Developed in association with the European Thoracic Oncology






















- Slides: 54

Developed in association with the European Thoracic Oncology Platform 27 -30 September 2011 | Stockholm, Sweden ECCO-ESMO 2011 Supported by Eli Lilly & Company

Special thanks to the ETOP reviewers • Enriqueta Felip, Barcelona, Spain • Solange Peters, Lausanne, Switzerland

Table of contents • Biomarkers • Early-stage/locally-advanced NSCLC • Metastatic NSCLC – 1 st line –Maintenance –Later lines –Elderly • Trial design

BIOMARKERS

#9003: Biomarker analysis in BO 21015, a Phase II randomized study of first-line bevacizumab combined with carboplatin-gemcitabine or carboplatin-paclitaxel in patients with advanced or recurrent non-squamous NSCLC – T Mok • Study objective – To explore correlation between biomarker candidates and best overall response to bevacizumab (BEV) plus chemotherapy in NSCLC • Study type/design – ABIGAIL: randomized, multicentre, Phase II trial – Up to 6 cycles (q 21 d) of BEV 7. 5 mg/kg or 15 mg/kg combined with carboplatin-gemcitabine (CG) or carboplatin-paclitaxel (CP) – Primary endpoint: Correlation of baseline plasma biomarker levels with best overall response to chemotherapy + BEV • b. FGF; E-selectin; ICAM; PLGF; VEGFA; VEGFR 1; VEGFR 2 • Patients – 303 patients with advanced/recurrent non-squamous NSCLC (biomarker evaluable population, n=287) – Median age ∼ 60 years; ∼ 60% male; ~65% ECOG PS 1; 85% white; ~30/~43/~27% never/former/current smokers Mok et al. EJC: 2011; (suppl; abstr 9003)

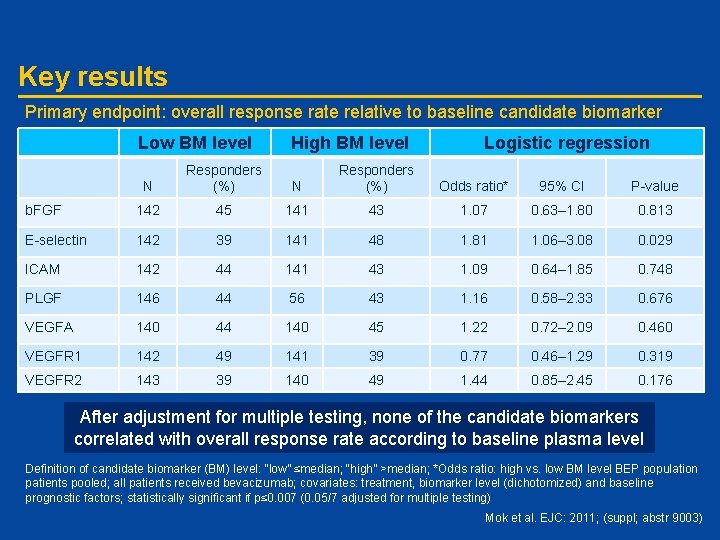
Key results Primary endpoint: overall response rate relative to baseline candidate biomarker status Low BM level High BM level Logistic regression N Responders (%) Odds ratio* 95% CI P-value b. FGF 142 45 141 43 1. 07 0. 63– 1. 80 0. 813 E-selectin 142 39 141 48 1. 81 1. 06– 3. 08 0. 029 ICAM 142 44 141 43 1. 09 0. 64– 1. 85 0. 748 PLGF 146 44 56 43 1. 16 0. 58– 2. 33 0. 676 VEGFA 140 44 140 45 1. 22 0. 72– 2. 09 0. 460 VEGFR 1 142 49 141 39 0. 77 0. 46– 1. 29 0. 319 VEGFR 2 143 39 140 49 1. 44 0. 85– 2. 45 0. 176 After adjustment for multiple testing, none of the candidate biomarkers correlated with overall response rate according to baseline plasma level Definition of candidate biomarker (BM) level: “low” ≤median; “high” >median; *Odds ratio: high vs. low BM level BEP population patients pooled; all patients received bevacizumab; covariates: treatment, biomarker level (dichotomized) and baseline prognostic factors; statistically significant if p≤ 0. 007 (0. 05/7 adjusted for multiple testing) Mok et al. EJC: 2011; (suppl; abstr 9003)

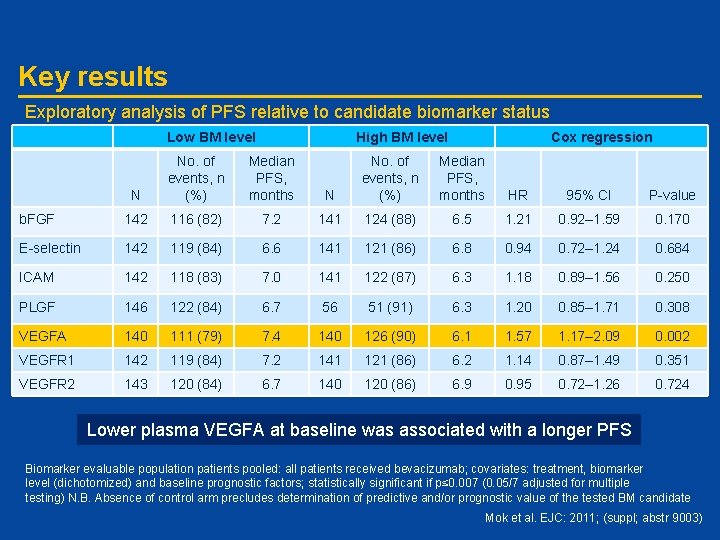
Key results Exploratory analysis of PFS relative to candidate biomarker status Low BM level High BM level N No. of events, n (%) Median PFS, months b. FGF 142 116 (82) E-selectin 142 ICAM Cox regression N No. of events, n (%) Median PFS, months HR 95% CI P-value 7. 2 141 124 (88) 6. 5 1. 21 0. 92– 1. 59 0. 170 119 (84) 6. 6 141 121 (86) 6. 8 0. 94 0. 72– 1. 24 0. 684 142 118 (83) 7. 0 141 122 (87) 6. 3 1. 18 0. 89– 1. 56 0. 250 PLGF 146 122 (84) 6. 7 56 51 (91) 6. 3 1. 20 0. 85– 1. 71 0. 308 VEGFA 140 111 (79) 7. 4 140 126 (90) 6. 1 1. 57 1. 17– 2. 09 0. 002 VEGFR 1 142 119 (84) 7. 2 141 121 (86) 6. 2 1. 14 0. 87– 1. 49 0. 351 VEGFR 2 143 120 (84) 6. 7 140 120 (86) 6. 9 0. 95 0. 72– 1. 26 0. 724 Lower plasma VEGFA at baseline was associated with a longer PFS Biomarker evaluable population patients pooled: all patients received bevacizumab; covariates: treatment, biomarker level (dichotomized) and baseline prognostic factors; statistically significant if p≤ 0. 007 (0. 05/7 adjusted for multiple testing) N. B. Absence of control arm precludes determination of predictive and/or prognostic value of the tested BM candidate Mok et al. EJC: 2011; (suppl; abstr 9003)

Conclusions • None of the biomarkers studied correlated with overall response rate • Low baseline VEGFA levels were associated with longer PFS in exploratory analysis (p=0. 002) – However, predictive and/or prognostic value cannot be determined in this study due to lack of control arm • Efficacy and safety profiles were similar to those observed in previous studies of BEV in advanced non-squamous NSCLC • Exploratory analyses for further biomarkers are ongoing Mok et al. EJC: 2011; (suppl; abstr 9003)

#26 LBA: Results of a pilot external quality assurance scheme for somatic EGFR mutation testing in non-small cell lung cancer managed by EMQN, ESMO, ESP, and ETOP – N Normanno • Study objective – To define the methodology and detect any inherent material problems in assessing EGFR mutation analyses in the Pan-EU EQA – Collaboration of experts in pathology (ESP), genetics (EMQN) and lung cancer (ETOP, ESMO, AIOM) • Study type/design – Pilot phase of the Pan-EU EQA for somatic EGFR mutation analyses (registration of open phase ends 30 September 2011) – 25 laboratories across 13 countries: • Provided with 10 formalin fixed paraffin embedded samples from NSCLC cell lines with different EGFR mutations • Registered with the EMQN • Performed DNA extraction and analysis using their usual method – Laboratory results were scored for accuracy ESP, European Society of Pathology; EMQN, European Molecular Genetics Quality Network; ETOP, European Thoracic Oncology Platform ESMO, European Society for Medical Oncology; AIMO, Italian Association of Medical Oncology; EQA, External Quality Assessment Normanno et al. EJC: 2011; (suppl; abstr 26 LBA)

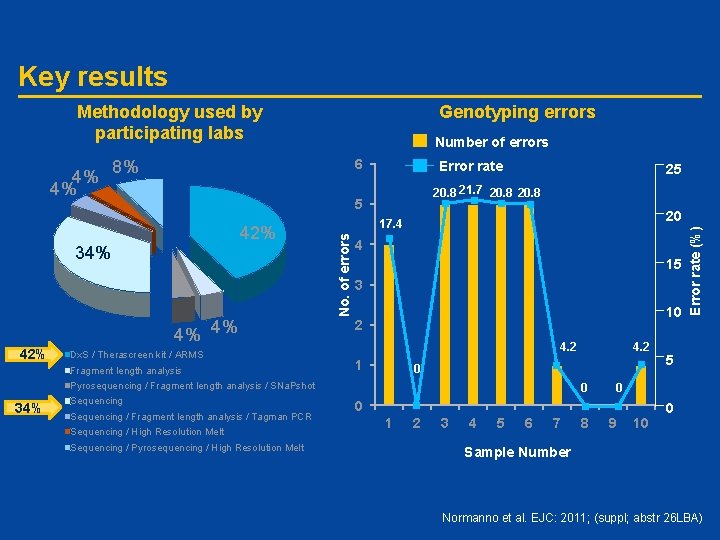
Key results Methodology used by participating labs 6 Error rate 20. 8 21. 7 20. 8 5 42% 4% 4% Dx. S / Therascreen kit / ARMS Fragment length analysis 25 20 17. 4 4 15 3 10 2 4. 2 1 0 Pyrosequencing / Fragment length analysis / SNa. Pshot 34% Sequencing / Fragment length analysis / Tagman PCR Sequencing / High Resolution Melt Sequencing / Pyrosequencing / High Resolution Melt 4. 2 0 2 3 4 5 6 7 8 5 0 0 1 Error rate (%) 8% 34% 42% Number of errors No. of errors 4% 4% Genotyping errors 9 10 0 Sample Number Normanno et al. EJC: 2011; (suppl; abstr 26 LBA)

Conclusions • The standard of genotyping in EGFR mutation testing for NSCLC is good, with a low level of “true” diagnostic errors • Incidence of clerical errors was high suggesting that some laboratories had failures in checking processes • The standard of reporting is more variable with many laboratories reporting the genotyping result in isolation of any interpretation • Robust EQA will harmonize reporting and analytical practices – Benefit patients with NSCLC – Transferable to future mutational testing EQA, External Quality Assessment Normanno et al. EJC: 2011; (suppl; abstr 26 LBA)

#9092: Efficacy of tyrosine kinase inhibitor for non-adenocarcinoma NSCLC patients with EGFR mutation – S Cho • Study objective – To determine the incidence of EGFR mutations and evaluate the efficacy of EGFR TKIs in non-adenocarcinoma NSCLC patients with EGFR mutation • Study type/design – Assessment of single centre data: assessment of patients with non-adenocarcinoma NSCLC (n=250) – Somatic mutation in exons 18 to 21 of EGFR were detected using a polymerase chain reaction (PCR)-based assay • Patients – Twenty patients had EGFR mutation – Twelve of them were treated with EGFR-TKI: seven patients had deletion mutation in exon 19 and one had L 858 R mutation Cho et al. EJC: 2011; (suppl; abstr 9092)

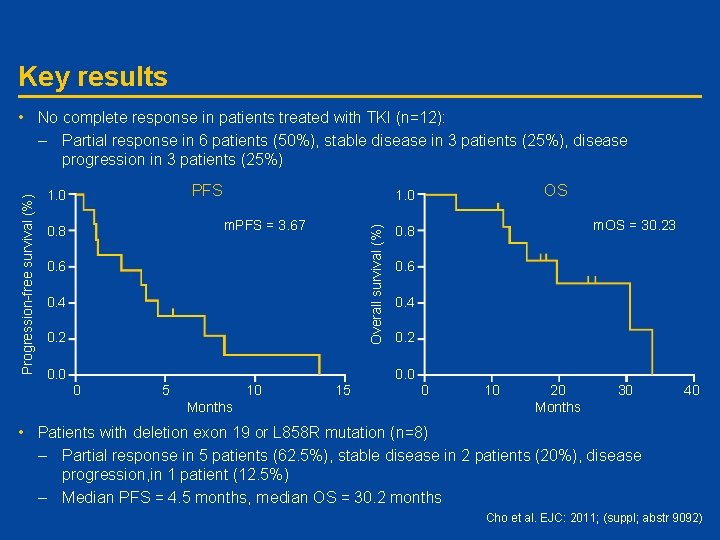
Key results • No complete response in patients treated with TKI (n=12): PFS 1. 0 m. PFS = 3. 67 0. 8 OS 1. 0 Overall survival (%) Progression-free survival (%) – Partial response in 6 patients (50%), stable disease in 3 patients (25%), disease progression in 3 patients (25%) 0. 6 0. 4 0. 2 0. 0 m. OS = 30. 23 0. 8 0. 6 0. 4 0. 2 0. 0 0 5 10 15 0 Months 10 20 Months 30 40 • Patients with deletion exon 19 or L 858 R mutation (n=8) – Partial response in 5 patients (62. 5%), stable disease in 2 patients (20%), disease progression, in 1 patient (12. 5%) – Median PFS = 4. 5 months, median OS = 30. 2 months Cho et al. EJC: 2011; (suppl; abstr 9092)

Conclusions • Disease control rate was significant in non-adenocarcinoma patients treated with EGFR-TKIs, although median PFS was lower than in patients with adenocarcinoma • In patients with exon 19 or L 858 R mutations, there was a more prolonged median PFS, suggesting that these active mutations may support positive treatment outcome in patients with non-adenocarcinoma • A further large study is warranted to confirm these findings Cho et al. EJC: 2011; (suppl; abstr 9092)

EARLY-STAGE LOCALLY-ADVANCED NSCLC

1 st line METASTATIC NSCLC

#9001: A retrospective subgroup analysis of EGFR immunohistochemistry (IHC) expression by Histo-Score correlated to outcomes from the BMS 099 1 st line phase III NSCLC trial of cetuximab (Cet) plus carboplatin/taxane – T Lynch • Study objective – To investigate the predictive role of tumour EGFR expression levels in the efficacy of cetuximab plus first-line chemotherapy in advanced NSCLC • Study type/design – BMS 099: Phase III trial – Tumour EGFR expression levels measured in tumour tissue specimens using EGFR IHC Histo-Score (H-Score) – Subjects were classified into high (≥ 200) and low (<200) EGFR expression groups • Patients – 676 patients with advanced NSCLC – Tissue specimens were available for 148 of 676 subjects Lynch et al. EJC: 2011; (suppl; abstr 9001)

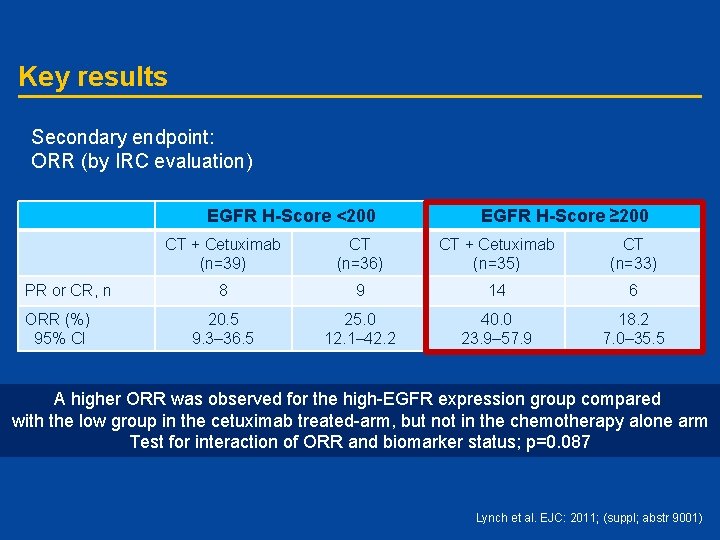
Key results Secondary endpoint: ORR (by IRC evaluation) EGFR H-Score <200 PR or CR, n ORR (%) 95% CI EGFR H-Score ≥ 200 CT + Cetuximab (n=39) CT (n=36) CT + Cetuximab (n=35) CT (n=33) 8 9 14 6 20. 5 9. 3– 36. 5 25. 0 12. 1– 42. 2 40. 0 23. 9– 57. 9 18. 2 7. 0– 35. 5 A higher ORR was observed for the high-EGFR expression group compared with the low group in the cetuximab treated-arm, but not in the chemotherapy alone arm Test for interaction of ORR and biomarker status; p=0. 087 Lynch et al. EJC: 2011; (suppl; abstr 9001)

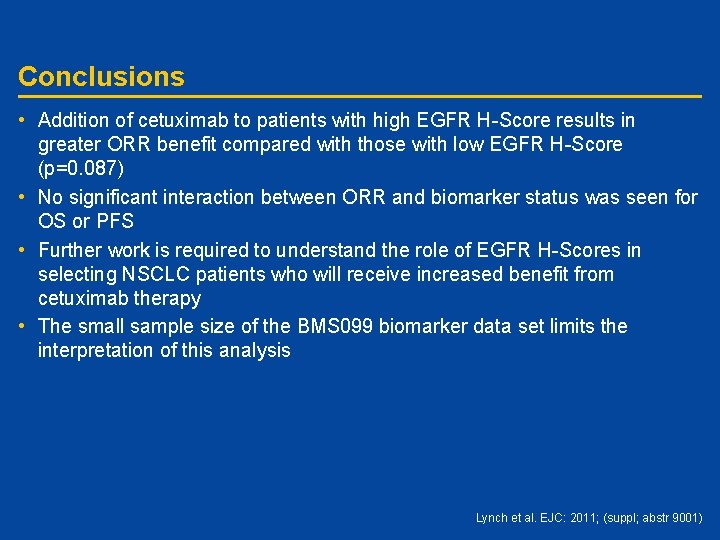
Conclusions • Addition of cetuximab to patients with high EGFR H-Score results in greater ORR benefit compared with those with low EGFR H-Score (p=0. 087) • No significant interaction between ORR and biomarker status was seen for OS or PFS • Further work is required to understand the role of EGFR H-Scores in selecting NSCLC patients who will receive increased benefit from cetuximab therapy • The small sample size of the BMS 099 biomarker data set limits the interpretation of this analysis Lynch et al. EJC: 2011; (suppl; abstr 9001)

#9028: Gemcitabine and cisplatin followed by concurrent gemcitabine and radiotherapy or sequential radiotherapy alone in unresectable stage III NSCLC – G Kerner • Study objective – To evaluate the outcome of concurrent and sequential chemoradiotherapy in unresectable stage III NSCLC • Study type/design – 2 -arm study • Arm A: Gemcitabine (G) + cisplatin (C) followed by concurrent G+ radiotherapy • Arm B: G + C followed by radiotherapy alone – Doses • Concurrent: initially G 1125 mg/m 2 on days 1/8 and C 80 mg/m 2 on day 1 of each 21 day cycle; then weekly G 300 mg/m 2 with 5 weeks of radiotherapy (60 Gy) • Sequential: 2– 4 cycles of chemotherapy then 5 weeks of radiotherapy alone – Endpoints: PFS and OS • Patients – 214 patients received concurrent chemoradiation and 69 received sequential chemoradiation – Median age 62 -64 years ; 70 -74% male; patients on sequential therapy had lower PS at baseline than concurrent therapy group (majority ECOG 1 vs ECOG 0); 2 -7/40 -41/52 -58% never/former/current smokers Kerner et al. EJC: 2011; (suppl; abstr 9028)

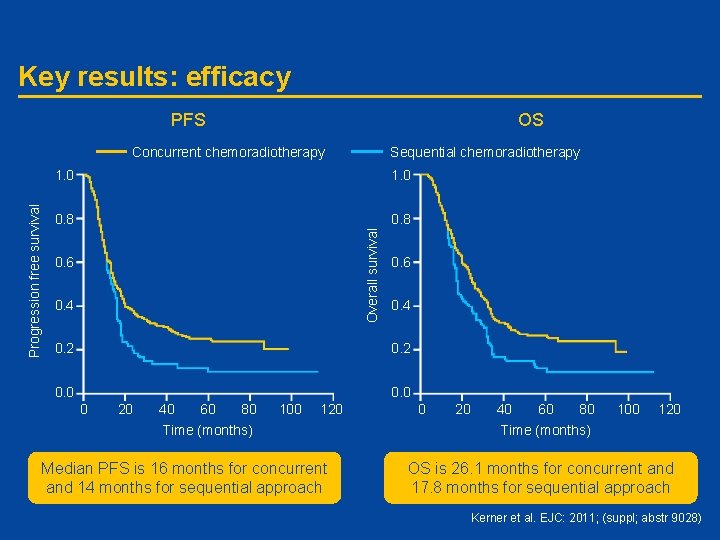
Key results: efficacy PFS OS Sequential chemoradiotherapy 1. 0 0. 8 Overall survival Progression free survival Concurrent chemoradiotherapy 0. 6 0. 4 0. 2 0. 0 0 20 40 60 80 Time (months) 100 120 Median PFS is 16 months for concurrent and 14 months for sequential approach 0 20 40 60 80 Time (months) 100 120 OS is 26. 1 months for concurrent and 17. 8 months for sequential approach Kerner et al. EJC: 2011; (suppl; abstr 9028)

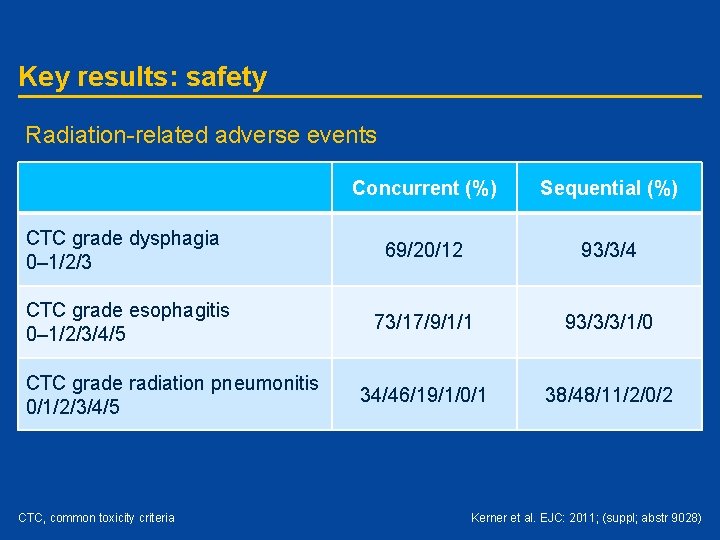
Key results: safety Radiation-related adverse events Concurrent (%) Sequential (%) CTC grade dysphagia 0– 1/2/3 69/20/12 93/3/4 CTC grade esophagitis 0– 1/2/3/4/5 73/17/9/1/1 93/3/3/1/0 34/46/19/1/0/1 38/48/11/2/0/2 CTC grade radiation pneumonitis 0/1/2/3/4/5 CTC, common toxicity criteria Kerner et al. EJC: 2011; (suppl; abstr 9028)

Conclusions • Concurrent chemoradiotherapy with gemcitabine as radiosensitizer gives comparable results as reported for highdose chemoradiotherapy regimens • 24% of patients were not fit enough to be treated with concurrent chemoradiotherapy schedules • Concurrent chemoradiotherapy is associated with more (low CTC grade) dysphagia and oesophagitis complaints CTC, common toxicity criteria Kerner et al. EJC: 2011; (suppl; abstr 9028)

Maintenance METASTATIC NSCLC

#34 LBA: Final efficacy outcomes for patients with advanced nonsquamous non-small cell lung cancer randomized to continuation maintenance with bevacizumab or bev+pemetrexed after first-line bevcisplatin-pemetrexed treatment – F Barlesi • Study objective – To investigate the use of pemetrexed (Pem) in addition to standard bevacizumab (BEV) continuation maintenance therapy in NSCLC • Study type/design – AVAPERL study: randomized, open-label Phase III trial – 4 cycles (q 3 w) of BEV 7. 5 mg/kg + Pem 500 mg/m 2 + cisplatin 75 mg/m 2 followed by randomization to maintenance therapy: • Arm A: BEV q 3 w until PD • Arm B: BEV + Pem q 3 w until PD – Endpoint: PFS from beginning of induction therapy • Patients – 125 patients randomized to BEV, 128 patients to Bev+Pem – Median age 60 years; ~57% male; 46 -55% ECOG PS 1; 86 -92% adenocarcinoma; ~26/~50/~24% never/former/current smokers Barseli et al. EJC: 2011; (suppl; abstr 34 LBA)

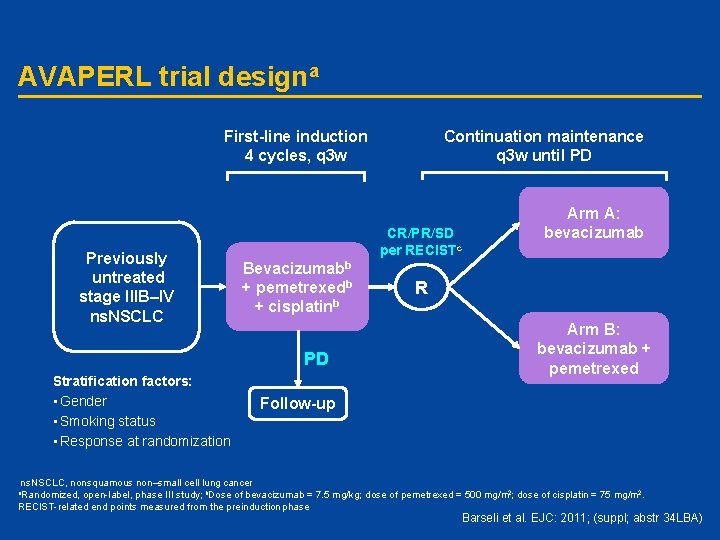
AVAPERL trial designa First-line induction 4 cycles, q 3 w Previously untreated stage IIIB–IV ns. NSCLC CR/PR/SD per RECISTc Bevacizumabb + pemetrexedb + cisplatinb PD Stratification factors: • Gender • Smoking status • Response at randomization Continuation maintenance q 3 w until PD Arm A: bevacizumab R Arm B: bevacizumab + pemetrexed Follow-up ns. NSCLC, nonsquamous non–small cell lung cancer open-label, phase III study; b. Dose of bevacizumab = 7. 5 mg/kg; dose of pemetrexed = 500 mg/m 2; dose of cisplatin = 75 mg/m 2. RECIST-related end points measured from the preinduction phase a. Randomized, Barseli et al. EJC: 2011; (suppl; abstr 34 LBA)

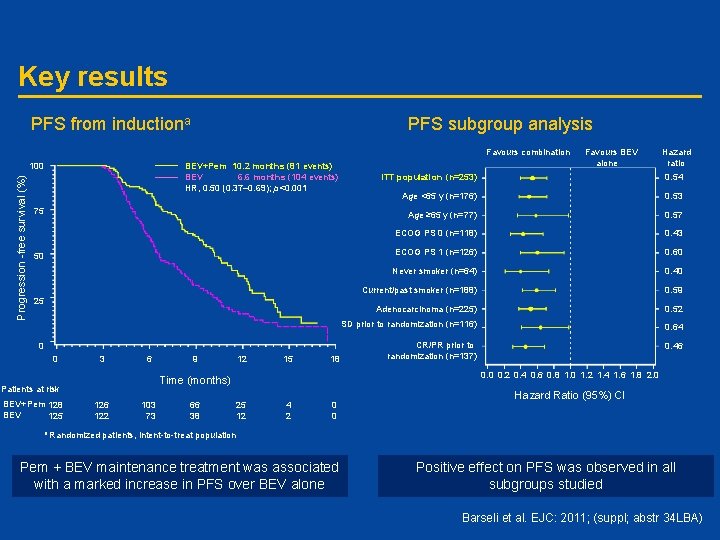
Key results PFS from inductiona PFS subgroup analysis Favours combination BEV+Pem 10. 2 months (81 events) BEV 6. 6 months (104 events) HR, 0. 50 (0. 37– 0. 69); p<0. 001 Progression -free survival (%) 100 75 50 25 0 0 3 6 12 15 18 126 122 a Randomized 103 73 66 38 Hazard ratio ITT population (n=253) 0. 54 Age <65 y (n=176) 0. 53 Age ≥ 65 y (n=77) 0. 57 ECOG PS 0 (n=118) 0. 43 ECOG PS 1 (n=126) 0. 60 Never smoker (n=64) 0. 40 Current/past smoker (n=188) 0. 59 Adenocarcinoma (n=225) 0. 52 SD prior to randomization (n=116) 0. 64 CR/PR prior to randomization (n=137) 0. 46 0. 0 0. 2 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4 1. 6 1. 8 2. 0 Time (months) Patients at risk BEV+Pem 128 BEV 125 9 Favours BEV alone 25 12 4 2 0 0 Hazard Ratio (95%) Cl patients, intent-to-treat population Pem + BEV maintenance treatment was associated with a marked increase in PFS over BEV alone Positive effect on PFS was observed in all subgroups studied Barseli et al. EJC: 2011; (suppl; abstr 34 LBA)

Conclusions • Continuation maintenance with BEV+Pem achieved a PFS benefit of unprecedented magnitude (10. 2 months; HR, 0. 50; p<0. 001) over BEV alone • Both regimens were well tolerated but AEs occurred more frequently in the combination treatment group, with some differences due to toxicities commonly attributed to chemotherapy • OS data available so far favour BEV+Pem maintenance treatment • Overall, the AVAPERL results strongly favour the use of BEV+Pem as continuation maintenance therapy in patients with non-squamous NSCLC Barseli et al. EJC: 2011; (suppl; abstr 34 LBA)

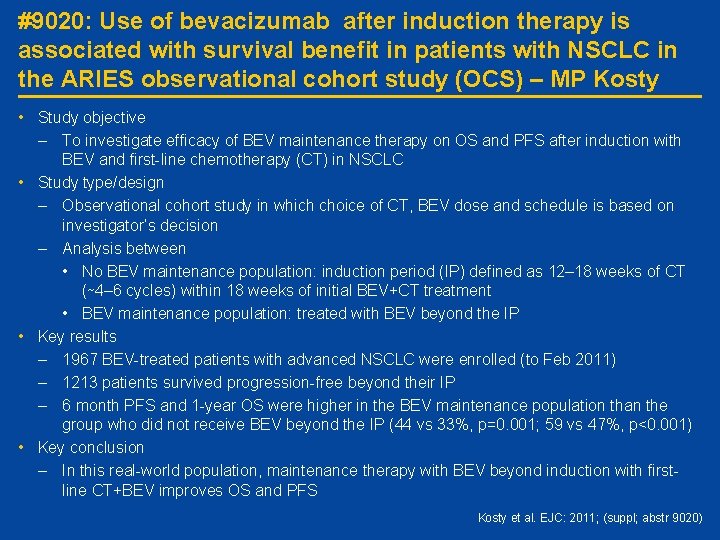
#9020: Use of bevacizumab after induction therapy is associated with survival benefit in patients with NSCLC in the ARIES observational cohort study (OCS) – MP Kosty • Study objective – To investigate efficacy of BEV maintenance therapy on OS and PFS after induction with BEV and first-line chemotherapy (CT) in NSCLC • Study type/design – Observational cohort study in which choice of CT, BEV dose and schedule is based on investigator’s decision – Analysis between • No BEV maintenance population: induction period (IP) defined as 12– 18 weeks of CT (∼ 4– 6 cycles) within 18 weeks of initial BEV+CT treatment • BEV maintenance population: treated with BEV beyond the IP • Key results – 1967 BEV-treated patients with advanced NSCLC were enrolled (to Feb 2011) – 1213 patients survived progression-free beyond their IP – 6 month PFS and 1 -year OS were higher in the BEV maintenance population than the group who did not receive BEV beyond the IP (44 vs 33%, p=0. 001; 59 vs 47%, p<0. 001) • Key conclusion – In this real-world population, maintenance therapy with BEV beyond induction with firstline CT+BEV improves OS and PFS Kosty et al. EJC: 2011; (suppl; abstr 9020)

Later lines METASTATIC NSCLC

#27 LBA: A Phase II study of sorafenib in patients with locally advanced and/or metastatic (stage IIIB or IV) non-small cell lung cancer (NSCLC) with a K-Ras mutation – A Dingemans • Study objective – To investigate the use of sorafenib in NSCLC with K-Ras mutation • Study type/design – Single arm Phase II study – Inclusion criteria: stage IV NSCLC with proven K-Ras mutation; progression after ≥ 1 platinum doublet; ECOG 0 -2; asymptomatic brain metastasis allowed – All patients were treated with sorafenib (400 mg, bid) – Primary endpoint: rate of no progression at 6 weeks – Secondary endpoint: PFS • Patients – 57 patients with stage IV NSCLC received at least one dose of sorafenib – Mean age 58. 5 years; 28% male; 21/77% former/current smokers – ECOG PS 0/1/2: 40/53/7% – 2 nd/3 rd/≥ 4 th line of treatment: 54/28/18% – 81% adenocarcinoma – Median duration of treatment 9 weeks (range 0 -61 weeks) Dingemans et al. EJC: 2011; (suppl; abstr 27 LBA)

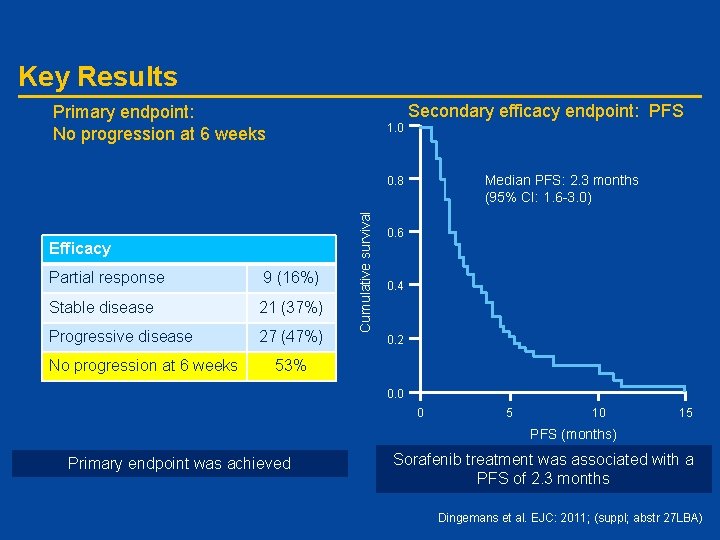
Key Results Secondary efficacy endpoint: PFS Primary endpoint: No progression at 6 weeks 1. 0 Median PFS: 2. 3 months (95% CI: 1. 6 -3. 0) Efficacy Partial response 9 (16%) Stable disease 21 (37%) Progressive disease 27 (47%) No progression at 6 weeks Cumulative survival 0. 8 0. 6 0. 4 0. 2 53% 0. 0 0 5 10 15 PFS (months) Primary endpoint was achieved Sorafenib treatment was associated with a PFS of 2. 3 months Dingemans et al. EJC: 2011; (suppl; abstr 27 LBA)

Conclusions • Sorafenib shows activity in K-Ras mutated NSCLC – Primary endpoint was achieved with no progression within 6 weeks observed in 53% of patients – Treatment was associated with median PFS of 2. 3 months • Sorafenib was well tolerated with few cases of grade 3 or more toxicity • WHO-PS 2 patients showed rapid deterioration (PFS of 1. 2 months) • Further work is required to – Identify subgroup of patients who will show prolonged benefit to treatment – Understand the effect of specific K-Ras mutation Dingemans et al. EJC: 2011; (suppl; abstr 27 LBA)

#9012: A Phase Ib study to evaluate the PI 3 -kinase inhibitor GDC-0941 with paclitaxel (P) and carboplatin (C), with and without bevacizumab (BEV), in patients with advanced non-small cell lung cancer (NSCLC) – H. Groen • Study objective – To establish the safety and tolerability of GDC-0941 with paclitaxel (P) and carboplatin (C), with and without bevacizumab (BEV) • Study type/design – 2 -arm phase Ib study • Arm A: GDC-0941 + C + P ineligible for BEV • Arm B: GDC-0941 + C + P eligible for BEV – Doses: GDC-09411, 60 to 330 mg PO qd days 1 -14 of a 21 -day cycle; P, 200 mg/m 2 for 4 -6 cycles; C, AUC 6 mg/m. L. min for 4 -6 cycles; BEV, 15 mg/kg every 3 weeks • Patients – 22 first-/second-line patients with advanced NSCLC – Median age 60 years; 68% male; 68% ECOG 1; 77% nonsquamous; 73% first line therapy; 5/68/27% never/former/current smoker Groen et al. EJC: 2011; (suppl; abstr 9012)

Key results • Tolerability – Treatment-related adverse events seen in ≥ 20% of patients (n=20, safety cutoff 25 Feb 2011) were alopecia, asthenia, nausea, stomatitis, neutropenia, rash, decreased appetite (anorexia), leukopenia, peripheral neuropathy, paresthesia, epistaxis and arthralgia – All were grade 1 or 2 except for neutropenia. • Efficacy – Partial responses were seen in 4 of 5 squamous patients, including 1 patient with a pathologic complete response – Partial responses were seen in 7 of 20 non-squamous patients Groen et al. EJC: 2011; (suppl; abstr 9012)

Conclusions • GDC-0941, P and C (±BEV) combination was well tolerated at doses consistent with preclinical activity • Evaluation of 330 mg GDC-0941 + C + P±BEV is ongoing • Randomized studies are planned Groen et al. EJC: 2011; (suppl; abstr 9012)

#9014: Randomized Phase II trial of NGR-h. TNF and chemotherapy in chemo-naive patients with non-small cell lung cancer (NSCLC) preliminary results – V. Gregorc • Study objective – To investigate effect of the vascular targeting agent NGR-h. TNF on PFS in NSCLC • Study type/design – Ongoing recruitment into randomized Phase II trial of chemo-naïve patients with stage IIIb-IV NSCLC (including those with brain metastasis), stratified by histology (nonsquamous vs squamous) and PS (0 vs 1) • Arm A: Chemotherapy for up to 6 cycles plus NGR-h. TNF 0. 8 µg/m 2 day 1 until progression • Arm B: Chemotherapy alone • Key results – 112 patients enrolled to date and data from 100 (n=50 per group) have been analyzed – Tolerability was similar between the groups except for higher incidence of chills (Grade 1) in the combination group – No difference was seen in PFS between the two treatment arms: • Nonsquamous histology; 6. 2/5. 4 months in Arms A/B • Squamous histology; 4. 5/2. 7 in Arms A/B • Key conclusion – NGR-h. TNF and chemotherapy can be given together without safety issues – Efficacy data are immature but currently do not appear promising NGR-h. TNF=tumour-homing peptide with human tumour necrosis factor Gregorc et al. EJC: 2011; (suppl; abstr 9014)

#9017: Initial detection of the double EGFR mutation (L 858 R or deletion in exon 19 [del 19] plus T 790 M) in NSCLC patients with brain metastases and the influence of first-line chemotherapy on outcome to erlotinib - C. Rolfo • Study objective – • • To investigate PFS achieved with erlotinib treatment in NSCLC harbouring dual activating mutation and T 790 M and according to site of metastasis Study type/design – Assessment of T 790 M mutations by Taq. Man assay Key results – 129 patients with advanced NSCLC – De novo T 790 M mutations were identified in 35% (45 of 129) of EGFR-mutant patients before receiving erlotinib – PFS was 12 months for patients with T 790 M mutation and 18 months for those without (p=0. 05) – Among patients with the T 790 M mutation, PFS was lower in those with brain metastasis than those without (1 vs 13 months, p=0. 002) – In patients without the T 790 M mutation, PFS was not significantly affected by brain metastasis – T 790 M mutation status had no effect on PFS in patients with bone, lung, liver or pleura metastases • Key conclusion – – The T 790 M mutation in EGFR-TKI naïve patients is a marker for poor prognosis, particularly in patients with brain metastases Initial chemotherapy can play a role in the management of these patients Rolfo et al. EJC: 2011; (suppl; abstr 9017)

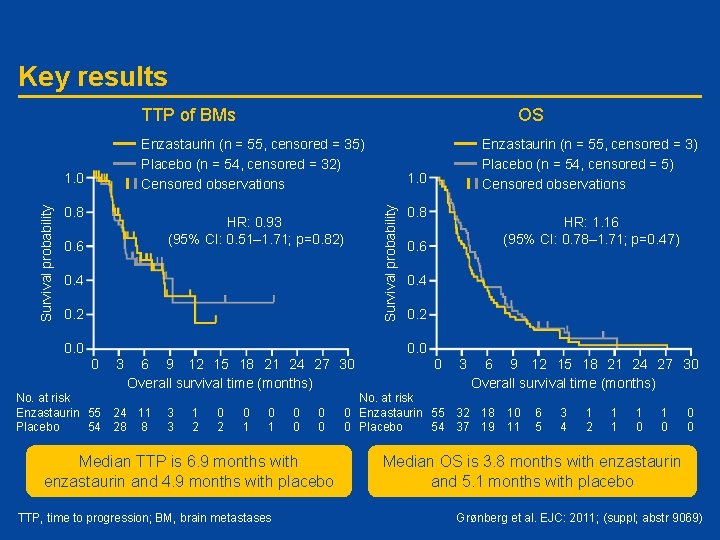
#9069: Randomized Phase II study of maintenance enzastaurin following whole brain radiation therapy in the treatment of brain metastases from lung cancer - the MENZA study – B. Grønberg • Study objective – To determine if maintenance enzastaurin (E) improves outcome of whole brain radiation therapy (WBRT) in lung cancer with brain metastases (BM) • Study type/design – MENZA trial: randomized, double-blind Phase II study of patients who had received WBRT (20 or 30 Gy) • Arm A: E (1125 mg on day 1 followed by 500 mg qd until progression) • Arm B: Placebo until progression – Primary outcome measure was time to progression (TTP) of BMs • Patients – 109 patients enrolled at 11 hospitals (Dec 2006 to April 2010) – Median age 61 -65 years; 60% male; ~70/~30% ECOG 0 -1/2 Grønberg et al. EJC: 2011; (suppl; abstr 9069)

Key results TTP of BMs Enzastaurin (n = 55, censored = 35) Placebo (n = 54, censored = 32) Censored observations 0. 8 HR: 0. 93 (95% CI: 0. 51– 1. 71; p=0. 82) 0. 6 0. 4 0. 2 0. 0 0 3 6 9 12 15 18 21 24 27 30 Overall survival time (months) No. at risk Enzastaurin 55 24 11 Placebo 54 28 8 3 3 1 2 0 1 0 0 0 0 Median TTP is 6. 9 months with enzastaurin and 4. 9 months with placebo TTP, time to progression; BM, brain metastases Enzastaurin (n = 55, censored = 3) Placebo (n = 54, censored = 5) Censored observations 1. 0 Survival probability OS 0. 8 HR: 1. 16 (95% CI: 0. 78– 1. 71; p=0. 47) 0. 6 0. 4 0. 2 0. 0 0 3 6 9 12 15 18 21 24 27 30 Overall survival time (months) No. at risk 0 Enzastaurin 55 32 18 0 Placebo 54 37 19 10 11 6 5 3 4 1 2 1 1 1 0 0 0 Median OS is 3. 8 months with enzastaurin and 5. 1 months with placebo Grønberg et al. EJC: 2011; (suppl; abstr 9069)

Conclusions • Efficacy – Enzastaurin did not delay disease progression or improve survival of lung cancer patients after WBRT – It was not associated with any benefit in quality of life • Tolerability – Enzastaurin was well tolerated in this population WBRT, whole brain radiation therapy Grønberg et al. EJC: 2011; (suppl; abstr 9069)

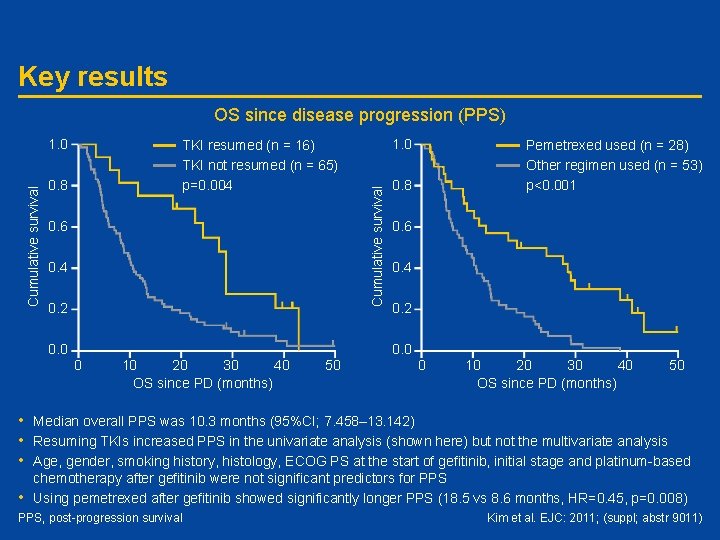
#9011: Survival in patients with non-small cell lung cancer which is clinically acquired resistance to gefitinib – natural history since progression – H Kim • Study objective – To investigate factors which affect survival of patients with NSCLC who have clinically-acquired resistance to tyrosine kinase inhibitors (TKIs) • Study type/design – Retrospective review of 81 advanced NSCLC patients who experienced disease progression following tumour response and durable (≥ 6 months) disease stabilization from first-line or second-line gefitinib • Arm A: Resumed TKIs • Arm B: TKIs not resumed – Primary outcome measure was post-progression survival (PPS) • Patients – 16 patients resumed TKIs (gefitinib n=11; erlotinib n=5) and 65 did not – Most patients were female never-smokers with adenocarcinoma at stage IV with ECOG PS 0 -1 Kim et al. EJC: 2011; (suppl; abstr 9011)

Key results OS since disease progression (PPS) TKI resumed (n = 16) TKI not resumed (n = 65) p=0. 004 0. 8 0. 6 0. 4 0. 2 0. 0 1. 0 Cumulative survival 1. 0 Pemetrexed used (n = 28) Other regimen used (n = 53) p<0. 001 0. 8 0. 6 0. 4 0. 2 0. 0 0 10 20 30 40 OS since PD (months) 50 • Median overall PPS was 10. 3 months (95%CI; 7. 458– 13. 142) • Resuming TKIs increased PPS in the univariate analysis (shown here) but not the multivariate analysis • Age, gender, smoking history, histology, ECOG PS at the start of gefitinib, initial stage and platinum-based chemotherapy after gefitinib were not significant predictors for PPS • Using pemetrexed after gefitinib showed significantly longer PPS (18. 5 vs 8. 6 months, HR=0. 45, p=0. 008) PPS, post-progression survival Kim et al. EJC: 2011; (suppl; abstr 9011)

Conclusions • Resuming treatment with TKIs who are believed to have clinically-acquired resistance can have benefits on survival in some NSCLC patients • Pemetrexed may improve outcome in patients with acquired resistance to gefitinib • Further work is required to improve our management of this patient group Kim et al. EJC: 2011; (suppl; abstr 9011)

#9018: Prospective assessment of combined pemetrexed and erlotinib or gefitinib therapy after the relapse to erlotinib or gefitinib in patients with advanced non-small cell lung cancer having an active epidermal growth factor receptor mutation – K Okishio • Study objective – To evaluate the efficacy and toxicity of pemetrexed combined with erlotinib or gefitinib after the relapse to erlotinib or gefitinib in patients with advanced NSCLC who have an active EGFR mutation • Study type/design – Phase II trial – Treatment: Pemetrexed (500 mg/m 2) was administered on day 1, followed by erlotinib or gefitinib on days 2– 16 (cycle repeated every 3 weeks until disease progression) – Primary outcome measure was disease control rate (DCR) • Patients – 27 patients with stage IV NSCLC (population evaluable for toxicity and efficacy, n=22) – Median age 67 years; 74% female; 74% ECOG PS 1; 96% adenocarcinoma; median number of prior chemotherapy regimens was 2 Okishio et al. EJC: 2011; (suppl; abstr 9018)

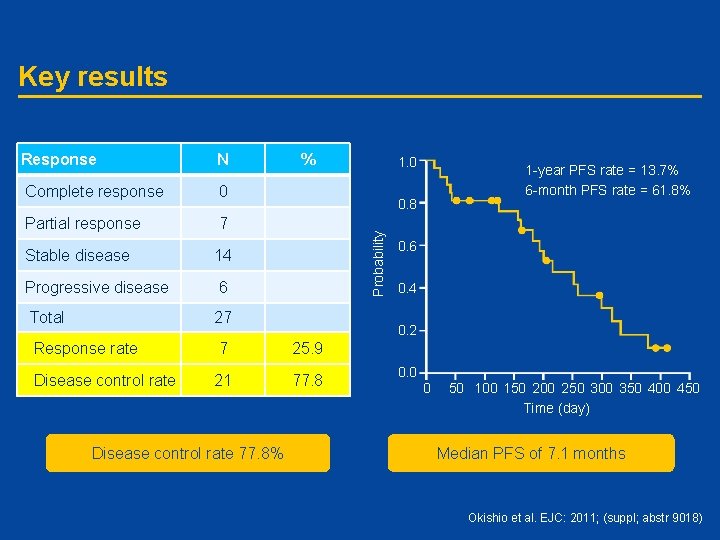
Key results Response N Complete response 0 Partial response 7 Stable disease 14 Progressive disease 6 Total 27 Response rate 7 25. 9 Disease control rate 21 77. 8 Disease control rate 77. 8% % 1. 0 1 -year PFS rate = 13. 7% 6 -month PFS rate = 61. 8% Probability 0. 8 0. 6 0. 4 0. 2 0. 0 0 50 100 150 200 250 300 350 400 450 Time (day) Median PFS of 7. 1 months Okishio et al. EJC: 2011; (suppl; abstr 9018)

Conclusions • Pemetrexed plus erlotinib or gefitinib combination treatment leads to a high DCR in patients with advanced NSCLC who have an active EGFR mutation • Combination treatment showed acceptable toxicity, with Grade 3/4 toxicities neutropenia, leucopenia, and anemia occurring in 22, 14 and 7% of patients, respectively • A Phase III trial for pemetrexed alone versus pemetrexed plus EGFR-TKI after relapse to EGFR-TKI is warranted DCR, disease control rate Okishio et al. EJC: 2011; (suppl; abstr 9018)

Elderly METASTATIC NSCLC

#9072: Pemetrexed (Pem) maintenance therapy in elderly patients with good performance status (PS) - analysis of PARAMOUNT Phase III study of Pem versus placebo in advanced nonsquamous NSCLC – C Gridelli • Study objective – • • • To further investigate efficacy and safety of Pem maintenance therapy in elderly advanced nonsquamous NSCLC patients with good PS Study type/design – PARAMOUNT: A Phase III, randomized, double-blind, placebo-controlled study of Pem maintenance (following. Pem induction) therapy – Subgroup analysis of patients aged ≥ 70 and <70 years Key results – Median age 73 years in the older group (n=92) and 60 years in the younger group (n=447) – Patient characteristics were comparable except for PS (PS 0/1 in ≥ 70: 20%/79%; <70: 34%/66%) and sex (M/F ≥ 70: 66%/34%; <70: 56%/44%) – For the ≥ 70 cohort, Pem reduced the risk of progression by 65% (p=0. 00041); median PFS was 6. 4 months for Pem and 3. 0 months for placebo. – For the <70 cohort, Pem reduced the risk of progression by 31%; median PFS was 4. 0 months for Pem and 2. 8 months for placebo – Incidence of grade 3/4 toxicities was higher in the Pem arms than the placebo arms in both age cohorts – Patients in the ≥ 70 cohort experienced a higher incidence of Grade 3/4 toxicities than those in the <70 cohort Key conclusions – Pem maintenance treatment is effective in elderly patients with good PS – Grade 3/4 hematologic toxicities were more common in older patients Gridelli et al. EJC: 2011; (suppl; abstr 9072)

TRIAL DESIGN

Trial in preparation #9035: A national, multi center, randomized, open-label, Phase II study of erlotinib versus gemcitabine (GEM) plus cisplatin as neoadjuvant treatment in stage IIIA-N 2 NSCLC patients with activating EGFR mutations (C-TONG 1103) – W. Zhong • Study objective – To investigate the efficacy and safety of erlotinib versus GEM plus cisplatin (GC) as neoadjuvant treatment in NSCLC • Study type/design – Randomized, open-label Phase II study in stage IIIA-N 2 NSCLC with activating mutations • Arm A: Erlotinib (150 mg qd for 42 days) • Arm B: GC (GEM 1250 mg/m 2 IV on days 1/8, and cisplatin 75 mg/m 2 on day 1 of a 3 -week schedule for 2 cycles) – Thoracotomy will take place after 6 weeks of treatment – Post-surgery treatment: erlotinib 150 mg/day for 1 year or 2 further cycles of GC – Primary endpoint: objective response rate (ORR) in neoadjuvant treatment • Patients – Target of 90 patients will be recruited over 18 months Zhong et al. EJC: 2011; (suppl; abstr 9035)

Study design • Treatment naïve • IIIA-N 2 NSCLC • N 2 confirmed by Erlotinib 150 mg/day x 6 weeks mediastinoscopy/ EBUS/PET-CT R 1: 1 • EGFR activating mutation • ECOG 0– 1 GEM 1250 mg/m 2 • Age ≥ 18 Y Days 1/8 + (n=90) Cis 75 mg/m 2 Day 1 q 3 w x 2 cycles Non. PD Surgery Erlotinib 150 mg/day 1 year Surgery GEM/Cis q 3 w x 2 cycles EBUS, endobronchial ultrasound; GEM, gemcitabine; Cis , cisplatin Primary endpoint • ORR Secondary endpoint • Lymph node • PFS downgrade rate • OS • Complete • Qo. L resection rate • Safety • p. CR Exploratory research • 24 & 48 week DFS rate • Biomarker profile Zhong et al. EJC: 2011; (suppl; abstr 9035)

Conclusions • Induction erlotinib therapy in IIIA-N 2 NSCLC with EGFR activating mutation is a promising strategy • The study is planned to start in September 2011 Zhong et al. EJC: 2011; (suppl; abstr 9035)

Developed in association with the European Thoracic Oncology Platform 27 -30 September 2011 | Stockholm, Sweden ECCO-ESMO 2011 Supported by Eli Lilly & Company
 European diagnostic manufacturers association
European diagnostic manufacturers association European parents association
European parents association European university association
European university association Ema magnetism
Ema magnetism European biomass industry association
European biomass industry association European diy retail association
European diy retail association Driver diagram palliative care
Driver diagram palliative care Body planes and cavities
Body planes and cavities Which of the following elevates the ribs?
Which of the following elevates the ribs? Thoracic configuration
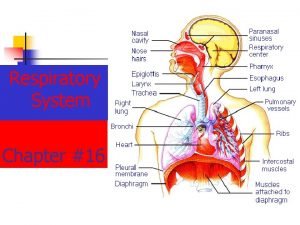
Thoracic configuration Thoracic cavity
Thoracic cavity Thyroid isotope scan
Thyroid isotope scan Thoracic mri images
Thoracic mri images Subcostal angle
Subcostal angle Latissimus dorsi innervation
Latissimus dorsi innervation Bean size lymph node
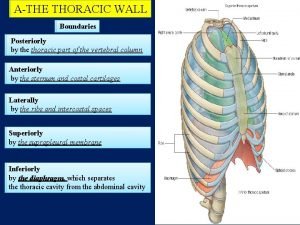
Bean size lymph node Thoracic wall boundaries
Thoracic wall boundaries Thoracic aorta supplies blood to
Thoracic aorta supplies blood to Thoracic surgeon
Thoracic surgeon Cervical facet referral pattern
Cervical facet referral pattern Thoracic duct
Thoracic duct Rajesh shah thoracic surgeon
Rajesh shah thoracic surgeon Mental anatomical term
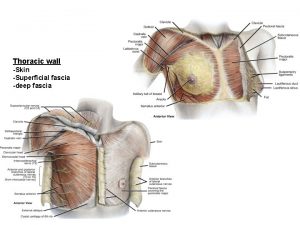
Mental anatomical term Superficial fascia of thorax
Superficial fascia of thorax Popularly called lymph glands
Popularly called lymph glands Thoracic surgery
Thoracic surgery Diaphragm cupola
Diaphragm cupola Branches of descending aorta
Branches of descending aorta Cervical lateral flexion
Cervical lateral flexion Muscles of thoracic spine
Muscles of thoracic spine Thoracic cage anterior view
Thoracic cage anterior view Thoracic membranes
Thoracic membranes Superior mediastinum contents
Superior mediastinum contents Thoracic membranes
Thoracic membranes Pig thoracic cavity diagram
Pig thoracic cavity diagram Soft spongy cone shaped organs in the thoracic cavity
Soft spongy cone shaped organs in the thoracic cavity Thoracic cavity
Thoracic cavity Costal groove of rib
Costal groove of rib Thorcic
Thorcic Intercostal muscles
Intercostal muscles Anatomy of thorax
Anatomy of thorax Thoracic nerves
Thoracic nerves Thoracic kyphosis
Thoracic kyphosis Internal thoracic artery
Internal thoracic artery Posterior axilla
Posterior axilla Kati mori london marathon
Kati mori london marathon Volume of thoracic cavity
Volume of thoracic cavity Rib counterstrain
Rib counterstrain Thoracic outlet wedge pillow
Thoracic outlet wedge pillow O2 flow rate chart
O2 flow rate chart Plumb line posture
Plumb line posture Thoracic inlet
Thoracic inlet Atheromatous thoracic aorta
Atheromatous thoracic aorta Difference between cervical thoracic and lumbar vertebrae
Difference between cervical thoracic and lumbar vertebrae Fascia thoracolumbalis
Fascia thoracolumbalis