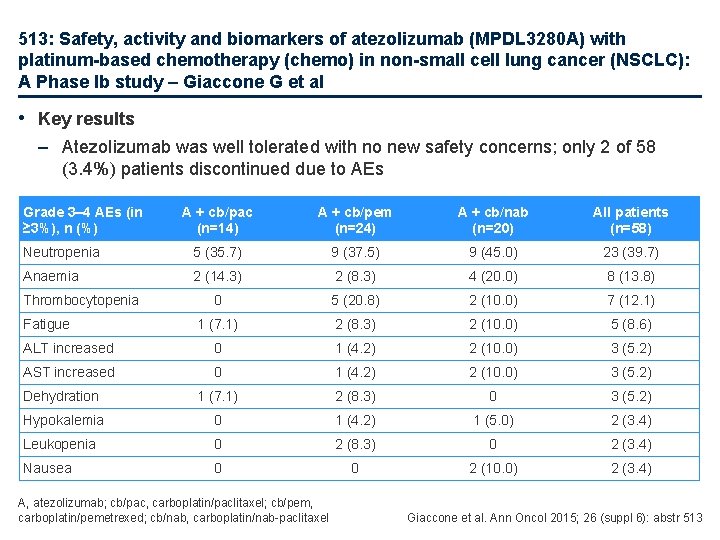
513: Safety, activity and biomarkers of atezolizumab (MPDL 3280 A) with platinum-based chemotherapy (chemo) in non-small cell lung cancer (NSCLC): A Phase Ib study – Giaccone G et al • Key results – Atezolizumab was well tolerated with no new safety concerns; only 2 of 58 (3. 4%) patients discontinued due to AEs Grade 3– 4 AEs (in ≥ 3%), n (%) A + cb/pac (n=14) A + cb/pem (n=24) A + cb/nab (n=20) All patients (n=58) Neutropenia 5 (35. 7) 9 (37. 5) 9 (45. 0) 23 (39. 7) Anaemia 2 (14. 3) 2 (8. 3) 4 (20. 0) 8 (13. 8) 0 5 (20. 8) 2 (10. 0) 7 (12. 1) 1 (7. 1) 2 (8. 3) 2 (10. 0) 5 (8. 6) ALT increased 0 1 (4. 2) 2 (10. 0) 3 (5. 2) AST increased 0 1 (4. 2) 2 (10. 0) 3 (5. 2) Dehydration 1 (7. 1) 2 (8. 3) 0 3 (5. 2) Hypokalemia 0 1 (4. 2) 1 (5. 0) 2 (3. 4) Leukopenia 0 2 (8. 3) 0 2 (3. 4) Nausea 0 0 2 (10. 0) 2 (3. 4) Thrombocytopenia Fatigue A, atezolizumab; cb/pac, carboplatin/paclitaxel; cb/pem, carboplatin/pemetrexed; cb/nab, carboplatin/nab-paclitaxel Giaccone et al. Ann Oncol 2015; 26 (suppl 6): abstr 513

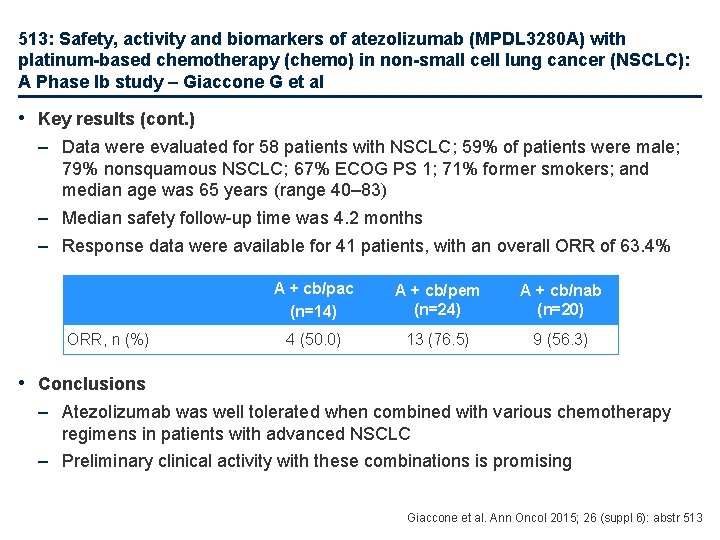
513: Safety, activity and biomarkers of atezolizumab (MPDL 3280 A) with platinum-based chemotherapy (chemo) in non-small cell lung cancer (NSCLC): A Phase Ib study – Giaccone G et al • Key results (cont. ) – Data were evaluated for 58 patients with NSCLC; 59% of patients were male; 79% nonsquamous NSCLC; 67% ECOG PS 1; 71% former smokers; and median age was 65 years (range 40– 83) – Median safety follow-up time was 4. 2 months – Response data were available for 41 patients, with an overall ORR of 63. 4% ORR, n (%) A + cb/pac (n=14) A + cb/pem (n=24) A + cb/nab (n=20) 4 (50. 0) 13 (76. 5) 9 (56. 3) • Conclusions – Atezolizumab was well tolerated when combined with various chemotherapy regimens in patients with advanced NSCLC – Preliminary clinical activity with these combinations is promising Giaccone et al. Ann Oncol 2015; 26 (suppl 6): abstr 513

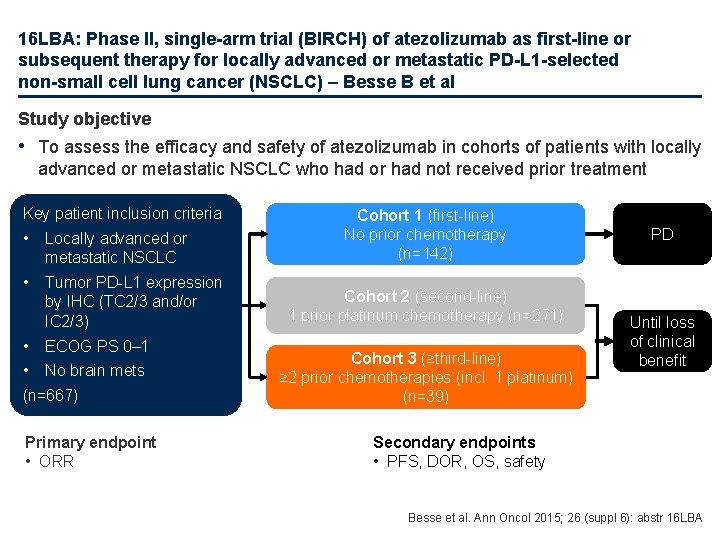
16 LBA: Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L 1 -selected non-small cell lung cancer (NSCLC) – Besse B et al Study objective • To assess the efficacy and safety of atezolizumab in cohorts of patients with locally advanced or metastatic NSCLC who had or had not received prior treatment Key patient inclusion criteria • Locally advanced or metastatic NSCLC • Tumor PD-L 1 expression by IHC (TC 2/3 and/or IC 2/3) • ECOG PS 0– 1 • No brain mets (n=667) Primary endpoint • ORR Cohort 1 (first-line) No prior chemotherapy (n=142) Cohort 2 (second-line) 1 prior platinum chemotherapy (n=271) Cohort 3 (≥third-line) ≥ 2 prior chemotherapies (incl. 1 platinum) (n=39) PD Until loss of clinical benefit Secondary endpoints • PFS, DOR, OS, safety Besse et al. Ann Oncol 2015; 26 (suppl 6): abstr 16 LBA

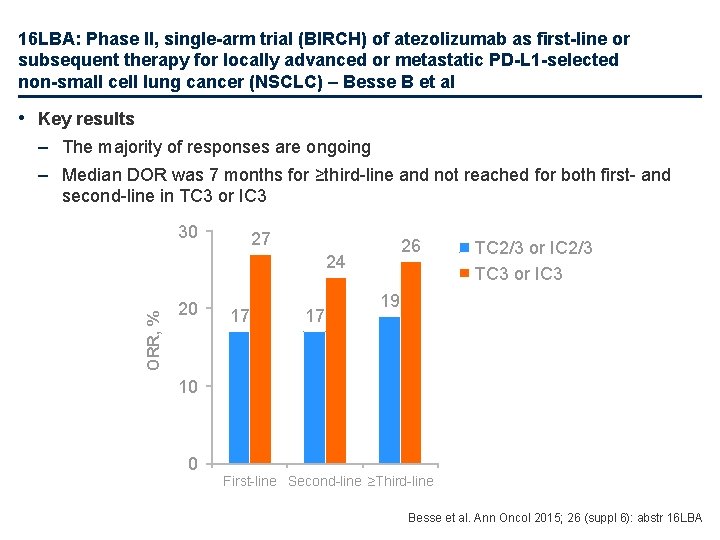
16 LBA: Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L 1 -selected non-small cell lung cancer (NSCLC) – Besse B et al • Key results – The majority of responses are ongoing – Median DOR was 7 months for ≥third-line and not reached for both first- and second-line in TC 3 or IC 3 30 27 26 ORR, % 24 20 17 17 TC 2/3 or IC 2/3 TC 3 or IC 3 19 10 0 First-line Second-line ≥Third-line Besse et al. Ann Oncol 2015; 26 (suppl 6): abstr 16 LBA

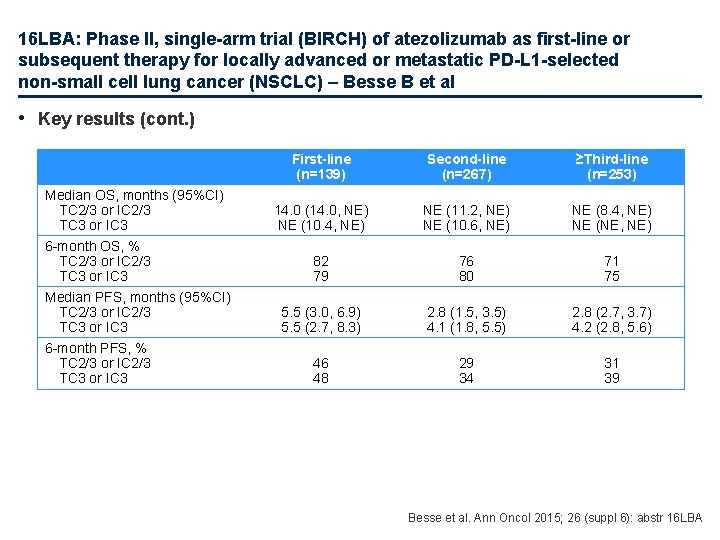
16 LBA: Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L 1 -selected non-small cell lung cancer (NSCLC) – Besse B et al • Key results (cont. ) Median OS, months (95%CI) TC 2/3 or IC 2/3 TC 3 or IC 3 6 -month OS, % TC 2/3 or IC 2/3 TC 3 or IC 3 Median PFS, months (95%CI) TC 2/3 or IC 2/3 TC 3 or IC 3 6 -month PFS, % TC 2/3 or IC 2/3 TC 3 or IC 3 First-line (n=139) Second-line (n=267) ≥Third-line (n=253) 14. 0 (14. 0, NE) NE (10. 4, NE) NE (11. 2, NE) NE (10. 6, NE) NE (8. 4, NE) NE (NE, NE) 82 79 76 80 71 75 5. 5 (3. 0, 6. 9) 5. 5 (2. 7, 8. 3) 2. 8 (1. 5, 3. 5) 4. 1 (1. 8, 5. 5) 2. 8 (2. 7, 3. 7) 4. 2 (2. 8, 5. 6) 46 48 29 34 31 39 Besse et al. Ann Oncol 2015; 26 (suppl 6): abstr 16 LBA

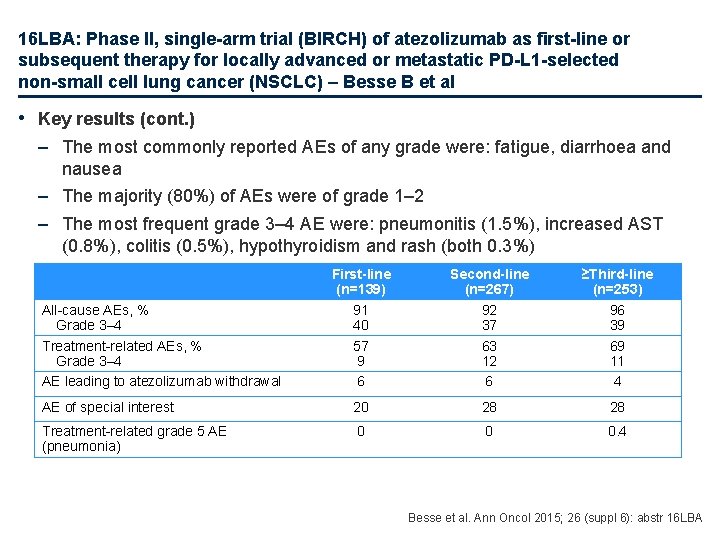
16 LBA: Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L 1 -selected non-small cell lung cancer (NSCLC) – Besse B et al • Key results (cont. ) – The most commonly reported AEs of any grade were: fatigue, diarrhoea and nausea – The majority (80%) of AEs were of grade 1– 2 – The most frequent grade 3– 4 AE were: pneumonitis (1. 5%), increased AST (0. 8%), colitis (0. 5%), hypothyroidism and rash (both 0. 3%) First-line (n=139) 91 40 57 9 6 Second-line (n=267) 92 37 63 12 6 ≥Third-line (n=253) 96 39 69 11 4 AE of special interest 20 28 28 Treatment-related grade 5 AE (pneumonia) 0 0 0. 4 All-cause AEs, % Grade 3– 4 Treatment-related AEs, % Grade 3– 4 AE leading to atezolizumab withdrawal Besse et al. Ann Oncol 2015; 26 (suppl 6): abstr 16 LBA

16 LBA: Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L 1 -selected non-small cell lung cancer (NSCLC) – Besse B et al • Conclusions – In PD-L 1 -selected patients with advanced NSCLC, atezolizumab monotherapy demonstrated clinically meaningful efficacy, although the data are not yet mature – The safety profile of atezolizumab was consistent with previous trials, with no unexpected signals or differences in toxicity – Higher PD-L 1 expression on IC and TC was correlated with higher responses; further prospective validation is required to evaluate whether this can select patients with NSCLC who could benefit from atezolizumab treatment Besse et al. Ann Oncol 2015; 26 (suppl 6): abstr 16 LBA

1328: Lung cancer in very elderly adults: Outcomes of population over 80 in our institution – Ortega Granados AL et al • Study objective – To examine patient characteristics and outcomes in elderly patients with lung cancer • Study design – Retrospective observational study of patients with lung cancer referred to the Centre of Medical Oncology (Jaén, Spain) during 2010– 2013 and followed until April 2015 • Patients were required to be ≥ 80 years of age and have lung cancer • Key results – Of 672 patients seen during 2010– 2013, 41 (6. 1%) were aged ≥ 80 years and of these 78% were male and 88% had NSCLC – 34% did not receive any oncogenic treatment, while 7. 3% had curative-intent treatment. In the first-line group 68% had chemotherapy (48% platinum doublet and 20% monotherapy) and 24% received tyrosine kinase inhibitors – Median survival time was 11. 2 months and median OS was 8 months; survival was significantly longer in never-smokers compared with smokers (p=0. 035) • Conclusion – Survival in elderly patients with lung cancer was better with active cancer treatment + best supportive care than only palliative care; smoking influenced survival Ortega Granados et al. Ann Oncol 2015; 26 (suppl 6): abstr 1328

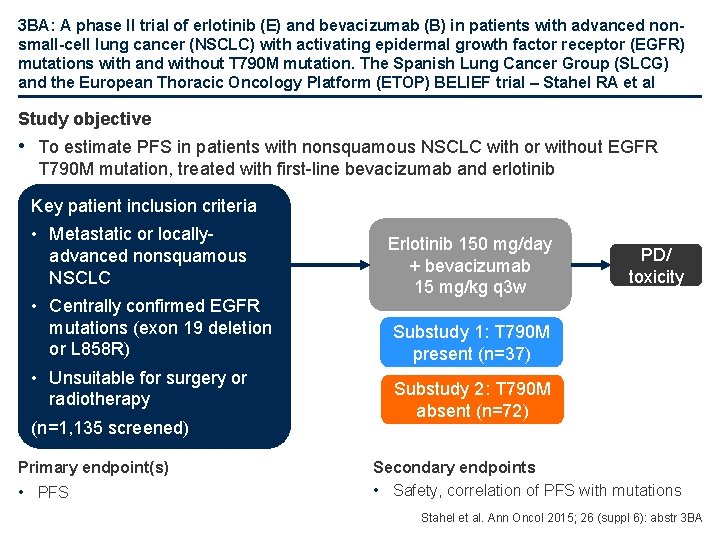
3 BA: A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced nonsmall-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T 790 M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial – Stahel RA et al Study objective • To estimate PFS in patients with nonsquamous NSCLC with or without EGFR T 790 M mutation, treated with first-line bevacizumab and erlotinib Key patient inclusion criteria • Metastatic or locallyadvanced nonsquamous NSCLC • Centrally confirmed EGFR mutations (exon 19 deletion or L 858 R) • Unsuitable for surgery or radiotherapy (n=1, 135 screened) Primary endpoint(s) • PFS Erlotinib 150 mg/day + bevacizumab 15 mg/kg q 3 w PD/ toxicity Substudy 1: T 790 M present (n=37) Substudy 2: T 790 M absent (n=72) Secondary endpoints • Safety, correlation of PFS with mutations Stahel et al. Ann Oncol 2015; 26 (suppl 6): abstr 3 BA

3 BA: A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced nonsmall-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T 790 M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial – Stahel RA et al • Key results – Overall, 38. 5% of patients were male; 66. 1% had never smoked; 5. 5% were PS 2; median age was 66 years – The presence of T 790 M at diagnosis was documented in 34% of patients with classical activating EGFR mutations using the sensitive PC 9 cut-off method (and 54% with the ultrasensitive DLD 1 cut-off method) – Median follow-up was 16. 0 months in the T 790 M+ group with 13. 1 months spent on treatment; median follow-up in the T 790 M- cohort was 18. 6 months with 8. 7 months on treatment – Toxicity was as expected with most AEs being of grade 1 and 2 in severity Stahel et al. Ann Oncol 2015; 26 (suppl 6): abstr 3 BA

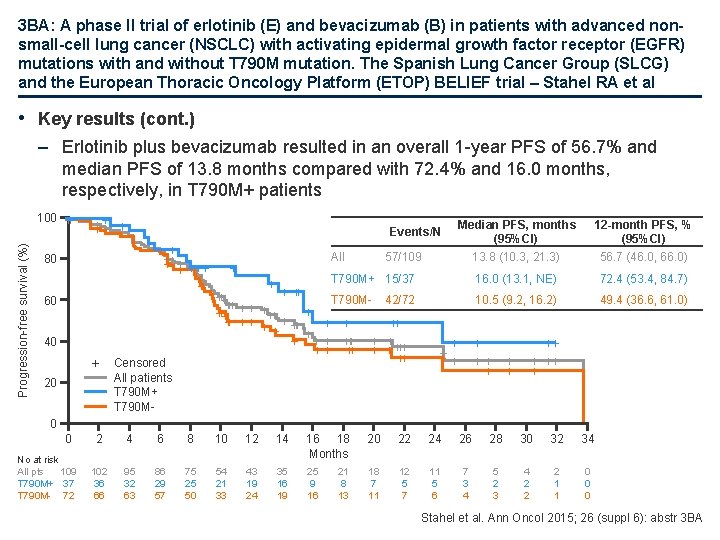
3 BA: A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced nonsmall-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T 790 M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial – Stahel RA et al • Key results (cont. ) – Erlotinib plus bevacizumab resulted in an overall 1 -year PFS of 56. 7% and median PFS of 13. 8 months compared with 72. 4% and 16. 0 months, respectively, in T 790 M+ patients Progression-free survival (%) 100 + + + ++++ ++ ++ + 80 60 40 + 20 Median PFS, months (95%CI) 13. 8 (10. 3, 21. 3) 12 -month PFS, % (95%CI) 56. 7 (46. 0, 66. 0) T 790 M+ 15/37 16. 0 (13. 1, NE) 72. 4 (53. 4, 84. 7) 42/72 10. 5 (9. 2, 16. 2) 49. 4 (36. 6, 61. 0) Events/N Censored All patients T 790 M+ T 790 M- All + + 57/109 ++ +++ T 790 M+++ + + ++ + + + +++ + +++++ ++ + ++ + 0 0 No at risk All pts 109 T 790 M+ 37 T 790 M- 72 2 4 6 8 10 12 14 16 18 Months 20 22 24 26 28 30 32 34 102 36 66 95 32 63 86 29 57 75 25 50 54 21 33 43 19 24 35 16 19 25 9 16 18 7 11 12 5 7 11 5 6 7 3 4 5 2 3 4 2 2 2 1 1 0 0 0 21 8 13 Stahel et al. Ann Oncol 2015; 26 (suppl 6): abstr 3 BA

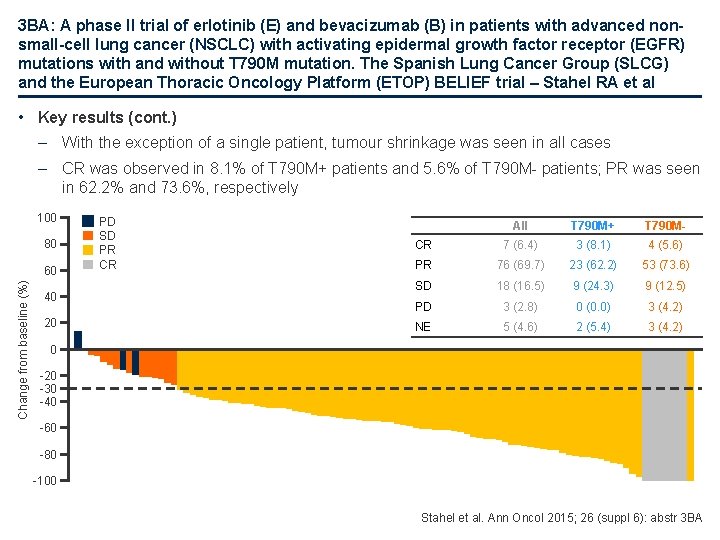
3 BA: A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced nonsmall-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T 790 M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial – Stahel RA et al • Key results (cont. ) – With the exception of a single patient, tumour shrinkage was seen in all cases – CR was observed in 8. 1% of T 790 M+ patients and 5. 6% of T 790 M- patients; PR was seen in 62. 2% and 73. 6%, respectively 100 80 Change from baseline (%) 60 40 20 PD SD PR CR All T 790 M+ T 790 M- CR 7 (6. 4) 3 (8. 1) 4 (5. 6) PR 76 (69. 7) 23 (62. 2) 53 (73. 6) SD 18 (16. 5) 9 (24. 3) 9 (12. 5) PD 3 (2. 8) 0 (0. 0) 3 (4. 2) NE 5 (4. 6) 2 (5. 4) 3 (4. 2) 0 -20 -30 -40 -60 -80 -100 Stahel et al. Ann Oncol 2015; 26 (suppl 6): abstr 3 BA

3 BA: A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced nonsmall-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T 790 M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial – Stahel RA et al • Conclusions – The combination of erlotinib and bevacizumab has manageable toxicity and PFS observed overall and in the T 790 M+ patients are similar and promising – These overall results are in keeping with the results of JO 25567 which demonstrated a significant benefit in PFS in Asian patients with EGFR mutated NSCLC when adding bevacizumab to erlotinib – The results support the use of bevacizumab in combination with erlotinib as a first-line treatment of Caucasian patients with NSCLC and activating EGFR mutations Stahel et al. Ann Oncol 2015; 26 (suppl 6): abstr 3 BA

Advanced NSCLC Not radically treatable stage III and stage IV Later lines

516: Chimeric Antigen Receptor-Modified T Cells for the immunotherapy of patients with Her-1 expressing advanced relapsed/refractory Non-Small Cell Lung Cancer – Feng K-C et al • Study objective – To evaluate the safety, feasibility and efficacy of CART cells to target Her-1 in advanced relapsed or multidrug resistant Her-1+ NSCLC • Study design – Phase 1 clinical study of patients with histologically confirmed advanced NSCLC with Her-1 expression (>50%), ECOG PS 0− 2 and at least 2 failed cycles of chemotherapy or targeted therapy – Patients with Her-1 positive, advanced relapsed or refractory NSCLC received CART-Her-1 cells with or without conditioning chemotherapy – Autologous CART-Her-1 cells were generated from 50− 80 m. L peripheral blood after a 10− 12 -day in vitro expansion, and the total CAR-expressing T cell number of 1 x 106/kg was set as an output control – Clinical responses were evaluated with RECIST 1. 1 – IHC staining was used to calculate the frequency of Her-1 positive cells CART, chimeric antigen receptor-modified T Feng et al. Ann Oncol 2015; 26 (suppl 6): abstr 516

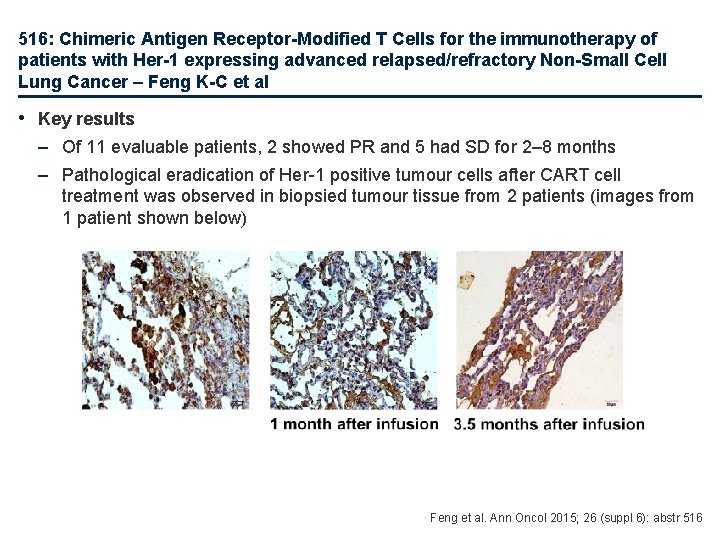
516: Chimeric Antigen Receptor-Modified T Cells for the immunotherapy of patients with Her-1 expressing advanced relapsed/refractory Non-Small Cell Lung Cancer – Feng K-C et al • Key results – Of 11 evaluable patients, 2 showed PR and 5 had SD for 2– 8 months – Pathological eradication of Her-1 positive tumour cells after CART cell treatment was observed in biopsied tumour tissue from 2 patients (images from 1 patient shown below) Feng et al. Ann Oncol 2015; 26 (suppl 6): abstr 516

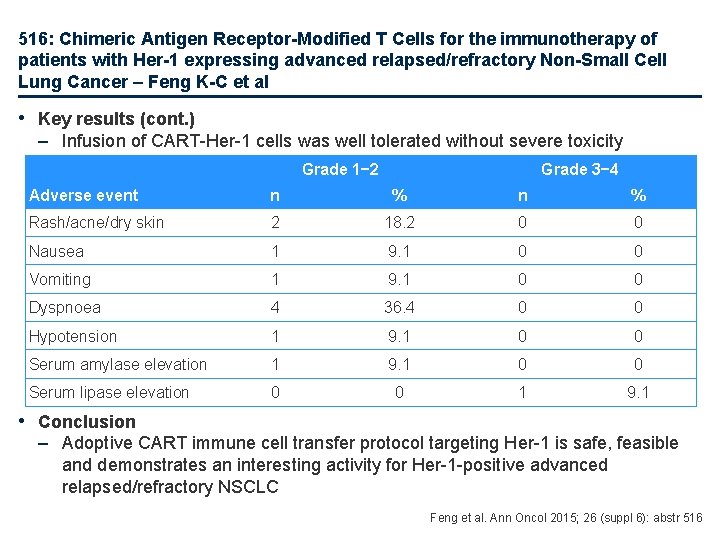
516: Chimeric Antigen Receptor-Modified T Cells for the immunotherapy of patients with Her-1 expressing advanced relapsed/refractory Non-Small Cell Lung Cancer – Feng K-C et al • Key results (cont. ) – Infusion of CART-Her-1 cells was well tolerated without severe toxicity Grade 1− 2 Grade 3− 4 Adverse event n % Rash/acne/dry skin 2 18. 2 0 0 Nausea 1 9. 1 0 0 Vomiting 1 9. 1 0 0 Dyspnoea 4 36. 4 0 0 Hypotension 1 9. 1 0 0 Serum amylase elevation 1 9. 1 0 0 Serum lipase elevation 0 0 1 9. 1 • Conclusion – Adoptive CART immune cell transfer protocol targeting Her-1 is safe, feasible and demonstrates an interesting activity for Her-1 -positive advanced relapsed/refractory NSCLC Feng et al. Ann Oncol 2015; 26 (suppl 6): abstr 516

3015: Chemotherapy and complete surgical resection are prognostic factors of survival in stage IV NSCLC with synchronous isolated metastasis – Toffart AC et al • Study objective – To assess survival of patients with synchronous isolated metastasis (SIM) stage IV NSCLC according to treatments received • Study design – This retrospective analysis examined patients with SIM stage IV NSCLC (treated after 1993) using the database of the Multidisciplinary Thoracic Oncology Group of the Grenoble Teaching Hospital; data was screened across the period 1980– 2012 – Data were collected for major prognostic characteristics at diagnosis (gender, age, performance status, histological diagnosis), TNM stage with details of metastatic site, characteristics and date of cancer treatment, plus survival follow-up – OS curves were obtained using the Kaplan-Meier method and compared between groups using the log-rank test. Cox models were used for multivariate analyses • Key results – Of 6, 760 patients with NSCLC in the database, 109 patients with SIM were identified • 96. 3% had ECOG PS 0– 2 • 57. 8% had adenocarcinoma • Most had clinical T and N classification of ≤ 2 Toffart et al. Ann Oncol 2015; 26 (suppl 6): abstr 3015

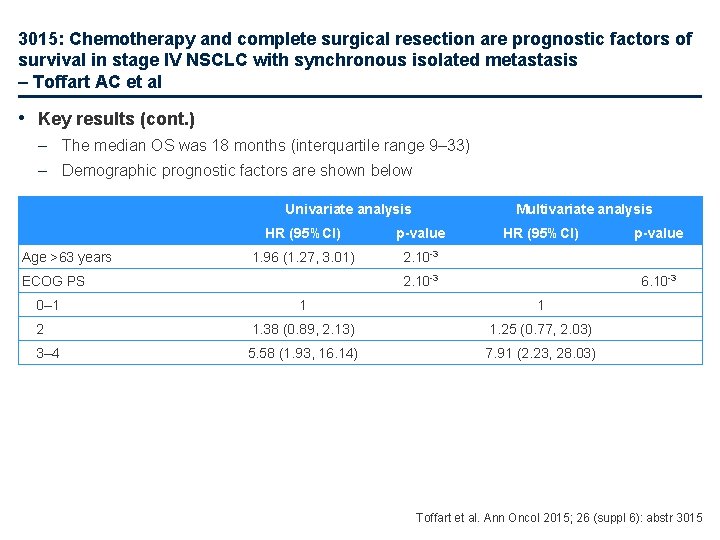
3015: Chemotherapy and complete surgical resection are prognostic factors of survival in stage IV NSCLC with synchronous isolated metastasis – Toffart AC et al • Key results (cont. ) – The median OS was 18 months (interquartile range 9– 33) – Demographic prognostic factors are shown below Univariate analysis Age >63 years HR (95%CI) p-value 1. 96 (1. 27, 3. 01) 2. 10 -3 ECOG PS 0– 1 Multivariate analysis HR (95%CI) 2. 10 -3 p-value 6. 10 -3 1 1 2 1. 38 (0. 89, 2. 13) 1. 25 (0. 77, 2. 03) 3– 4 5. 58 (1. 93, 16. 14) 7. 91 (2. 23, 28. 03) Toffart et al. Ann Oncol 2015; 26 (suppl 6): abstr 3015

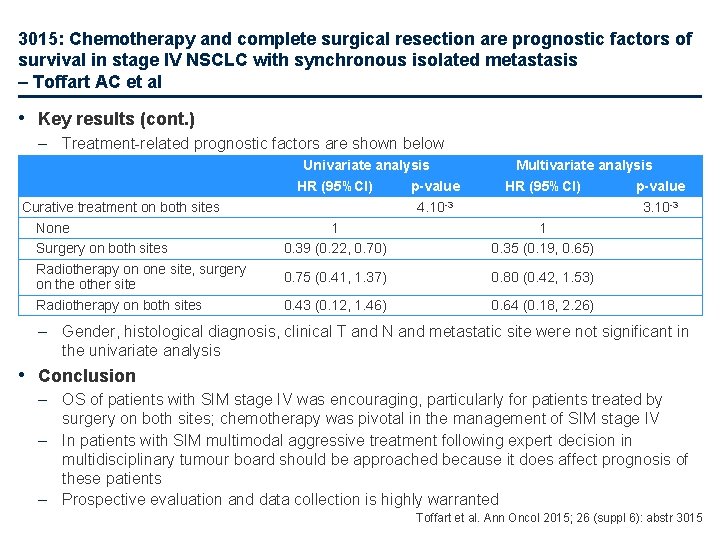
3015: Chemotherapy and complete surgical resection are prognostic factors of survival in stage IV NSCLC with synchronous isolated metastasis – Toffart AC et al • Key results (cont. ) – Treatment-related prognostic factors are shown below Curative treatment on both sites None Surgery on both sites Radiotherapy on one site, surgery on the other site Radiotherapy on both sites Univariate analysis HR (95%CI) p-value 4. 10 -3 1 0. 39 (0. 22, 0. 70) Multivariate analysis HR (95%CI) p-value 3. 10 -3 1 0. 35 (0. 19, 0. 65) 0. 75 (0. 41, 1. 37) 0. 80 (0. 42, 1. 53) 0. 43 (0. 12, 1. 46) 0. 64 (0. 18, 2. 26) – Gender, histological diagnosis, clinical T and N and metastatic site were not significant in the univariate analysis • Conclusion – OS of patients with SIM stage IV was encouraging, particularly for patients treated by surgery on both sites; chemotherapy was pivotal in the management of SIM stage IV – In patients with SIM multimodal aggressive treatment following expert decision in multidisciplinary tumour board should be approached because it does affect prognosis of these patients – Prospective evaluation and data collection is highly warranted Toffart et al. Ann Oncol 2015; 26 (suppl 6): abstr 3015

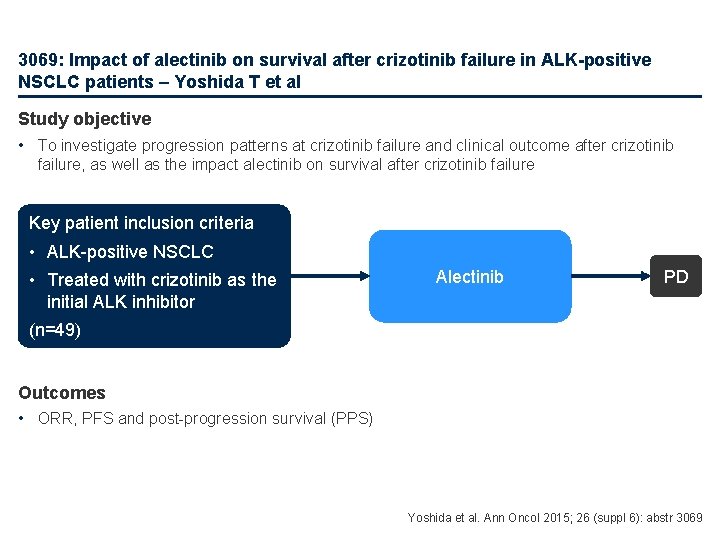
3069: Impact of alectinib on survival after crizotinib failure in ALK-positive NSCLC patients – Yoshida T et al Study objective • To investigate progression patterns at crizotinib failure and clinical outcome after crizotinib failure, as well as the impact alectinib on survival after crizotinib failure Key patient inclusion criteria • ALK-positive NSCLC • Treated with crizotinib as the initial ALK inhibitor Alectinib PD (n=49) Outcomes • ORR, PFS and post-progression survival (PPS) Yoshida et al. Ann Oncol 2015; 26 (suppl 6): abstr 3069

3069: Impact of alectinib on survival after crizotinib failure in ALK-positive NSCLC patients – Yoshida T et al • Key results – Following crizotinib treatment, the ORR was 65%, median PFS was 9. 9 months; a response (CR + PR) was observed in 32 of 49 patients, 8 patients had SD and 8 PD • Multivariate analyses revealed PS score ≥ 2 and crizotinib response to be significant predictive factors for PPS (p=0. 0238 and p=0. 0325, respectively) – Of the 35 patients who progressed, 12 received alectinib • Median PPS was 10. 5 months • Patients who received alectinib had a significantly longer PPS than those who did not (4. 5 months vs. NR, p=0. 0003) • Ten of the 12 (83%) patients had a PR (p<0. 0001), two had SD • Multivariate analysis demonstrated that alectinib treatment after crizotinib failure significantly improved PPS (HR 0. 021 [95%CI 0. 0001, 0. 1394]; p<0. 0001) • Conclusion – After crizotinib failure, alectinib was associated with a significantly longer disease control rate and an interesting post-crizotinib survival in patients with ALK+ NSCLC Yoshida et al. Ann Oncol 2015; 26 (suppl 6): abstr 3069

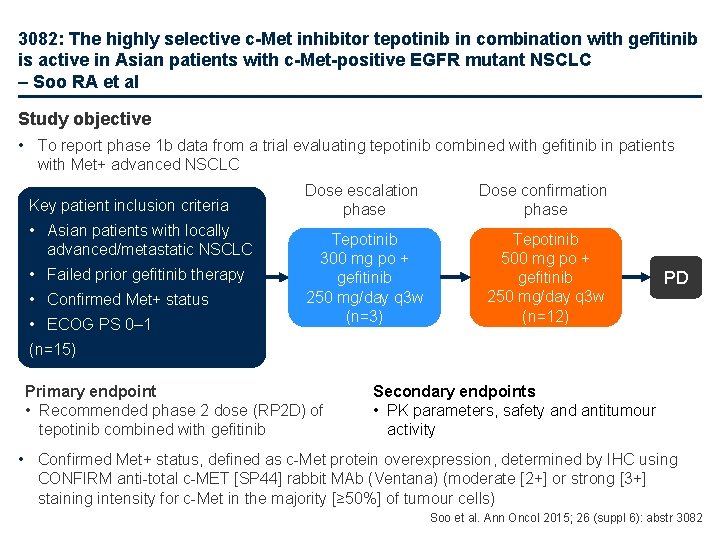
3082: The highly selective c-Met inhibitor tepotinib in combination with gefitinib is active in Asian patients with c-Met-positive EGFR mutant NSCLC – Soo RA et al Study objective • To report phase 1 b data from a trial evaluating tepotinib combined with gefitinib in patients with Met+ advanced NSCLC Key patient inclusion criteria • Asian patients with locally advanced/metastatic NSCLC • Failed prior gefitinib therapy • Confirmed Met+ status • ECOG PS 0– 1 Dose escalation phase Dose confirmation phase Tepotinib 300 mg po + gefitinib 250 mg/day q 3 w (n=3) Tepotinib 500 mg po + gefitinib 250 mg/day q 3 w (n=12) PD (n=15) Primary endpoint • Recommended phase 2 dose (RP 2 D) of tepotinib combined with gefitinib Secondary endpoints • PK parameters, safety and antitumour activity • Confirmed Met+ status, defined as c-Met protein overexpression, determined by IHC using CONFIRM anti-total c-MET [SP 44] rabbit MAb (Ventana) (moderate [2+] or strong [3+] staining intensity for c-Met in the majority [≥ 50%] of tumour cells) Soo et al. Ann Oncol 2015; 26 (suppl 6): abstr 3082

3082: The highly selective c-Met inhibitor tepotinib in combination with gefitinib is active in Asian patients with c-Met-positive EGFR mutant NSCLC – Soo RA et al • Key results – Of the 15 patients enrolled, 12 had discontinued treatment as of 01 June 2015, mostly due to progressive disease – No dose-limiting toxicities were observed with either dose of tepotinib – The dose of 500 mg/day was confirmed as the RP 2 D – AEs were generally mild – A total of 11 (73. 3%) patients had any tepotinib-related TEAE (3 in the 300 mg group and 8 in the 500 mg group) • Amylase increase, diarrhoea, decreased appetite and dyspesia occurred in ≥ 20% of all patients – The likelihood of response to treatment was greater as tumour c-Met expression increased (for IHC 2+, 1 PR, 5 SD and 2 PD; IHC 3+, 4 PR, 1 SD and 1 PD) • Conclusions – When used in combination with gefitinib, tepotinib should be used at 500 mg/day in Asian patients with advanced NSCLC – Antitumour response appeared to be greater in patients with elevated c-Met Soo et al. Ann Oncol 2015; 26 (suppl 6): abstr 3082 expression but requires further investigation

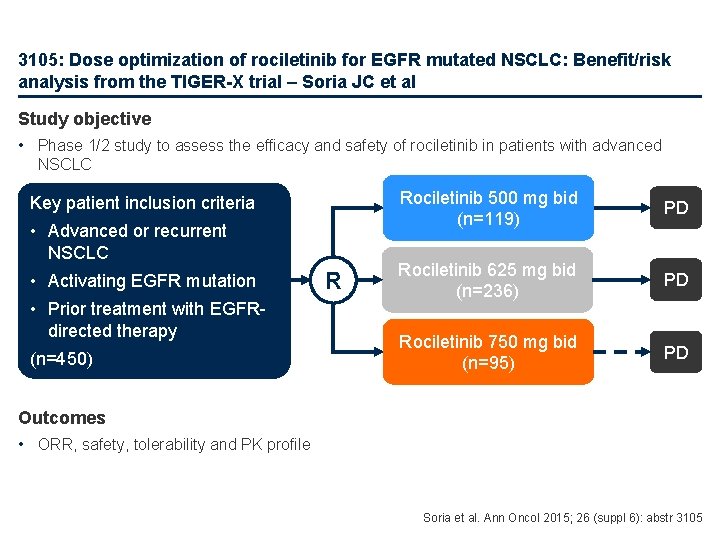
3105: Dose optimization of rociletinib for EGFR mutated NSCLC: Benefit/risk analysis from the TIGER-X trial – Soria JC et al Study objective • Phase 1/2 study to assess the efficacy and safety of rociletinib in patients with advanced NSCLC Key patient inclusion criteria • Advanced or recurrent NSCLC • Activating EGFR mutation • Prior treatment with EGFRdirected therapy (n=450) R Rociletinib 500 mg bid (n=119) PD Rociletinib 625 mg bid (n=236) PD Rociletinib 750 mg bid (n=95) PD Outcomes • ORR, safety, tolerability and PK profile Soria et al. Ann Oncol 2015; 26 (suppl 6): abstr 3105

3105: Dose optimization of rociletinib for EGFR mutated NSCLC: Benefit/risk analysis from the TIGER-X trial – Soria JC et al • Key results – Increasing dose was not associated with increased RECIST responses 500 mg bida (n=48) 625 mg bid (n=114) 750 mg bid (n=77) Total (n=239) Objective response rate, % (95%CI) 60 (45, 74) 54 (44, 63) 46 (34, 57) 52 (46, 59) Disease control rate, % (95%CI) 90 (77, 97) 84 (76, 90) 82 (71, 90) 85 (79, 89) – Hyperglycaemia was the most common AE occurring in 35%, 45% and 59% of patients receiving rociletinib 500, 625 and 750 mg bid, respectively; corresponding values for grade ≥ 3 hyperglycaemia were 17%, 24% and 36%, respectively • Diarrhoea, nausea, fatigue and QTc prolongation were the other four most common AEs – The concentration of the metabolite M 502 was associated with rociletinib-induced hyperglycaemia • Conclusions – All three doses of rociletinib were well tolerated and had activity in patients with advanced NSCLC – There was no dose-response effect for antitumour activity – Rociletinib at 500 mg bid was associated with the best risk-benefit profile aincludes patients who switched from 900 mg bid free-based formulation Soria et al. Ann Oncol 2015; 26 (suppl 6): abstr 3105

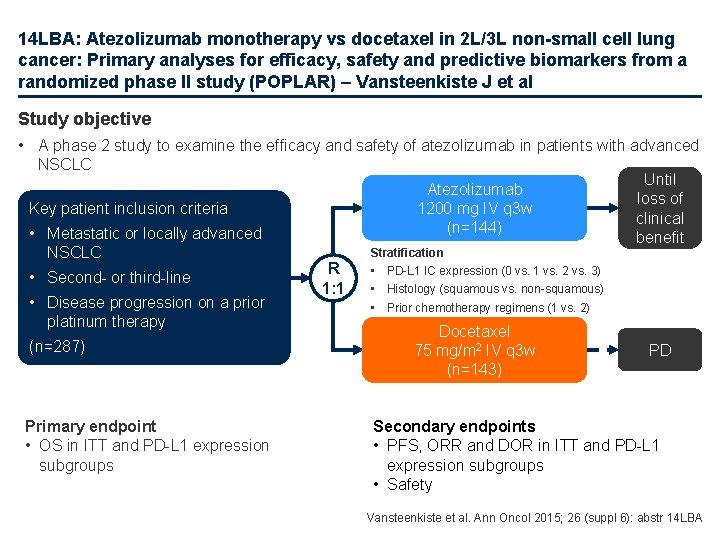
14 LBA: Atezolizumab monotherapy vs docetaxel in 2 L/3 L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR) – Vansteenkiste J et al Study objective • A phase 2 study to examine the efficacy and safety of atezolizumab in patients with advanced NSCLC Atezolizumab 1200 mg IV q 3 w (n=144) Key patient inclusion criteria • Metastatic or locally advanced NSCLC • Second- or third-line • Disease progression on a prior platinum therapy (n=287) Primary endpoint • OS in ITT and PD-L 1 expression subgroups Stratification R 1: 1 Until loss of clinical benefit • PD-L 1 IC expression (0 vs. 1 vs. 2 vs. 3) • Histology (squamous vs. non-squamous) • Prior chemotherapy regimens (1 vs. 2) Docetaxel 75 mg/m 2 IV q 3 w (n=143) PD Secondary endpoints • PFS, ORR and DOR in ITT and PD-L 1 expression subgroups • Safety Vansteenkiste et al. Ann Oncol 2015; 26 (suppl 6): abstr 14 LBA

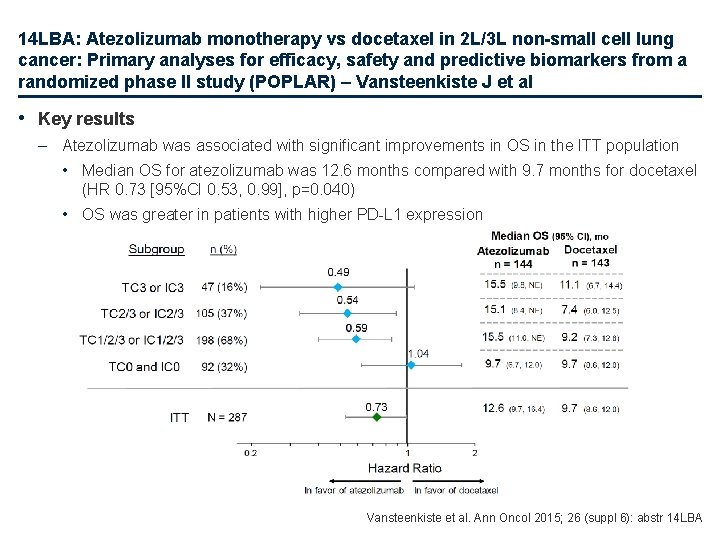
14 LBA: Atezolizumab monotherapy vs docetaxel in 2 L/3 L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR) – Vansteenkiste J et al • Key results – Atezolizumab was associated with significant improvements in OS in the ITT population • Median OS for atezolizumab was 12. 6 months compared with 9. 7 months for docetaxel (HR 0. 73 [95%CI 0. 53, 0. 99], p=0. 040) • OS was greater in patients with higher PD-L 1 expression Vansteenkiste et al. Ann Oncol 2015; 26 (suppl 6): abstr 14 LBA

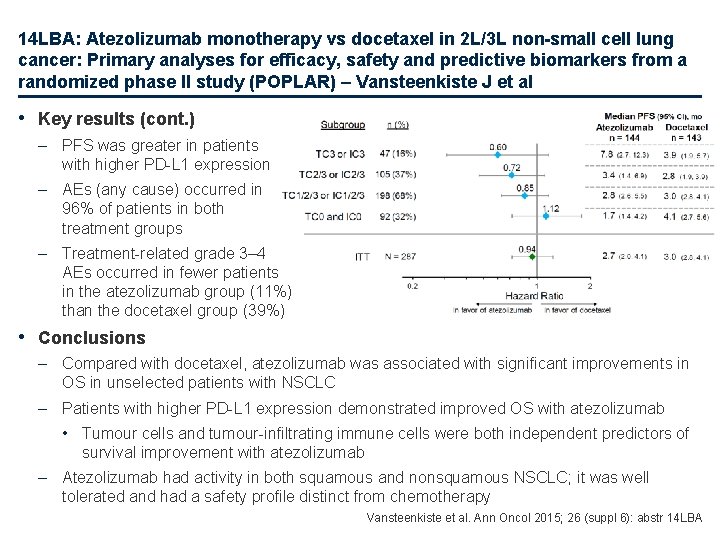
14 LBA: Atezolizumab monotherapy vs docetaxel in 2 L/3 L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR) – Vansteenkiste J et al • Key results (cont. ) – PFS was greater in patients with higher PD-L 1 expression – AEs (any cause) occurred in 96% of patients in both treatment groups – Treatment-related grade 3– 4 AEs occurred in fewer patients in the atezolizumab group (11%) than the docetaxel group (39%) • Conclusions – Compared with docetaxel, atezolizumab was associated with significant improvements in OS in unselected patients with NSCLC – Patients with higher PD-L 1 expression demonstrated improved OS with atezolizumab • Tumour cells and tumour-infiltrating immune cells were both independent predictors of survival improvement with atezolizumab – Atezolizumab had activity in both squamous and nonsquamous NSCLC; it was well tolerated and had a safety profile distinct from chemotherapy Vansteenkiste et al. Ann Oncol 2015; 26 (suppl 6): abstr 14 LBA

15 LBA: High tumoral IFNγ m. RNA, PD-L 1 protein, and combined IFNγ m. RNA/ PD-L 1 protein expression associates with response to durvalumab (anti-PD-L 1) monotherapy in NSCLC patients – Higgs B et al • Study objective – To perform post-hoc analyses on results from a phase 1/2 study to determine factors that predict response to durvalumab (MEDI 4736) • Study design – Study 1108 was a non-randomised, open-label, phase 1/2 multicentre study that enrolled patients with stage IIIB/IV squamous and nonsquamous NSCLC – A total of 228 NSCLC patients (n=102 squamous; n=126 nonsquamous) were treated with durvalumab 10 mg/kg q 2 w, most (n=201) were previously treated • Of these 200 patients were evaluable for a response – Predictive biomarkers were assessed • IHC for tumoural PD-L 1 was conducted on pre-treatment fresh or archival biopsies (SP 263 assay; n=176) • Frozen tumour samples with sufficient m. RNA quality were profiled (n=122) with 100 pre -selected genes using Fluidigm Biomark™. • Matched m. RNA and PD-L 1 IHC data were available for 112 patient biopsies Higgs et al. Ann Oncol 2015; 26 (suppl 6): abstr 15 LBA

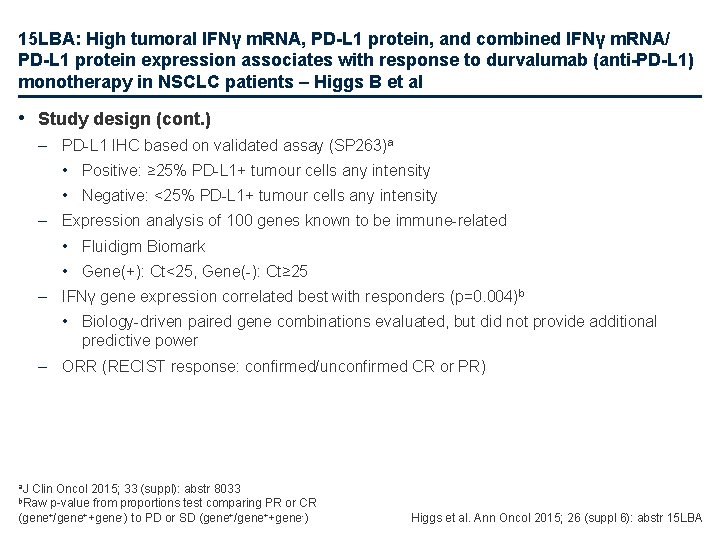
15 LBA: High tumoral IFNγ m. RNA, PD-L 1 protein, and combined IFNγ m. RNA/ PD-L 1 protein expression associates with response to durvalumab (anti-PD-L 1) monotherapy in NSCLC patients – Higgs B et al • Study design (cont. ) – PD-L 1 IHC based on validated assay (SP 263)a • Positive: ≥ 25% PD-L 1+ tumour cells any intensity • Negative: <25% PD-L 1+ tumour cells any intensity – Expression analysis of 100 genes known to be immune-related • Fluidigm Biomark • Gene(+): Ct<25, Gene(-): Ct≥ 25 – IFNγ gene expression correlated best with responders (p=0. 004)b • Biology-driven paired gene combinations evaluated, but did not provide additional predictive power – ORR (RECIST response: confirmed/unconfirmed CR or PR) a. J Clin Oncol 2015; 33 (suppl): abstr 8033 b. Raw p-value from proportions test comparing PR or CR (gene+/gene++gene-) to PD or SD (gene+/gene++gene-) Higgs et al. Ann Oncol 2015; 26 (suppl 6): abstr 15 LBA

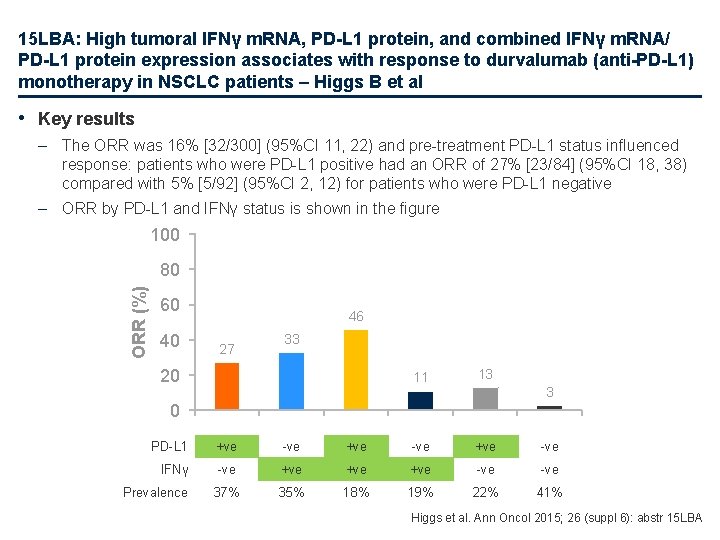
15 LBA: High tumoral IFNγ m. RNA, PD-L 1 protein, and combined IFNγ m. RNA/ PD-L 1 protein expression associates with response to durvalumab (anti-PD-L 1) monotherapy in NSCLC patients – Higgs B et al • Key results – The ORR was 16% [32/300] (95%CI 11, 22) and pre-treatment PD-L 1 status influenced response: patients who were PD-L 1 positive had an ORR of 27% [23/84] (95%CI 18, 38) compared with 5% [5/92] (95%CI 2, 12) for patients who were PD-L 1 negative – ORR by PD-L 1 and IFNγ status is shown in the figure 100 ORR (%) 80 60 40 46 27 33 20 11 13 3 0 PD-L 1 +ve -ve IFNγ -ve +ve +ve -ve 37% 35% 18% 19% 22% 41% Prevalence Higgs et al. Ann Oncol 2015; 26 (suppl 6): abstr 15 LBA

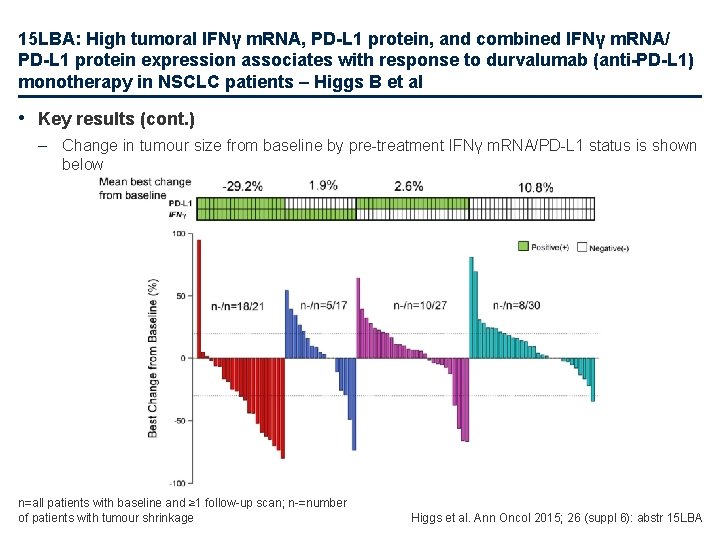
15 LBA: High tumoral IFNγ m. RNA, PD-L 1 protein, and combined IFNγ m. RNA/ PD-L 1 protein expression associates with response to durvalumab (anti-PD-L 1) monotherapy in NSCLC patients – Higgs B et al • Key results (cont. ) – Change in tumour size from baseline by pre-treatment IFNγ m. RNA/PD-L 1 status is shown below n=all patients with baseline and ≥ 1 follow-up scan; n-=number of patients with tumour shrinkage Higgs et al. Ann Oncol 2015; 26 (suppl 6): abstr 15 LBA

15 LBA: High tumoral IFNγ m. RNA, PD-L 1 protein, and combined IFNγ m. RNA/ PD-L 1 protein expression associates with response to durvalumab (anti-PD-L 1) monotherapy in NSCLC patients – Higgs B et al • Conclusions – Post-hoc analyses indicated that in patients with NSCLC, IFNγ and PD-L 1 status/expression are predictive of response to durvalumab • Patients positive for PD-L 1 had better response than patients negative for PD-L 1 • Similarly, patients who were IFNγ m. RNA positive had better overall response than IFNγ m. RNA negative patients • Patients positive for both IFNγ and PD-L 1 were even more likely to benefit from treatment with durvalumab than patients negative for these biomarkers – Further prospective studies are required to confirm these observations Higgs et al. Ann Oncol 2015; 26 (suppl 6): abstr 15 LBA

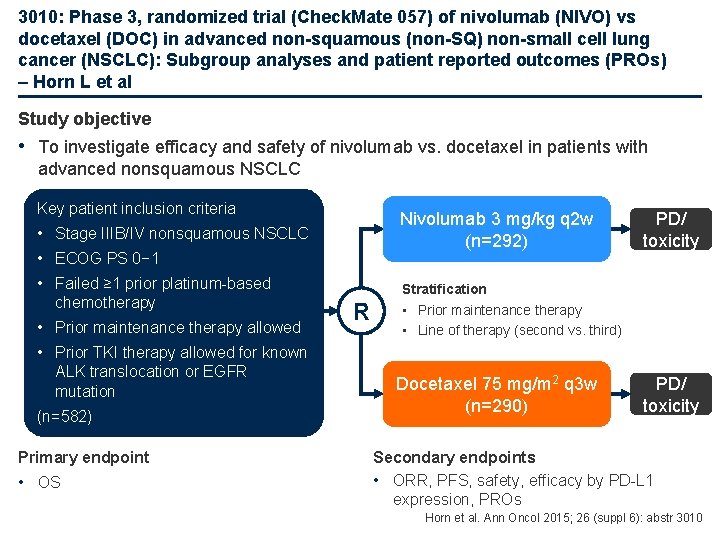
3010: Phase 3, randomized trial (Check. Mate 057) of nivolumab (NIVO) vs docetaxel (DOC) in advanced non-squamous (non-SQ) non-small cell lung cancer (NSCLC): Subgroup analyses and patient reported outcomes (PROs) – Horn L et al Study objective • To investigate efficacy and safety of nivolumab vs. docetaxel in patients with advanced nonsquamous NSCLC Key patient inclusion criteria Nivolumab 3 mg/kg q 2 w (n=292) • Stage IIIB/IV nonsquamous NSCLC • ECOG PS 0− 1 • Failed ≥ 1 prior platinum-based chemotherapy • Prior maintenance therapy allowed • Prior TKI therapy allowed for known ALK translocation or EGFR mutation (n=582) Primary endpoint • OS R PD/ toxicity Stratification • Prior maintenance therapy • Line of therapy (second vs. third) Docetaxel 75 mg/m 2 q 3 w (n=290) PD/ toxicity Secondary endpoints • ORR, PFS, safety, efficacy by PD-L 1 expression, PROs Horn et al. Ann Oncol 2015; 26 (suppl 6): abstr 3010

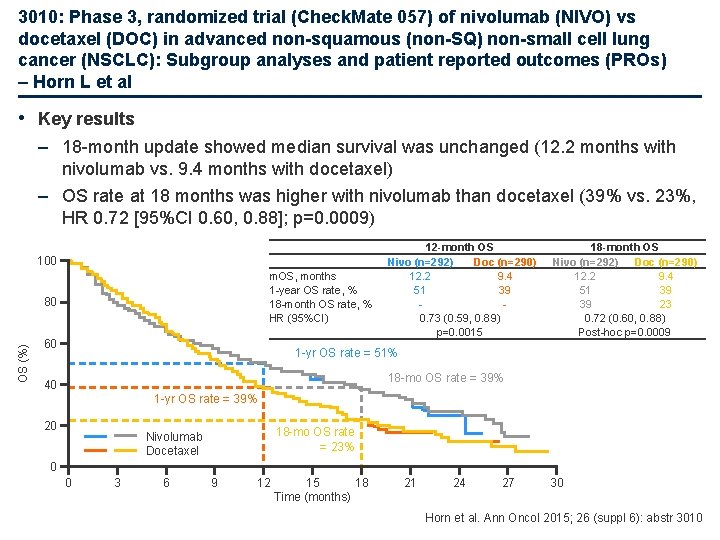
3010: Phase 3, randomized trial (Check. Mate 057) of nivolumab (NIVO) vs docetaxel (DOC) in advanced non-squamous (non-SQ) non-small cell lung cancer (NSCLC): Subgroup analyses and patient reported outcomes (PROs) – Horn L et al • Key results – 18 -month update showed median survival was unchanged (12. 2 months with nivolumab vs. 9. 4 months with docetaxel) – OS rate at 18 months was higher with nivolumab than docetaxel (39% vs. 23%, HR 0. 72 [95%CI 0. 60, 0. 88]; p=0. 0009) 100 m. OS, months 1 -year OS rate, % 18 -month OS rate, % HR (95%CI) OS (%) 80 60 12 -month OS Nivo (n=292) Doc (n=290) 12. 2 9. 4 51 39 0. 73 (0. 59, 0. 89) p=0. 0015 18 -month OS Nivo (n=292) Doc (n=290) 12. 2 9. 4 51 39 39 23 0. 72 (0. 60, 0. 88) Post-hoc p=0. 0009 1 -yr OS rate = 51% 18 -mo OS rate = 39% 40 1 -yr OS rate = 39% 20 18 -mo OS rate = 23% Nivolumab Docetaxel 0 0 3 6 9 12 15 18 Time (months) 21 24 27 30 Horn et al. Ann Oncol 2015; 26 (suppl 6): abstr 3010

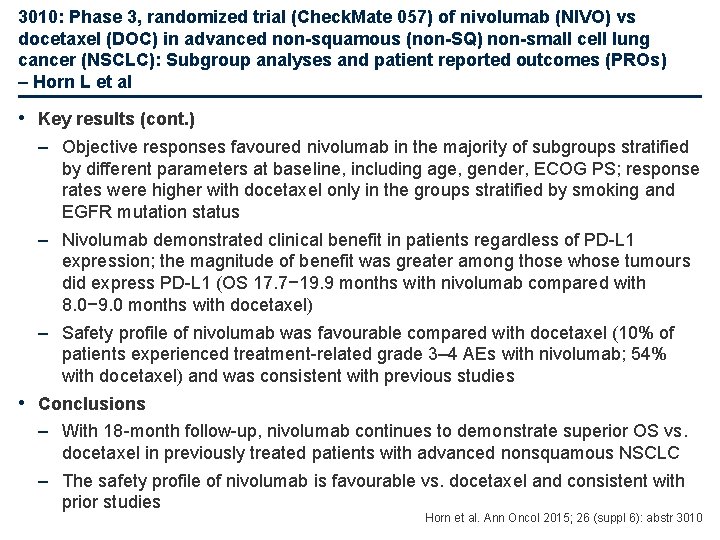
3010: Phase 3, randomized trial (Check. Mate 057) of nivolumab (NIVO) vs docetaxel (DOC) in advanced non-squamous (non-SQ) non-small cell lung cancer (NSCLC): Subgroup analyses and patient reported outcomes (PROs) – Horn L et al • Key results (cont. ) – Objective responses favoured nivolumab in the majority of subgroups stratified by different parameters at baseline, including age, gender, ECOG PS; response rates were higher with docetaxel only in the groups stratified by smoking and EGFR mutation status – Nivolumab demonstrated clinical benefit in patients regardless of PD-L 1 expression; the magnitude of benefit was greater among those whose tumours did express PD-L 1 (OS 17. 7− 19. 9 months with nivolumab compared with 8. 0− 9. 0 months with docetaxel) – Safety profile of nivolumab was favourable compared with docetaxel (10% of patients experienced treatment-related grade 3– 4 AEs with nivolumab; 54% with docetaxel) and was consistent with previous studies • Conclusions – With 18 -month follow-up, nivolumab continues to demonstrate superior OS vs. docetaxel in previously treated patients with advanced nonsquamous NSCLC – The safety profile of nivolumab is favourable vs. docetaxel and consistent with prior studies Horn et al. Ann Oncol 2015; 26 (suppl 6): abstr 3010

3011: Evaluation of overall health status in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel in Check. Mate 017 – Reck M et al • Study objective – To investigate the effect on quality of life of nivolumab vs. docetaxel in patients with squamous NSCLC • Study design – Check. Mate 017 was a randomised, open-label, phase 3 study that evaluated the efficacy and safety of second-line nivolumab 3 mg/kg q 2 w (n=135) vs. docetaxel 75 mg/m 2 q 3 w (n=137) in advanced squamous NSCLC – Health status on treatment was assessed q 4 w for nivolumab and q 3 w for docetaxel for 6 months and q 6 w thereafter, plus 2 post-treatment visits – Quality of life was evaluated using the EQ-index (scaled 0− 1) and the EQ-VAS (scaled 1− 100); higher scores indicate better health status – The minimally important differences are ≥ 0. 08 points for the EQ-5 D and ≥ 7 points for the EQ-5 D VAS EQ-5 D index, Euro. QOL Group’s preference-based health state utility measure; EQ-VAS, Euro. QOL visual analogue scale Reck et al. Ann Oncol 2015; 26 (suppl 6): abstr 3011

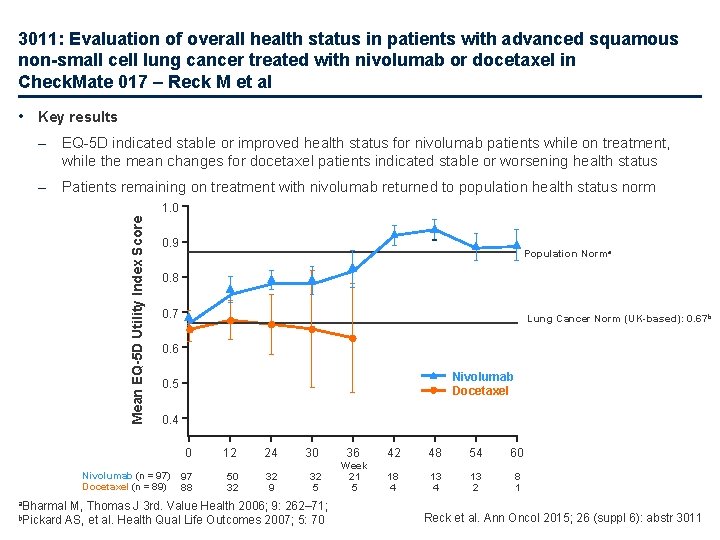
3011: Evaluation of overall health status in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel in Check. Mate 017 – Reck M et al • Key results – EQ-5 D indicated stable or improved health status for nivolumab patients while on treatment, while the mean changes for docetaxel patients indicated stable or worsening health status – Patients remaining on treatment with nivolumab returned to population health status norm Mean EQ-5 D Utility Index Score 1. 0 0. 9 Population Norma 0. 8 0. 7 Lung Cancer Norm (UK-based): 0. 67 b 0. 6 Nivolumab Docetaxel 0. 5 0. 4 0 Nivolumab (n = 97) Docetaxel (n = 89) 97 88 12 50 32 24 32 9 30 32 5 a. Bharmal M, Thomas J 3 rd. Value Health 2006; 9: 262– 71; b. Pickard AS, et al. Health Qual Life Outcomes 2007; 5: 70 36 42 48 54 60 Week 21 5 18 4 13 2 8 1 Reck et al. Ann Oncol 2015; 26 (suppl 6): abstr 3011

3011: Evaluation of overall health status in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel in Check. Mate 017 – Reck M et al • Key results (cont. ) – Changes from baseline in EQ-5 D index scores exceeded the MID to show improvement with nivolumab beyond 42 weeks and deterioration with docetaxel at 36 weeks – Time to first disease-related deterioration was slower with nivolumab compared with docetaxel with HR (95%CI) 0. 55 (0. 36, 0. 84) for EQ-5 D index and 0. 59 (0. 40, 0. 87) with EQ-VAS – Using the LCSS Symptom Burden Index by week 12, 20. 0% of patients receiving nivolumab demonstrated clinically meaningful improvement compared with 21. 9% of patients receiving docetaxel • Conclusions – Mean changes from baseline in general health status indicated stable or improved health status for patients receiving nivolumab compared with stable or worsening health status with docetaxel – The observed return to population health status norm with nivolumab suggests that prolonged survival occurs with a resumption of normal life – Health status of patients on nivolumab deteriorated at a significantly slower rate than those on docetaxel MID, minimally important difference Reck et al. Ann Oncol 2015; 26 (suppl 6): abstr 3011

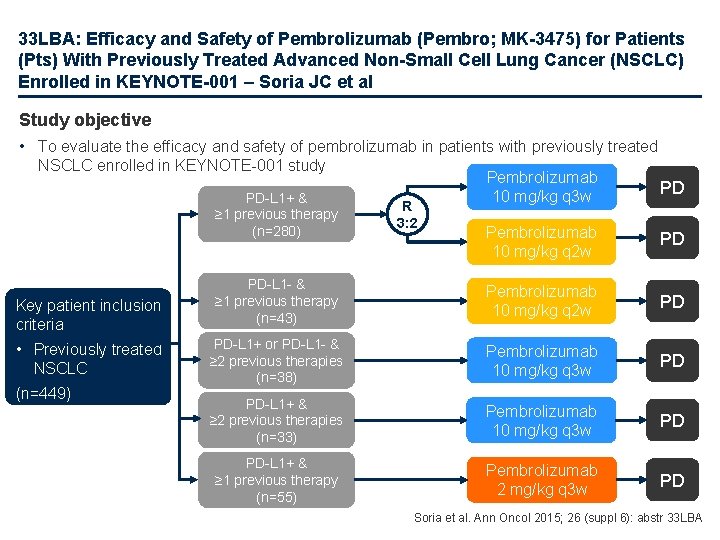
33 LBA: Efficacy and Safety of Pembrolizumab (Pembro; MK-3475) for Patients (Pts) With Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC) Enrolled in KEYNOTE-001 – Soria JC et al Study objective • To evaluate the efficacy and safety of pembrolizumab in patients with previously treated NSCLC enrolled in KEYNOTE-001 study Pembrolizumab 10 mg/kg q 3 w PD Pembrolizumab 10 mg/kg q 2 w PD PD-L 1 - & ≥ 1 previous therapy (n=43) Pembrolizumab 10 mg/kg q 2 w PD PD-L 1+ or PD-L 1 - & ≥ 2 previous therapies (n=38) Pembrolizumab 10 mg/kg q 3 w PD PD-L 1+ & ≥ 2 previous therapies (n=33) Pembrolizumab 10 mg/kg q 3 w PD PD-L 1+ & ≥ 1 previous therapy (n=55) Pembrolizumab 2 mg/kg q 3 w PD PD-L 1+ & ≥ 1 previous therapy (n=280) Key patient inclusion criteria • Previously treated NSCLC (n=449) R 3: 2 Soria et al. Ann Oncol 2015; 26 (suppl 6): abstr 33 LBA

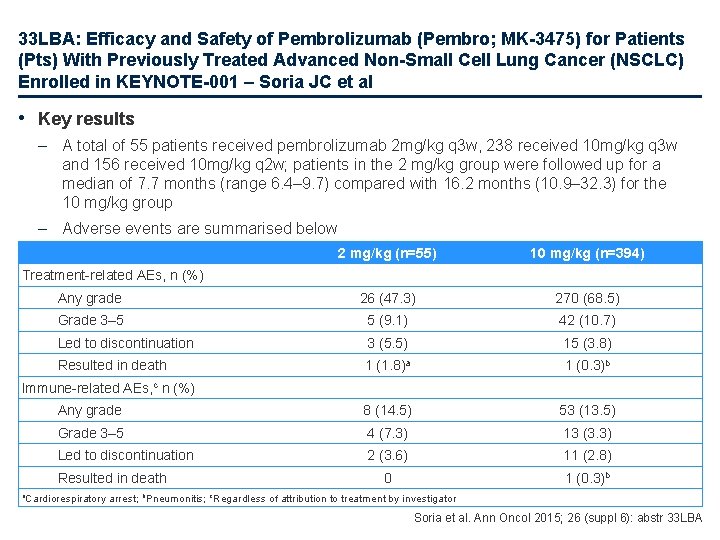
33 LBA: Efficacy and Safety of Pembrolizumab (Pembro; MK-3475) for Patients (Pts) With Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC) Enrolled in KEYNOTE-001 – Soria JC et al • Key results – A total of 55 patients received pembrolizumab 2 mg/kg q 3 w, 238 received 10 mg/kg q 3 w and 156 received 10 mg/kg q 2 w; patients in the 2 mg/kg group were followed up for a median of 7. 7 months (range 6. 4– 9. 7) compared with 16. 2 months (10. 9– 32. 3) for the 10 mg/kg group – Adverse events are summarised below 2 mg/kg (n=55) 10 mg/kg (n=394) Any grade 26 (47. 3) 270 (68. 5) Grade 3– 5 5 (9. 1) 42 (10. 7) Led to discontinuation 3 (5. 5) 15 (3. 8) Resulted in death 1 (1. 8)a 1 (0. 3)b Any grade 8 (14. 5) 53 (13. 5) Grade 3– 5 4 (7. 3) 13 (3. 3) Led to discontinuation 2 (3. 6) 11 (2. 8) 0 1 (0. 3)b Treatment-related AEs, n (%) Immune-related AEs, c n (%) Resulted in death a. Cardiorespiratory arrest; b. Pneumonitis; c. Regardless of attribution to treatment by investigator Soria et al. Ann Oncol 2015; 26 (suppl 6): abstr 33 LBA

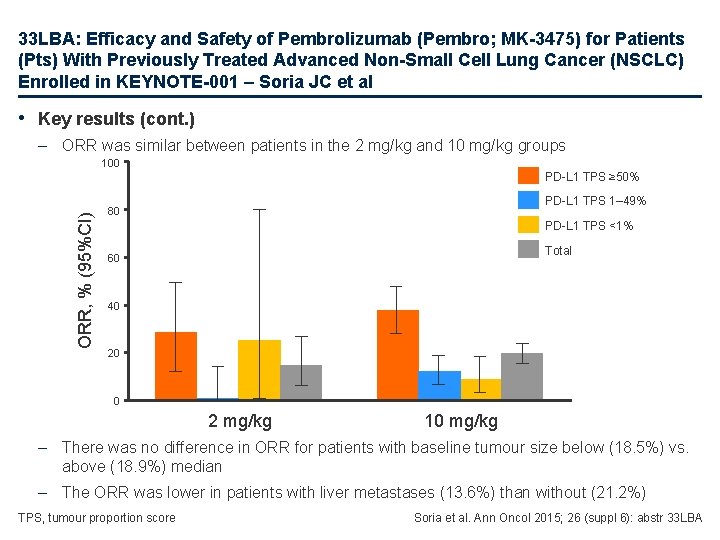
33 LBA: Efficacy and Safety of Pembrolizumab (Pembro; MK-3475) for Patients (Pts) With Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC) Enrolled in KEYNOTE-001 – Soria JC et al • Key results (cont. ) – ORR was similar between patients in the 2 mg/kg and 10 mg/kg groups 100 ORR, % (95%CI) PD-L 1 TPS ≥ 50% PD-L 1 TPS 1– 49% 80 PD-L 1 TPS <1% Total 60 40 20 0 2 mg/kg 10 mg/kg – There was no difference in ORR for patients with baseline tumour size below (18. 5%) vs. above (18. 9%) median – The ORR was lower in patients with liver metastases (13. 6%) than without (21. 2%) TPS, tumour proportion score Soria et al. Ann Oncol 2015; 26 (suppl 6): abstr 33 LBA

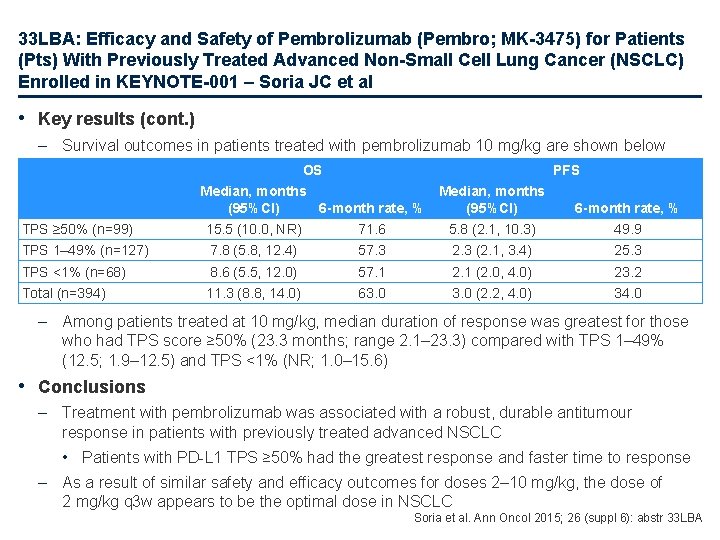
33 LBA: Efficacy and Safety of Pembrolizumab (Pembro; MK-3475) for Patients (Pts) With Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC) Enrolled in KEYNOTE-001 – Soria JC et al • Key results (cont. ) – Survival outcomes in patients treated with pembrolizumab 10 mg/kg are shown below OS PFS Median, months (95%CI) 6 -month rate, % TPS ≥ 50% (n=99) Median, months (95%CI) 6 -month rate, % 15. 5 (10. 0, NR) 71. 6 5. 8 (2. 1, 10. 3) 49. 9 TPS 1– 49% (n=127) 7. 8 (5. 8, 12. 4) 57. 3 2. 3 (2. 1, 3. 4) 25. 3 TPS <1% (n=68) 8. 6 (5. 5, 12. 0) 57. 1 2. 1 (2. 0, 4. 0) 23. 2 Total (n=394) 11. 3 (8. 8, 14. 0) 63. 0 (2. 2, 4. 0) 34. 0 – Among patients treated at 10 mg/kg, median duration of response was greatest for those who had TPS score ≥ 50% (23. 3 months; range 2. 1– 23. 3) compared with TPS 1– 49% (12. 5; 1. 9– 12. 5) and TPS <1% (NR; 1. 0– 15. 6) • Conclusions – Treatment with pembrolizumab was associated with a robust, durable antitumour response in patients with previously treated advanced NSCLC • Patients with PD-L 1 TPS ≥ 50% had the greatest response and faster time to response – As a result of similar safety and efficacy outcomes for doses 2– 10 mg/kg, the dose of 2 mg/kg q 3 w appears to be the optimal dose in NSCLC Soria et al. Ann Oncol 2015; 26 (suppl 6): abstr 33 LBA

Other malignancies SCLC and mesothelioma

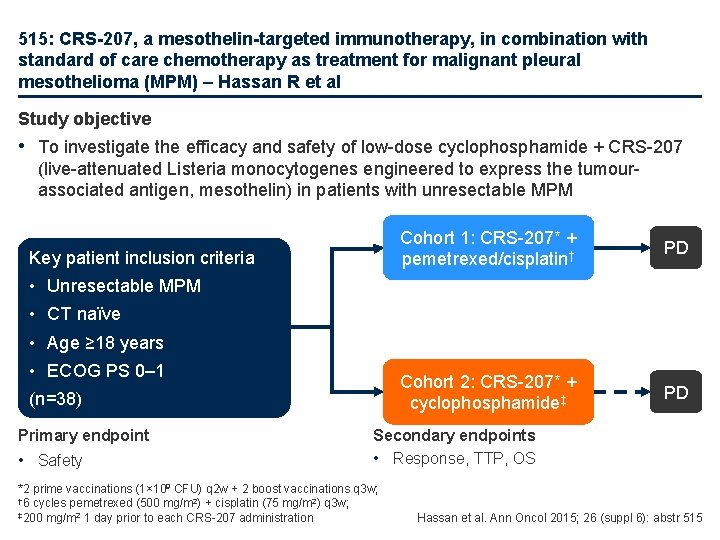
515: CRS-207, a mesothelin-targeted immunotherapy, in combination with standard of care chemotherapy as treatment for malignant pleural mesothelioma (MPM) – Hassan R et al Study objective • To investigate the efficacy and safety of low-dose cyclophosphamide + CRS-207 (live-attenuated Listeria monocytogenes engineered to express the tumourassociated antigen, mesothelin) in patients with unresectable MPM Key patient inclusion criteria Cohort 1: CRS-207* + pemetrexed/cisplatin† PD Cohort 2: CRS-207* + cyclophosphamide‡ PD • Unresectable MPM • CT naïve • Age ≥ 18 years • ECOG PS 0– 1 (n=38) Primary endpoint • Safety Secondary endpoints • Response, TTP, OS *2 prime vaccinations (1× 109 CFU) q 2 w + 2 boost vaccinations q 3 w; † 6 cycles pemetrexed (500 mg/m 2) + cisplatin (75 mg/m 2) q 3 w; ‡ 200 mg/m 2 1 day prior to each CRS-207 administration Hassan et al. Ann Oncol 2015; 26 (suppl 6): abstr 515

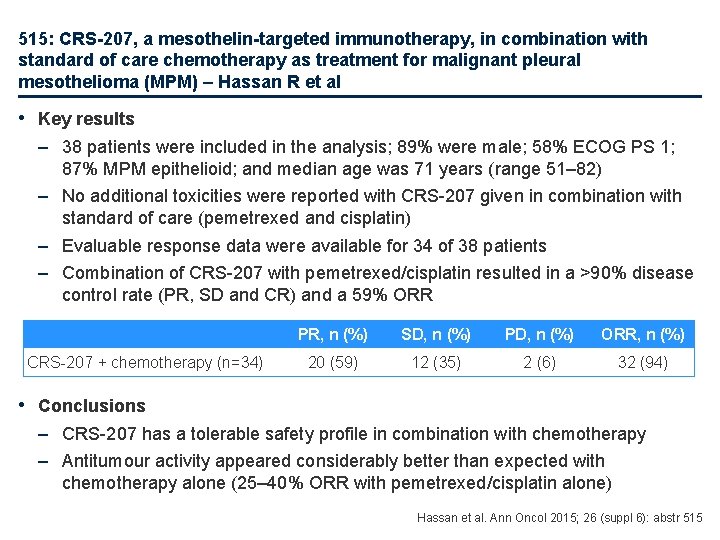
515: CRS-207, a mesothelin-targeted immunotherapy, in combination with standard of care chemotherapy as treatment for malignant pleural mesothelioma (MPM) – Hassan R et al • Key results – 38 patients were included in the analysis; 89% were male; 58% ECOG PS 1; 87% MPM epithelioid; and median age was 71 years (range 51– 82) – No additional toxicities were reported with CRS-207 given in combination with standard of care (pemetrexed and cisplatin) – Evaluable response data were available for 34 of 38 patients – Combination of CRS-207 with pemetrexed/cisplatin resulted in a >90% disease control rate (PR, SD and CR) and a 59% ORR CRS-207 + chemotherapy (n=34) PR, n (%) SD, n (%) PD, n (%) ORR, n (%) 20 (59) 12 (35) 2 (6) 32 (94) • Conclusions – CRS-207 has a tolerable safety profile in combination with chemotherapy – Antitumour activity appeared considerably better than expected with chemotherapy alone (25– 40% ORR with pemetrexed/cisplatin alone) Hassan et al. Ann Oncol 2015; 26 (suppl 6): abstr 515

3026: Lung toxicity after post-operative radiotherapy after EPP for mesothelioma and pneumonectomy for non-small cell lung cancer – Botticella A et al • Study objective – To assess whether patients with MPM treated with post-operative RT after extrapleural pneumonectomy (EPP) are more prone to develop lung toxicity vs. patients with NCSLC treated with post-operative RT after pneumonectomy • Study design – Patients with MPM who received post-operative RT after EPP (n=39) and those with NSCLC who received post-operative RT after pneumonectomy (n=10) were retrospectively reviewed – For patients with MPM and NSCLC, the respective prescription doses were 54 Gy and 54– 66 Gy in 2 Gy fractions delivered to the clinical target volume – Primary endpoint: lung toxicity. Dyspnoea was graded using the CTCAE v. 4. 03 and was recorded before RT, 45 days after the completion of RT and every 3 months thereafter until follow-up completion – The correlation between the dosimetric parameters and the toxicity (dyspnoea score) was investigated • Key results – Of 32 MPM patients assessable for acute toxicity, 11 had grade ≥ 2 dyspnoea vs. 0 for NSCLC – Of 28 MPM patients assessable for late toxicity, 10 had grade ≥ 2 dyspnoea vs. 0 for NSCLC – Mean lung dose was 7. 56 Gy (range 1. 6– 14. 8) in MPM patients vs. 5. 96 (3. 2– 14. 5) for NSCLC • Conclusion – Compared with NSCLC, more patients with MPM experienced grade ≥ 2 toxicity after post-operative radiotherapy; if used in multimodality treatment, radiotherapy doses should be strictly constrained in MPM Botticella et al. Ann Oncol 2015; 26 (suppl 6): abstr 3026

3052: Prophylactic cranial irradiation for extensive stage small cell lung cancer – Matutino ARB et al • Study objective – To evaluate the benefit associated with prophylactic cranial irradiation (PCI) in the treatment of patients with extensive stage SCLC (es. SCLC) • Study design – The electronic charts of patients with es. SCLC (2008– 2014) were retrospectively reviewed – All patients had baseline negative CNS evaluation and received ≥ 4 cycles of platinum-based chemotherapy, without progressive disease. Patients with early interruption of chemotherapy due to toxicity were excluded – Analyses were based on descriptive statistics and survival curves were calculated using Kaplan–Meier method • Key results – Among 48 eligible patients the median number of cycles of chemotherapy was 6 and 31% experienced a grade ≥ 3 toxicity – A total of 37. 5% received PCI; response rate to chemotherapy was greater in patients who had received PCI (100%) vs. those who did not (87%), while CNS recurrence rate was lower (17% vs. 53%, respectively); 89% of those who received PCI had second-line treatment vs. 77% of those who did not receive PCI • Conclusion – Screening and carefully selecting patients for PCI can improve OS and CNS disease control, as well as increasing the exposure of patients to second-line therapy Matutino et al. Ann Oncol 2015; 26 (suppl 6): abstr 3052

7 LBA: Safety, activity, and response durability assessment of single agent rovalpituzumab tesirine, a delta-like protein 3 (DLL 3)-targeted antibody drug conjugate (ADC), in small cell lung cancer (SCLC) – Pietanza MC et al • Study objective – To examine the safety and activity of rovalpituzumab tesirine in patients with recurrent SCLC • Study design – First-in-human phase 1 trial that enrolled patients with SCLC with recurrent disease after one or two previous lines of treatment – Rovalpituzumab tesirine was given at doses of 0. 05, 0. 1, 0. 2, 0. 3, 0. 4 and 0. 8 mg/kg q 3 w in cohorts of 1– 3 patients until dose limiting toxicities (DLTs) were observed • Initial PK analysis revealed an ADC half-life longer than predicted (~11 days) so a q 6 w regimen was introduced – A DLL 3 antibody was developed to assess antigen expression in archived tumour specimens. DLL 3 positive (DLL 3+) tumours were defined by IHC membrane-associated H -scores ≥ 180 on a scale of 300 • Key results – Data available as of July 2015 included 79 patients (34 q 3 w and 45 q 6 w) with a median age of 62 years (range 44– 81) Pietanza et al. Ann Oncol 2015; 26 (suppl 6): abstr 7 LBA

7 LBA: Safety, activity, and response durability assessment of single agent rovalpituzumab tesirine, a delta-like protein 3 (DLL 3)-targeted antibody drug conjugate (ADC), in small cell lung cancer (SCLC) – Pietanza MC et al • Key results (cont. ) – Maximum tolerated doses (MTD) of 0. 2 mg/kg q 3 w x 3 cycles and 0. 3 mg/kg q 6 w x 2 cycles were further evaluated in expansion cohorts – AEs occurring in >10% of patients are shown below 0. 2 mg/kg q 3 w 0. 3 mg/kg q 6 w 0. 2 mg/kg q 3 w + 0. 2 mg/kg q 6 w AE, % All Grade 3/4 Fatigue 24 4 30 7 28 6 Peripheral oedema 16 – 18 2 17 1 Maculopapular rash 12 – 16 7 14 4 Thrombocytopenia 4 – 20 14 14 9 Pleural effusion 16 12 11 – 13 4 Nausea 20 – 9 – 13 – Anaemia 12 – 11 2 12 1 Decreased appetite – – 18 – 12 – Erythema 8 – 14 – 12 – Photosensitivity reaction 8 4 14 – 12 1 – The dose of 0. 3 mg/kg q 6 w was selected as the recommended phase 2 dose Pietanza et al. Ann Oncol 2015; 26 (suppl 6): abstr 7 LBA

7 LBA: Safety, activity, and response durability assessment of single agent rovalpituzumab tesirine, a delta-like protein 3 (DLL 3)-targeted antibody drug conjugate (ADC), in small cell lung cancer (SCLC) – Pietanza MC et al • Key results (cont. ) – Biomarker analyses revealed a relationship between DLL 3 expression and ORR (table) Rovalpituzumab tesirine ORR, % All SCLC patients & dose levels DLL 3+SCLC phase 1 b cohorts Overall 23 44 H-score expression High (180+) Intermediate (90– 180) Low (0– 90) 70 11 19 2 nd line 24 44 3 rd line 20 45 ‘Sensitive’* to C/E 28 64 Refractory or ‘resistant’* C/E 16 23 *Clinical benefit on 1 L; began 2 L in ≥ 90 days; **Clinical benefit on 1 L; began 2 L in <90 days – The mean follow-up without progression at 0. 3 mg/kg q 6 w was 189+ days • Conclusions – Treatment with rovalpituzumab tesirine had antitumour activity and a manageable toxicity profile in patients with recurrent DLL 3+ SCLC – DLL 3 is the first predictive marker associated with drug efficacy in SCLC – The results support biomarker-guided phase 2 studies Pietanza et al. Ann Oncol 2015; 26 (suppl 6): abstr 7 LBA

Other malignancies Rare tumours

3023: Pan-European survey on thymic malignancies: A collaboration of the EORTC Lung Cancer Group (LCG) with the RYTHMIC network – Menis J et al • Study objective – To provide an overview of the current treatment strategies for thymic malignancies • Study design – A 25 -item survey was disseminated in the EORTC LCG and RYTHMIC networks • Key results – A total of 52 physicians from 11 countries completed the survey – A surgical sample was the most common method of making a diagnosis (45. 4%) followed by core needle biopsy (33. 6%) and open biopsy (15. 8%) – CAP is the preferred treatment for first-line chemotherapy for thymoma and thymic carcinoma – c-kit or EGFR mutation testing is not regularly performed – Mean ORR, PFS and OS for first-line treatment of thymoma was 50. 1%, 13. 9 months and 27. 3 months, while it was 41. 4%, 7. 9 months and 16. 2 months for thymic carcinoma • Mean values for second-line treatment were also greater for thymoma than thymic carcinoma • Conclusion – This pan-European survey provides insight into current treatment practices and will assist in developing future collaborative trials CAP, cisplatin + doxorubicin + cyclophosphamide Menis et al. Ann Oncol 2015; 26 (suppl 6): abstr 3023

5 LBA: Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: Efficacy and safety results from the placebo -controlled, double-blind, multicenter, Phase 3 RADIANT-4 study – Yao J et al Study objective • To assess the efficacy and safety of everolimus in patients with either advanced, nonfunctional progressive lung or gastrointestinal (GI) neuroendocrine tumors (NETs) Everolimus 10 mg/day (n=205) Key patient inclusion criteria • Well-differentiated (G 1/G 2), advanced (pathologically confirmed), progressive, nonfunctional lung or GI NET • No active or history of carcinoid syndrome Stratification • Prior SSA treatment (yes vs. no) R 2: 1 • Tumor origin (stratum A vs. B)* • WHO PS (0 vs. 1) • Enrolled within 6 months from radiologic progression Placebo (n=97) (n=302) Primary endpoint • PFS PD/ toxicity/ consent withdrawn Secondary endpoints • OS, ORR, DCR, safety *Stratum A (better prognosis) – appendix, caecum, jejunum, ileum, and NET of unknown primary; stratum B (worse prognosis) – lung, stomach, rectum and colon (except caecum) Yao et al. Ann Oncol 2015; 26 (suppl 6): abstr 5 LBA

5 LBA: Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: Efficacy and safety results from the placebo -controlled, double-blind, multicenter, Phase 3 RADIANT-4 study – Yao J et al • Key results PFS OS (first interim analysis) 100 HR 0. 48 (95%CI 0. 35, 0. 67); p<0. 00001 80 60 40 Censoring Times 20 Everolimus (n/N=113/205) Placebo (n/N=65/97) 0 0 2 4 6 8 10 12 Everolimus vs. placebo HR 0. 64 (95%CI 0. 40, 1. 05); p=0. 037 (NS)* 100 Placebo: 3. 9 months (95%CI 3. 58, 7. 43) Probability of overall survival (%) Probability of progressionfree survival (%) Everolimus: 11. 0 months (95%CI 9. 23, 13. 31) 80 60 40 Censoring Times 20 Everolimus (n/N=42/205) Placebo (n/N=28/97) 0 15 18 21 24 27 30 0 2 4 6 8 10 12 Months Placebo 97 18 21 24 27 30 Months No. of patients still at risk Everolimus 205 15 No. of patients still at risk 168 145 124 101 81 65 52 26 10 3 0 0 65 39 30 24 21 17 15 11 6 5 1 0 *p-value boundary for significance was = 0. 0002 Everolimus 205 Placebo 97 195 184 179 172 170 158 143 100 59 31 5 0 94 86 80 75 70 67 61 42 21 13 5 0 Yao et al. Ann Oncol 2015; 26 (suppl 6): abstr 5 LBA

5 LBA: Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: Efficacy and safety results from the placebo -controlled, double-blind, multicenter, Phase 3 RADIANT-4 study – Yao J et al • Key results (cont. ) – Everolimus improved PFS in all pre-defined subgroups compared with placebo as well for different levels of primary liver tumour burden and tumour origin Subgroups Prior SSA treatment Yes No Tumour origin Stratum A Stratum B Lung GI NET of unknown primary WHO PS 0 1 Liver tumour burden None ≤ 10% >10– 25% >25% n HR (95%CI) 157 145 0. 52 (0. 34, 0. 81) 0. 60 (0. 39, 0. 94) 153 149 90 175 36 0. 63 (0. 40, 1. 02) 0. 43 (0. 28, 0. 66) 0. 50 (0. 28, 0. 88) 0. 56 (0. 37, 0. 84) 0. 60 (0. 24, 1. 51) 216 86 0. 58 (0. 41, 0. 84) 0. 50 (0. 28, 0. 91) 48 180 37 35 0. 49 (0. 20, 1. 20) 0. 67 (0. 45, 1. 00) 0. 62 (0. 20, 1. 93) 0. 18 (0. 06, 0. 50) *Stratum A (better prognosis) – appendix, caecum, jejunum, ileum, and NET of unknown primary; stratum B (worse prognosis) – lung, stomach, rectum and colon (except caecum) Yao et al. Ann Oncol 2015; 26 (suppl 6): abstr 5 LBA

5 LBA: Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: Efficacy and safety results from the placebo -controlled, double-blind, multicenter, Phase 3 RADIANT-4 study – Yao J et al • Key results (cont. ) Drug-related AEs (of any grade occurring in ≥ 15% of patients), % Stomatitis Diarrhoea Fatigue Infections Rash Peripheral oedema Nausea Anaemia Decreased appetite Asthenia Non-infectious pneumonitis Dysgeusia Everolimus (n=202) All grades Grade 3– 4 63 9 31 7 31 3 29 7 27 1 26 2 17 1 16 4 16 1 15 1 Placebo (n=98) All grades Grade 3– 4 19 0 16 2 24 1 4 0 8 0 4 1 10 0 2 1 6 0 5 0 1 0 4 0 Yao et al. Ann Oncol 2015; 26 (suppl 6): abstr 5 LBA

5 LBA: Everolimus in advanced nonfunctional neuroendocrine tumors (NET) of lung or gastrointestinal (GI) origin: Efficacy and safety results from the placebo -controlled, double-blind, multicenter, Phase 3 RADIANT-4 study – Yao J et al • Conclusions – Everolimus demonstrated statistically significant and meaningful prolongation of PFS in patients with well-differentiated, advanced, progressive, nonfunctional lung or GI NET – The safety profile was consistent with the known safety profile of everolimus – Everolimus is the first targeted agent to show robust antitumor activity with an acceptable tolerability in a broad spectrum of NET including those from pancreas, lung and GI tract Yao et al. Ann Oncol 2015; 26 (suppl 6): abstr 5 LBA

Other malignancies Brain metastases

3061: Assessment of Brigatinib (AP 26113) CNS Activity in Patients (Pts) With ALK+ NSCLC and intracranial metastases in a Phase 1/2 Study – Camidge DR et al Study objective • To investigate the efficacy and safety of brigatinib in patients with ALK+ NSCLC and intracranial CNS metastases in a phase 1/2, single-arm, multicentre study Key patient inclusion criteria • ALK+ NSCLC • Advanced malignancies Brigatinib (total daily dose 30– 300 mg) PD (n=137) Primary endpoint • ORR during the phase 2 portion of the study • RR Secondary endpoints • Safety, tolerability, best target lesion response, TTP, PFS, DCR, OS Camidge et al. Ann Oncol 2015; 26 (suppl 6): abstr 3061

3061: Assessment of Brigatinib (AP 26113) CNS Activity in Patients (Pts) With ALK+ NSCLC and intracranial metastases in a Phase 1/2 Study – Camidge DR et al • Key results – Of the 137 enrolled patients, 79 were ALK+ and 50 of these had intracranial CNS metastases Patients with measurable (≥ 10 mm) Patients with only nonmeasurable intracranial CNS metastases (n=15) intracranial CNS metastases (n=31) ORR (CR + PR), n (%) 8 (53) 11 (35) CR, n (%) 1 (7) 11 (35) PR, n (%) 7 (47) NA DCR (CR + PR + SDa) 13 (87) 29 (94) SDa lasting >6 months, n (%) 3 (20) 15 (48) PD, n (%) 2 (13) 2 (6) a. SD for measurable and non-CR/non-PD for nonmeasurable intracranial CNS metastases – Treatment-emergent grade ≥ 3 events in ≥ 4% of ALK+ NSCLC patients with intracranial CNS metastases at baseline were: increased lipase (14%), hypertension (10%), increased amylase (6%), dyspnoea (6%) and fatigue (4%) • Conclusions – Brigatinib demonstrated antitumour activity and durable response in patients with ALK+ NSCLC and intracranial metastases; it was associated with a good ORR and PFS – A prospective evaluation of brigatinib in ALK+ NSCLC patients with intracranial CNS metastases is being conducted Camidge et al. Ann Oncol 2015; 26 (suppl 6): abstr 3061

3008: No benefit of temozolomide added to whole brain radiotherapy for non-small cell lung cancer metastases – Rajer M et al Study objective • To investigate the efficacy and safety of temozolomide added to WBRT for NSCLC brain metastases Key patient inclusion criteria • Cytologically or histologically confirmed NSCLC • Multiple brain metastases • PS 0− 2 • Not suitable for surgery or SRS (n=103) Temozolomide 75 mg/m 2 + WBRT 35 Gy* (n=51) PD WBRT 35 Gy* (n=52) PD R Endpoints • PFS, OS, symptom control, safety *Given in 14 fractions Rajer et al. Ann Oncol 2015; 26 (suppl 6): abstr 3008

3008: No benefit of temozolomide added to whole brain radiotherapy for non-small cell lung cancer metastases – Rajer M et al • Key results – Of 103 patients studied, 66 (64%) were males; median age was 60 years (range 39– 77) – The toxicity profile of temozolomide was as expected: • Thrombocytopenia occurred in 5 patients • Lymphocytopenia occurred in 8 patients • Pancytopenia occurred in a single patient • None of these AEs were grade 3 or 4 Rajer et al. Ann Oncol 2015; 26 (suppl 6): abstr 3008

3008: No benefit of temozolomide added to whole brain radiotherapy for non-small cell lung cancer metastases – Rajer M et al • Key results (cont. ) – Median PFS was 3 months in the combined temozolomide group and 4 months in the WBRT only group (p=0. 91) Cumulative survival 1. 0 ++ + 0. 8 0. 6 PFS + ++ ++ ++ + RT + temozolomide RT RT + temozolomide-censored RT-censored + + p=0. 91 ++ 0. 4 + + ++ 0. 2 0. 0 0 10 20 30 PFS (weeks) 40 50 60 Rajer et al. Ann Oncol 2015; 26 (suppl 6): abstr 3008

3008: No benefit of temozolomide added to whole brain radiotherapy for non-small cell lung cancer metastases – Rajer M et al • Key results (cont. ) – Median OS was 4 months in the combined temozolomide group and 9 months in the WBRT only group (p=0. 61) Cumulative survival 1. 0 RT + temozolomide RT RT + temozolomide-censored RT-censored OS 0. 8 0. 6 + 0. 4 + + 0. 2 + + + + p=0. 61 + + + 0. 0 0 10 20 30 OS (months) 40 50 60 Rajer et al. Ann Oncol 2015; 26 (suppl 6): abstr 3008

3008: No benefit of temozolomide added to whole brain radiotherapy for non-small cell lung cancer metastases – Rajer M et al • Conclusions – The addition of temozolomide to WBRT was not associated with any survival benefit in patients with NSCLC and multiple brain metastases – Although the treatment was well tolerated, the combination did not demonstrate any improved activity – The optimal treatment of these patients remains unclear – More clinical trials are needed to address the poor survival and quality of life of patients with NSCLC and brain metastases Rajer et al. Ann Oncol 2015; 26 (suppl 6): abstr 3008

3009: Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement – Varga A et al Study objective • Phase 1/2 study to investigate the efficacy and safety of rociletinib in patients with advanced EGFR+ NSCLC with a history of CNS disease Key patient inclusion criteria Phase 2 expansion cohorts • Advanced or recurrent EGFR+ NSCLC Rociletinib 500 mg bid (n=119) PD* Rociletinib 625 mg bid (n=236) PD* Rociletinib 750 mg bid (n=95) PD* • PD while on EGFR-directed therapy • T 790 M-positive biopsy at study entry • Asymptomatic treated CNS metastases allowed (n=450) Primary endpoint • ORR *Continued rociletinib treatment permitted if deemed clinically beneficial Varga et al. Ann Oncol 2015; 26 (suppl 6): abstr 3009

3009: Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement – Varga A et al • Key results – To date, 450 patients have entered the phase 2 TIGER-X study; 66% of patients are female; 28% have ECOG PS 0; 82% had received TKI immediately prior to enrolment; 41% had a history of CNS disease; and median age was 63 years AEs (in >10% of patients), % 500 mg bid (n=119) 625 mg bid (236) 750 mg bid (n=95) Hyperglycaemia 35 45 59 Diarrhoea 33 40 30 Nausea 19 34 37 Fatigue 19 28 30 QTc prolongation 13 23 26 Decreased appetite 15 16 25 Muscle spasms 14 13 21 Vomiting 8 16 14 Weight loss 10 9 17 – Hyperglycaemia was the only grade ≥ 3 AE observed in >10% of patients (17%) Varga et al. Ann Oncol 2015; 26 (suppl 6): abstr 3009

3009: Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement – Varga A et al • Key results (cont. ) – ORR to rociletinib was similar regardless of history of CNS disease (45% with history and 58% without), but data collection is ongoing SLD change from baseline (%) 100 80 60 40 20 0 – 20 – 40 – 60 – 80 – 100 History of CNS metastases Ongoing Centrally confirmed T 790 M-positive without history of CNS disease SLD change from baseline (%) Centrally confirmed T 790 M-positive with history of CNS disease 100 80 60 40 20 0 – 20 – 40 – 60 – 80 – 100 No history of CNS metastases Ongoing Varga et al. Ann Oncol 2015; 26 (suppl 6): abstr 3009

3009: Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement – Varga A et al • Key results (cont. ) – Continued treatment with rociletinib ≥ 14 days after disease progression was associated with longer median duration of treatment in those who had received brain radiation than those who did not (93 days compared with 69 days) Patients without brain radiation after first PD (n=30; 500, 625 or 750 bid) Patients with brain radiation after first PD (n=19; 500, 625 or 750 bid) Duration of treatment (weeks) 0 3 (c 2) 9 (c 4) 15 (c 6) 21 24 (c 9) 30 33(c 12)39 42(c 15)48 51(c 18)57 60 Median duration: 69 days after PD Pre-PD Post-PD 11/30 patients (36. 6%) ongoing after PD 0 3 (c 2) 9 (c 4) 15 (c 6) 21 24 (c 9) 30 33 (c 12) 39 42(c 15)48 51(c 18) 57 60 Median duration: 93 days after PD Pre-PD Post-PD 6/19 patients (31. 6%) ongoing after PD Varga et al. Ann Oncol 2015; 26 (suppl 6): abstr 3009

3009: Activity of rociletinib in EGFR mutant NSCLC patients with a history of CNS involvement – Varga A et al • Conclusions – Although it was not formally compared, rociletinib response rate does not appear to be affected by history of CNS disease (a factor often associated with poor prognosis) – Patients with a history of CNS metastases may have a higher rate of CNS progression than those with no history – Rociletinib may provide ongoing extracranial disease control in patients who received CNS radiation due to progressive disease – Rociletinib was generally well tolerated – The role of rociletinib in patients with NSCLC and CNS involvement is being further explored in the ongoing TIGER clinical development program Varga et al. Ann Oncol 2015; 26 (suppl 6): abstr 3009
 European diy retail association
European diy retail association European delirium association
European delirium association European diagnostic manufacturers association
European diagnostic manufacturers association European parents association
European parents association European university association
European university association European magnetism association
European magnetism association European biomass industry association
European biomass industry association Azgos vein
Azgos vein Scapular anastomosis
Scapular anastomosis Kati mori
Kati mori Goblet cells of respiratory epithelium
Goblet cells of respiratory epithelium Thoracic outlet wedge pillow
Thoracic outlet wedge pillow Anterior cervical counterstrain points
Anterior cervical counterstrain points British thoracic society oxygen guidelines
British thoracic society oxygen guidelines Genu recurvatum
Genu recurvatum Difference between cervical thoracic and lumbar vertebrae
Difference between cervical thoracic and lumbar vertebrae Typical intercostal nerve diagram
Typical intercostal nerve diagram Atheromatous thoracic aorta
Atheromatous thoracic aorta Lateral raphe of thoracolumbar fascia
Lateral raphe of thoracolumbar fascia Inferior articular process
Inferior articular process Sibson fascia
Sibson fascia Thoracic aortic aneurysm
Thoracic aortic aneurysm Chest x ray anatomy
Chest x ray anatomy Sternum jugular notch
Sternum jugular notch Jugular notch
Jugular notch Thoracic duct
Thoracic duct Transverse costal facet
Transverse costal facet Neurogeen thoracic outlet syndroom
Neurogeen thoracic outlet syndroom Arteries of thoracic wall
Arteries of thoracic wall Tactile fremitus
Tactile fremitus Body planes directions and cavities
Body planes directions and cavities Corpus callosum anatomy radiology
Corpus callosum anatomy radiology Which ventral cavity subdivision has no bony protection
Which ventral cavity subdivision has no bony protection Rule of 12 in thyroid
Rule of 12 in thyroid Thoracic wall
Thoracic wall Latissimus dorsi innervation
Latissimus dorsi innervation Left lymphatic duct
Left lymphatic duct Layers of chest wall
Layers of chest wall Thoracic aorta supplies blood to
Thoracic aorta supplies blood to Cervical facet referral pattern
Cervical facet referral pattern Thoracic surgeon
Thoracic surgeon Thoracic duct
Thoracic duct Rajesh shah thoracic surgeon
Rajesh shah thoracic surgeon Deep fascia of thorax
Deep fascia of thorax Popularly called lymph glands
Popularly called lymph glands Which membrane encloses the abdominopelvic viscera?
Which membrane encloses the abdominopelvic viscera? Main arteries in the body
Main arteries in the body Thoracic surgery
Thoracic surgery Cupola of diaphragm
Cupola of diaphragm Thoracic extension
Thoracic extension Thoracic cage anterior view
Thoracic cage anterior view Muscles of thoracic spine
Muscles of thoracic spine Body cavities labeled
Body cavities labeled Thoracic skin
Thoracic skin Thoracic membranes
Thoracic membranes Pig thoracic cavity diagram
Pig thoracic cavity diagram Soft, spongy, cone-shaped organs in the thoracic cavity.
Soft, spongy, cone-shaped organs in the thoracic cavity. Thoracic cavity
Thoracic cavity Petrous temporal bone
Petrous temporal bone Fascia of thorax
Fascia of thorax Anatomy of thorax
Anatomy of thorax Thoracic vertebrae not labeled
Thoracic vertebrae not labeled Thoracic kyphosis
Thoracic kyphosis Thoracic nerves
Thoracic nerves Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng Phép trừ bù
Phép trừ bù Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Dot
Dot Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ độ dài liên kết
độ dài liên kết Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Phối cảnh
Phối cảnh điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Lp html
Lp html Hệ hô hấp
Hệ hô hấp Các số nguyên tố
Các số nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chụp tư thế worms-breton
Chụp tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Tư thế ngồi viết
Tư thế ngồi viết V. c c
V. c c Thẻ vin
Thẻ vin Thể thơ truyền thống
Thể thơ truyền thống Chúa sống lại
Chúa sống lại Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Bmc oncology department
Bmc oncology department Via oncology epic
Via oncology epic Global oncology trends 2017 advances complexity and cost
Global oncology trends 2017 advances complexity and cost Ukons acute oncology guidelines
Ukons acute oncology guidelines Hematopoietic drugs wikipedia
Hematopoietic drugs wikipedia Unc pediatric hematology oncology fellowship
Unc pediatric hematology oncology fellowship Usdmf
Usdmf