Developed in association with the European Thoracic Oncology










- Slides: 84

Developed in association with the European Thoracic Oncology Platform IASLC 18 th World Conference on Lung Cancer October 15– 18, 2017 Yokohama, Japan Supported by Eli Lilly and Company has not influenced the content of this publication

Letter from Prof Rolf Stahel Dear Colleagues It is my pleasure to present this ETOP slide set which has been designed to highlight and summarise key findings in thoracic cancers from the major congresses in 2017. This slide set specifically focuses on the IASLC 18 th World Conference on Lung Cancer and is available in 4 languages – English, French, Chinese and Japanese. The area of clinical research in oncology is a challenging and ever changing environment. Within this environment we all value access to scientific data and research which helps to educate and inspire further advancements in our roles as scientists, clinicians and educators. I hope you find this review of the latest developments in thoracic cancers of benefit to you in your practice. If you would like to share your thoughts with us we would welcome your comments. Please send any correspondence to etop@etop. eu-org. I would like to thank our ETOP members Drs Solange Peters and Martin Reck for their roles as Editors – for prioritising abstracts and reviewing slide content. The slide set you see before you would not be possible without their commitment and hard work. And finally, we are also very grateful to Lilly Oncology for their financial, administerial and logistical support in the realisation of this complex yet rewarding activity. Yours sincerely, Rolf Stahel President, ETOP Foundation Council

ETOP Medical Oncology Slide Deck Editors 2017 Focus: advanced NSCLC (not radically treatable stage III & stage IV) Dr Solange Peters Multidisciplinary Oncology Center, Lausanne Cancer Center, Lausanne, Switzerland Focus: other malignancies, SCLC, mesothelioma, rare tumours Dr Martin Reck Department of Thoracic Oncology, Hospital Grosshansdorf, Germany

Contents • Biomarkers and screening • Early stage and locally advanced NSCLC – Stages I, II and III • Advanced NSCLC – Not radically treatable stage III and stage IV – First line – Later lines • Other malignancies – SCLC, mesothelioma and thymic epithelial tumours

Biomarkers

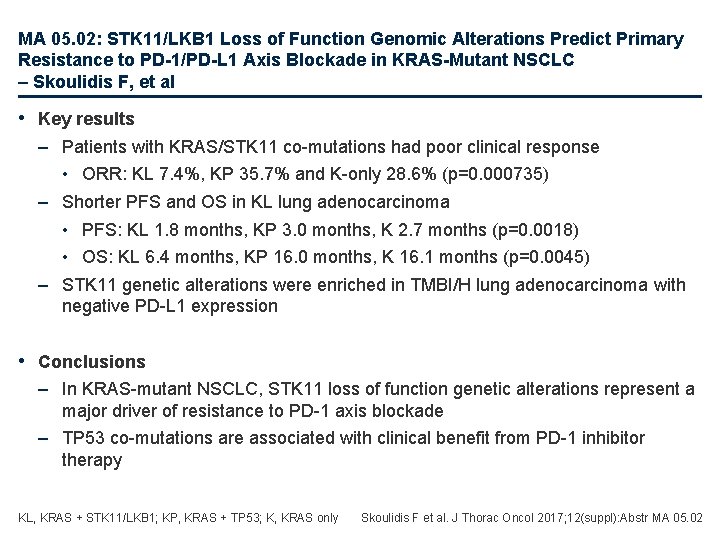
MA 05. 02: STK 11/LKB 1 Loss of Function Genomic Alterations Predict Primary Resistance to PD-1/PD-L 1 Axis Blockade in KRAS-Mutant NSCLC – Skoulidis F, et al • Study objective – To investigate the impact of STK 11 genomic alterations in the efficacy of PD-1/ PD-L 1 inhibitors in patients with KRAS mutant and wild-type lung adenocarcinoma • Methods – Data were retrospectively analysed from patients with lung adenocarcinoma treated with immune checkpoint inhibitors • 174 KRAS-mutant lung adenocarcinoma included – 146 nivolumab, 19 pembrolizumab, 9 anti-PD-1/PD-L 1 + anti-CTLA-4 – Tumour cell PD-L 1 expression was tested using the VENTANA PD-L 1 (SP 142) and the E 1 L 3 N IHC assay – TMB was classified as high (TMB-H), intermediate (TMB-I) or low (TMB-L) Skoulidis F et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 05. 02

MA 05. 02: STK 11/LKB 1 Loss of Function Genomic Alterations Predict Primary Resistance to PD-1/PD-L 1 Axis Blockade in KRAS-Mutant NSCLC – Skoulidis F, et al • Key results – Patients with KRAS/STK 11 co-mutations had poor clinical response • ORR: KL 7. 4%, KP 35. 7% and K-only 28. 6% (p=0. 000735) – Shorter PFS and OS in KL lung adenocarcinoma • PFS: KL 1. 8 months, KP 3. 0 months, K 2. 7 months (p=0. 0018) • OS: KL 6. 4 months, KP 16. 0 months, K 16. 1 months (p=0. 0045) – STK 11 genetic alterations were enriched in TMBI/H lung adenocarcinoma with negative PD-L 1 expression • Conclusions – In KRAS-mutant NSCLC, STK 11 loss of function genetic alterations represent a major driver of resistance to PD-1 axis blockade – TP 53 co-mutations are associated with clinical benefit from PD-1 inhibitor therapy KL, KRAS + STK 11/LKB 1; KP, KRAS + TP 53; K, KRAS only Skoulidis F et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 05. 02

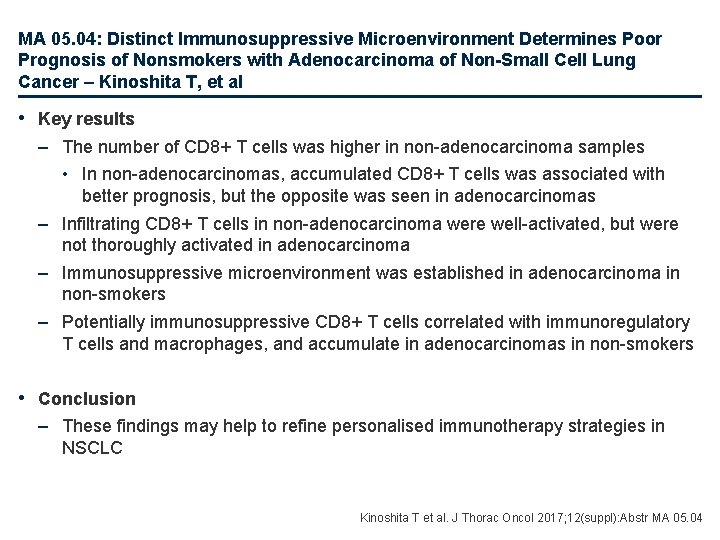
MA 05. 04: Distinct Immunosuppressive Microenvironment Determines Poor Prognosis of Nonsmokers with Adenocarcinoma of Non-Small Cell Lung Cancer – Kinoshita T, et al • Study objective – To investigate the prognostic significance of CD 8+ TILs according to histological types in resected NSCLC • Methods – Prognostic significance of CD 8+ TILs was studied by immunohistochemical analysis and immune-related gene expression analysis – Patients were divided by histological type: adenocarcinoma and nonadenocarcinoma, and by smoking status Kinoshita T et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 05. 04

MA 05. 04: Distinct Immunosuppressive Microenvironment Determines Poor Prognosis of Nonsmokers with Adenocarcinoma of Non-Small Cell Lung Cancer – Kinoshita T, et al • Key results – The number of CD 8+ T cells was higher in non-adenocarcinoma samples • In non-adenocarcinomas, accumulated CD 8+ T cells was associated with better prognosis, but the opposite was seen in adenocarcinomas – Infiltrating CD 8+ T cells in non-adenocarcinoma were well-activated, but were not thoroughly activated in adenocarcinoma – Immunosuppressive microenvironment was established in adenocarcinoma in non-smokers – Potentially immunosuppressive CD 8+ T cells correlated with immunoregulatory T cells and macrophages, and accumulate in adenocarcinomas in non-smokers • Conclusion – These findings may help to refine personalised immunotherapy strategies in NSCLC Kinoshita T et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 05. 04

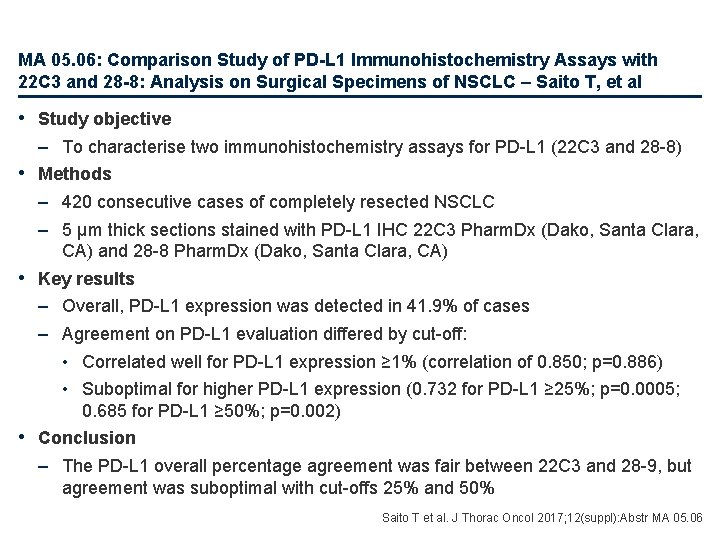
MA 05. 06: Comparison Study of PD-L 1 Immunohistochemistry Assays with 22 C 3 and 28 -8: Analysis on Surgical Specimens of NSCLC – Saito T, et al • Study objective – To characterise two immunohistochemistry assays for PD-L 1 (22 C 3 and 28 -8) • Methods – 420 consecutive cases of completely resected NSCLC – 5 μm thick sections stained with PD-L 1 IHC 22 C 3 Pharm. Dx (Dako, Santa Clara, CA) and 28 -8 Pharm. Dx (Dako, Santa Clara, CA) • Key results – Overall, PD-L 1 expression was detected in 41. 9% of cases – Agreement on PD-L 1 evaluation differed by cut-off: • Correlated well for PD-L 1 expression ≥ 1% (correlation of 0. 850; p=0. 886) • Suboptimal for higher PD-L 1 expression (0. 732 for PD-L 1 ≥ 25%; p=0. 0005; 0. 685 for PD-L 1 ≥ 50%; p=0. 002) • Conclusion – The PD-L 1 overall percentage agreement was fair between 22 C 3 and 28 -9, but agreement was suboptimal with cut-offs 25% and 50% Saito T et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 05. 06

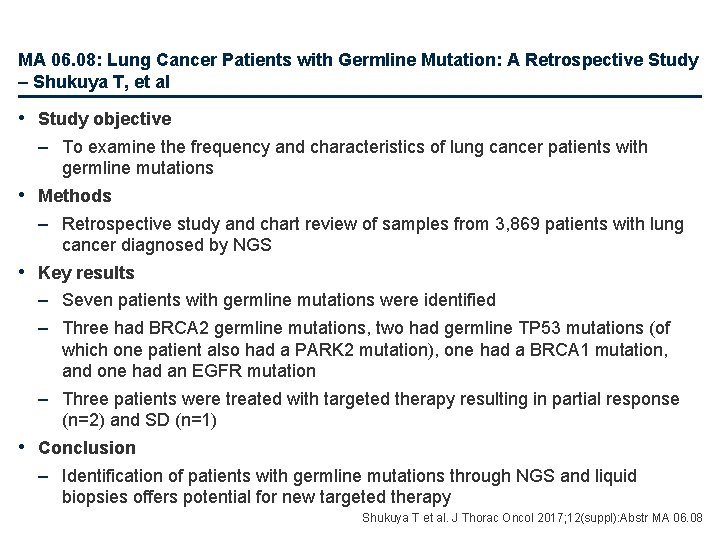
MA 06. 08: Lung Cancer Patients with Germline Mutation: A Retrospective Study – Shukuya T, et al • Study objective – To examine the frequency and characteristics of lung cancer patients with germline mutations • Methods – Retrospective study and chart review of samples from 3, 869 patients with lung cancer diagnosed by NGS • Key results – Seven patients with germline mutations were identified – Three had BRCA 2 germline mutations, two had germline TP 53 mutations (of which one patient also had a PARK 2 mutation), one had a BRCA 1 mutation, and one had an EGFR mutation – Three patients were treated with targeted therapy resulting in partial response (n=2) and SD (n=1) • Conclusion – Identification of patients with germline mutations through NGS and liquid biopsies offers potential for new targeted therapy Shukuya T et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 06. 08

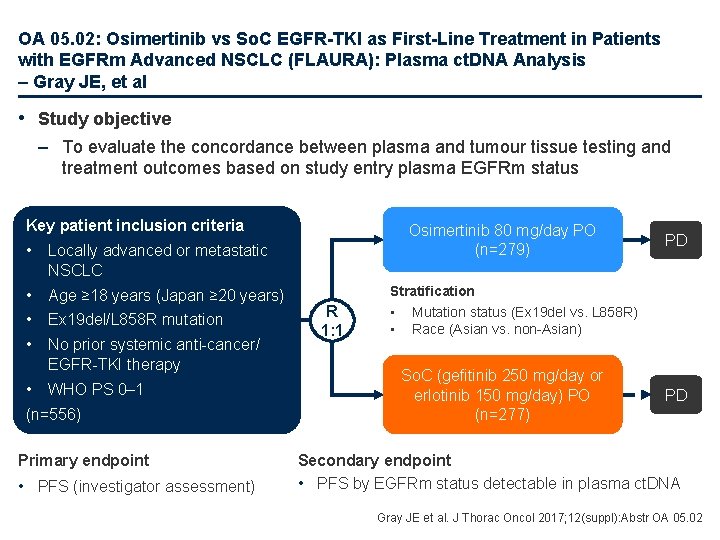
OA 05. 02: Osimertinib vs So. C EGFR-TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC (FLAURA): Plasma ct. DNA Analysis – Gray JE, et al • Study objective – To evaluate the concordance between plasma and tumour tissue testing and treatment outcomes based on study entry plasma EGFRm status Key patient inclusion criteria • Locally advanced or metastatic NSCLC • Age ≥ 18 years (Japan ≥ 20 years) • Ex 19 del/L 858 R mutation • No prior systemic anti-cancer/ EGFR-TKI therapy • WHO PS 0– 1 • (n=556) 5 Primary endpoint • PFS (investigator assessment) Osimertinib 80 mg/day PO (n=279) R 1: 1 PD Stratification • Mutation status (Ex 19 del vs. L 858 R) • Race (Asian vs. non-Asian) So. C (gefitinib 250 mg/day or erlotinib 150 mg/day) PO (n=277) PD Secondary endpoint • PFS by EGFRm status detectable in plasma ct. DNA Gray JE et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 05. 02

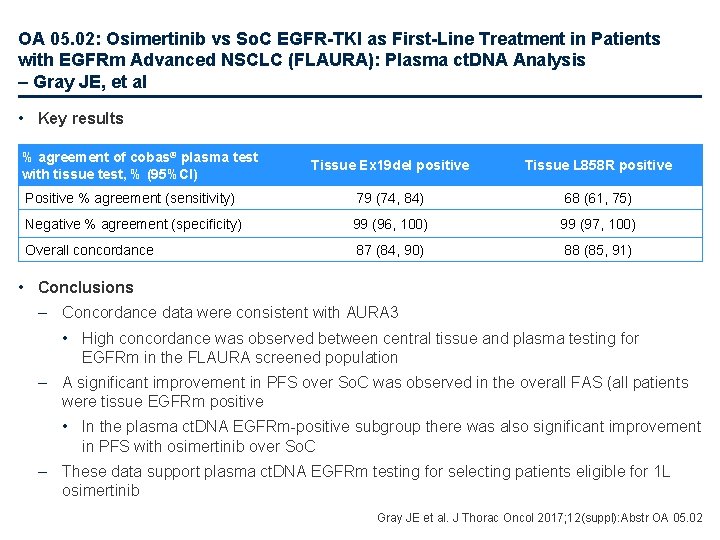
OA 05. 02: Osimertinib vs So. C EGFR-TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC (FLAURA): Plasma ct. DNA Analysis – Gray JE, et al • Key results % agreement of cobas® plasma test with tissue test, % (95%CI) Tissue Ex 19 del positive Tissue L 858 R positive Positive % agreement (sensitivity) 79 (74, 84) 68 (61, 75) Negative % agreement (specificity) 99 (96, 100) 99 (97, 100) Overall concordance 87 (84, 90) 88 (85, 91) • Conclusions – Concordance data were consistent with AURA 3 • High concordance was observed between central tissue and plasma testing for EGFRm in the FLAURA screened population – A significant improvement in PFS over So. C was observed in the overall FAS (all patients were tissue EGFRm positive • In the plasma ct. DNA EGFRm-positive subgroup there was also significant improvement in PFS with osimertinib over So. C – These data support plasma ct. DNA EGFRm testing for selecting patients eligible for 1 L osimertinib Gray JE et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 05. 02

MA 11. 02: Circulating Tumour DNA in Early Stage NSCLC: High Sensitivity Analysis in Low Burden Disease. LUCID Study Update – Ruiz-Valdepenas A, et al • Study objective – To investigate high sensitivity analysis of ct. DNA in patients with early stage NSCLC • Methods – Plasma samples collected from early stage NSCLC patients in the LUCID (LUng cancer CIrculating tumour DNA) study – Analysis of multiple mutations in parallel allows detection of very low levels of ct. DNA • Key results – Preliminary data show a median number of 306 mutations in tumour exome sequence of 19 patients – In the next stage, TAilored Panel Sequencing (TAPAS) will be used to sequence plasma samples with a bespoke patient-specific panel • Conclusions – These techniques will improve the detection of early stage cancer, detecting low levels of ct. DNA earlier than previously possible – It may also prove useful in identifying the presence of minimal residual disease after radical treatment Ruiz-Valdepenas A et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 11. 02

OA 07. 03 a: Impact of Tumor Mutation Burden on the Efficacy of Nivolumab or Nivolumab + Ipilimumab in Small Cell Lung Cancer: An Exploratory Analysis of Check. Mate 032 – Antonio S, et al • Study objective – To determine whether high tumour mutation burden (TMB) is associated with greater benefit for treatment with nivolumab with or without ipilimumab in patients with SCLC in the Check. Mate 032 • Methods – Patients from Check. Mate 032 with paired tumour/whole blood samples and TMB evaluable were included (133 from the nivolumab arm and 78 from the nivolumab + ipilimumab arm) – Whole exome sequencing was used to determine TMB which was calculated as the total number of missense mutations in the tumour – Patients were divided according to three TMB tertiles based on total number of missense mutations: low 0 to <143; medium 143 to 247; and high ≥ 248 Antonio S et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 07. 03 a

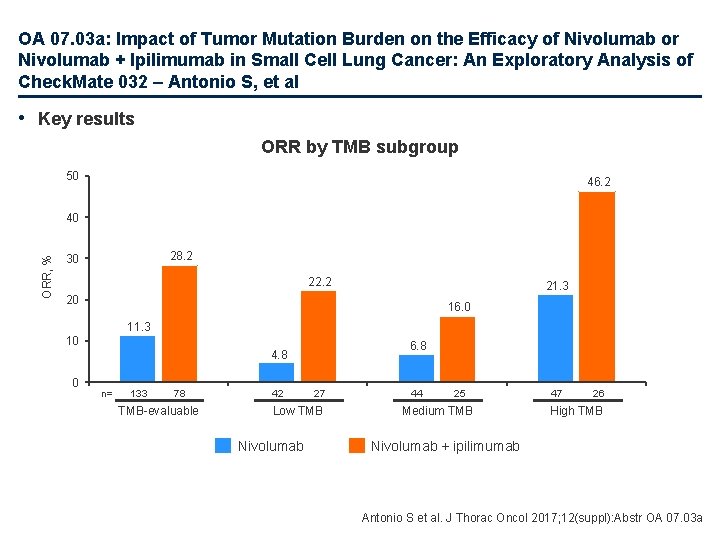
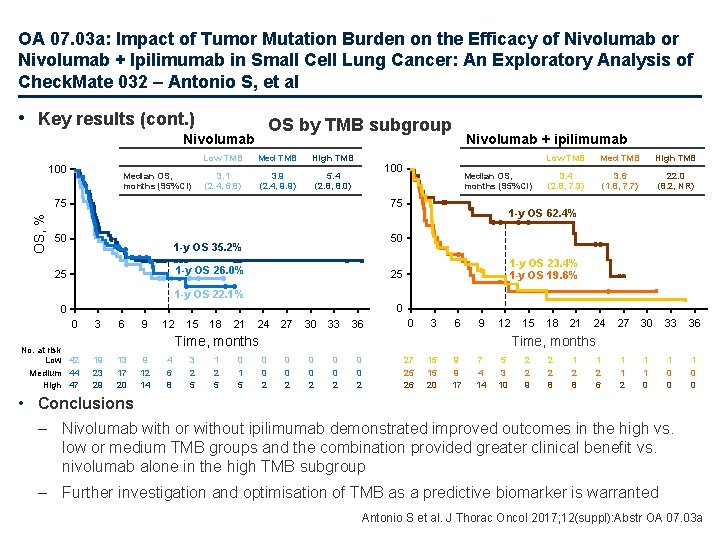
OA 07. 03 a: Impact of Tumor Mutation Burden on the Efficacy of Nivolumab or Nivolumab + Ipilimumab in Small Cell Lung Cancer: An Exploratory Analysis of Check. Mate 032 – Antonio S, et al • Key results ORR by TMB subgroup 50 46. 2 ORR, % 40 28. 2 30 22. 2 21. 3 20 16. 0 11. 3 10 0 6. 8 4. 8 n= 133 78 TMB-evaluable 42 27 Low TMB Nivolumab 44 25 Medium TMB 47 26 High TMB Nivolumab + ipilimumab Antonio S et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 07. 03 a

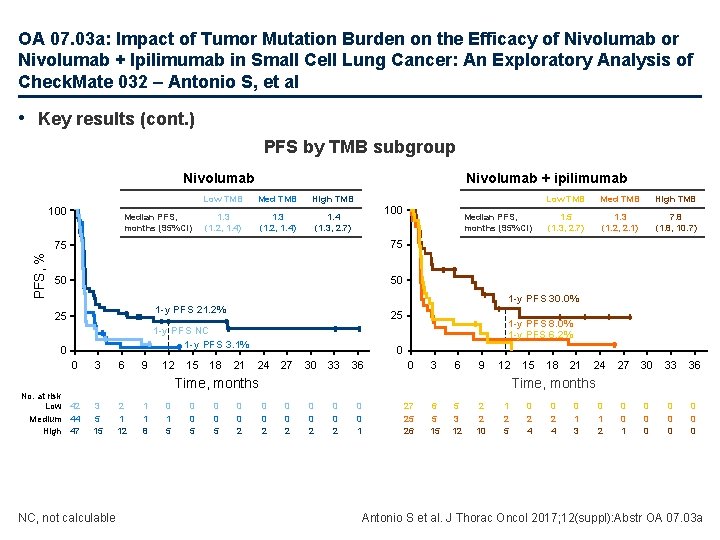
OA 07. 03 a: Impact of Tumor Mutation Burden on the Efficacy of Nivolumab or Nivolumab + Ipilimumab in Small Cell Lung Cancer: An Exploratory Analysis of Check. Mate 032 – Antonio S, et al • Key results (cont. ) PFS by TMB subgroup Nivolumab PFS, % 100 Median PFS, months (95%CI) Nivolumab + ipilimumab Low TMB Med TMB High TMB 1. 3 (1. 2, 1. 4) 1. 4 (1. 3, 2. 7) 100 75 75 50 50 25 1 -y PFS NC 1 -y PFS 3. 1% 0 0 No. at risk Low 42 Medium 44 High 47 3 6 9 12 15 18 NC, not calculable 2 1 1 8 0 1 5 0 0 5 High TMB 1. 5 (1. 3, 2. 7) 1. 3 (1. 2, 2. 1) 7. 8 (1. 8, 10. 7) 21 1 -y PFS 8. 0% 1 -y PFS 6. 2% 0 24 27 30 33 36 0 3 6 9 12 0 0 2 15 18 21 24 27 30 33 36 0 0 1 0 0 0 0 0 Time, months 3 5 15 Med TMB 1 -y PFS 30. 0% 1 -y PFS 21. 2% 25 Median PFS, months (95%CI) Low TMB 0 0 2 0 0 1 27 25 26 6 5 15 5 3 12 2 2 10 1 2 5 0 2 4 0 1 3 0 1 2 Antonio S et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 07. 03 a

OA 07. 03 a: Impact of Tumor Mutation Burden on the Efficacy of Nivolumab or Nivolumab + Ipilimumab in Small Cell Lung Cancer: An Exploratory Analysis of Check. Mate 032 – Antonio S, et al • Key results (cont. ) OS by TMB subgroup Nivolumab 100 Median OS, months (95%CI) Low TMB Med TMB High TMB 3. 1 (2. 4, 6. 8) 3. 9 (2. 4, 9. 9) 5. 4 (2. 8, 8. 0) 100 Median OS, months (95%CI) 75 75 OS, % Nivolumab + ipilimumab 50 Med TMB High TMB 3. 4 (2. 8, 7. 3) 3. 6 (1. 8, 7. 7) 22. 0 (8. 2, NR) 1 -y OS 62. 4% 50 1 -y OS 35. 2% 1 -y OS 26. 0% 25 Low TMB 1 -y OS 23. 4% 1 -y OS 19. 6% 25 1 -y OS 22. 1% 0 0 0 No. at risk Low 42 Medium 44 High 47 3 6 9 12 15 18 21 24 27 30 33 36 0 3 6 9 12 13 17 20 9 12 14 4 6 8 3 2 5 1 2 5 0 1 5 18 21 24 27 30 33 36 1 1 2 1 1 0 0 Time, months 19 23 29 15 0 0 2 0 0 2 27 25 26 15 15 20 9 9 17 7 4 14 5 3 10 2 2 9 2 2 8 1 2 6 • Conclusions – Nivolumab with or without ipilimumab demonstrated improved outcomes in the high vs. low or medium TMB groups and the combination provided greater clinical benefit vs. nivolumab alone in the high TMB subgroup – Further investigation and optimisation of TMB as a predictive biomarker is warranted Antonio S et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 07. 03 a

OA 09. 01: Characterizing the Genomic Landscape of EGFR C 797 S in Lung Cancer Using ct. DNA Next-Generation Sequencing – Piotrowska Z, et al • Study objective – To investigate the relative frequency of allelic configuration (cis vs. trans) of C 797 S with respect to T 790 M in clinical samples • Methods – Patients with lung adenocarcinoma and an EGFR C 797 S mutation were selected from the Guardant Health database (n=61, samples=141) – All patients had comprehensive ct. DNA testing using the Guardant 360 NGS assay – Cis/trans configuration for T 790 M and C 797 S was determined using Integrated Genomics Viewer software Piotrowska Z et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 09. 01

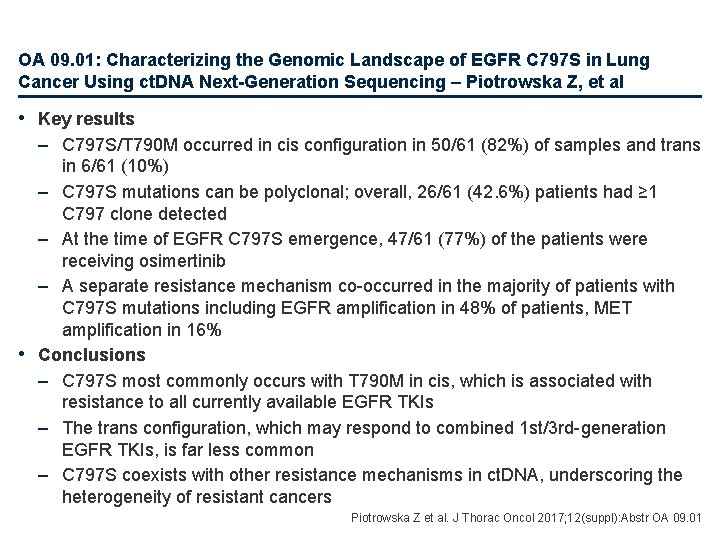
OA 09. 01: Characterizing the Genomic Landscape of EGFR C 797 S in Lung Cancer Using ct. DNA Next-Generation Sequencing – Piotrowska Z, et al • Key results – C 797 S/T 790 M occurred in cis configuration in 50/61 (82%) of samples and trans in 6/61 (10%) – C 797 S mutations can be polyclonal; overall, 26/61 (42. 6%) patients had ≥ 1 C 797 clone detected – At the time of EGFR C 797 S emergence, 47/61 (77%) of the patients were receiving osimertinib – A separate resistance mechanism co-occurred in the majority of patients with C 797 S mutations including EGFR amplification in 48% of patients, MET amplification in 16% • Conclusions – C 797 S most commonly occurs with T 790 M in cis, which is associated with resistance to all currently available EGFR TKIs – The trans configuration, which may respond to combined 1 st/3 rd-generation EGFR TKIs, is far less common – C 797 S coexists with other resistance mechanisms in ct. DNA, underscoring the heterogeneity of resistant cancers Piotrowska Z et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 09. 01

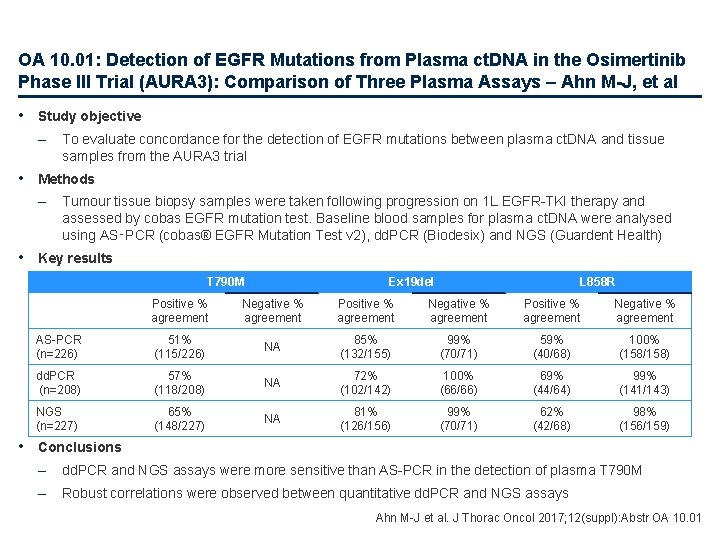
OA 10. 01: Detection of EGFR Mutations from Plasma ct. DNA in the Osimertinib Phase III Trial (AURA 3): Comparison of Three Plasma Assays – Ahn M-J, et al • Study objective – To evaluate concordance for the detection of EGFR mutations between plasma ct. DNA and tissue samples from the AURA 3 trial • Methods – Tumour tissue biopsy samples were taken following progression on 1 L EGFR-TKI therapy and assessed by cobas EGFR mutation test. Baseline blood samples for plasma ct. DNA were analysed using AS‑PCR (cobas® EGFR Mutation Test v 2), dd. PCR (Biodesix) and NGS (Guardent Health) • Key results T 790 M Ex 19 del L 858 R Positive % agreement Negative % agreement AS-PCR (n=226) 51% (115/226) NA 85% (132/155) 99% (70/71) 59% (40/68) 100% (158/158) dd. PCR (n=208) 57% (118/208) NA 72% (102/142) 100% (66/66) 69% (44/64) 99% (141/143) NGS (n=227) 65% (148/227) NA 81% (126/156) 99% (70/71) 62% (42/68) 98% (156/159) • Conclusions – dd. PCR and NGS assays were more sensitive than AS-PCR in the detection of plasma T 790 M – Robust correlations were observed between quantitative dd. PCR and NGS assays Ahn M-J et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 10. 01

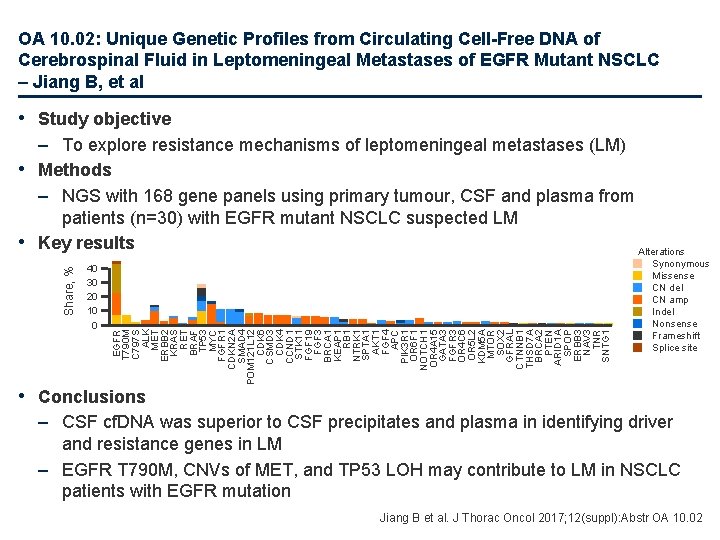
OA 10. 02: Unique Genetic Profiles from Circulating Cell-Free DNA of Cerebrospinal Fluid in Leptomeningeal Metastases of EGFR Mutant NSCLC – Jiang B, et al • Study objective 40 30 20 10 0 EGFR T 790 M C 797 S ALK MET ERBB 2 KRAS RET BRAF TP 53 MYC FGFR 1 CDKN 2 A SMAD 4 POM 121 L 12 CDK 6 CSMD 3 CDK 4 CCND 1 STK 11 FGF 19 FGF 3 BRCA 1 KEAP 1 RB 1 NTRK 1 SPTA 1 AKT 1 FGF 4 APC PIK 3 R 1 OR 6 F 1 NOTCH 1 OR 4 A 15 GATA 3 FGFR 3 OR 4 C 6 OR 5 L 2 KDM 5 A MTOR SOX 2 GFRAL CTNNB 1 THSD 7 A BRCA 2 PTEN ARID 1 A SPOP ERBB 3 NAV 3 TNR SNTG 1 Share, % – To explore resistance mechanisms of leptomeningeal metastases (LM) • Methods – NGS with 168 gene panels using primary tumour, CSF and plasma from patients (n=30) with EGFR mutant NSCLC suspected LM • Key results Alterations Synonymous Missense CN del CN amp Indel Nonsense Frameshift Splice site • Conclusions – CSF cf. DNA was superior to CSF precipitates and plasma in identifying driver and resistance genes in LM – EGFR T 790 M, CNVs of MET, and TP 53 LOH may contribute to LM in NSCLC patients with EGFR mutation Jiang B et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 10. 02

OA 10. 05: Non-Invasive Molecular Profiling in NSCLC by Targeted and Whole Exome Analysis of Plasma cf. DNA – Cheng ML, et al • Study objective – To develop a workflow strategy to enable effective selection of tumour samples for targeted and whole exome sequencing using cf. DNA • Methods – Plasma samples were collected from patients with NSCLC (n=20) who had received treatment (including chemotherapy, targeted therapy or immunotherapy) • The majority of patients (>70%) had stage III or IV disease – cf. DNA was extracted from plasma and analysis was performed using low-pass shallow whole genome sequencing (s. WGS) and MSK-IMPACT (targeting over 400 cancer-related genes) – Analysis of matched normal was performed for somatic variant calling Cheng ML et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 10. 05

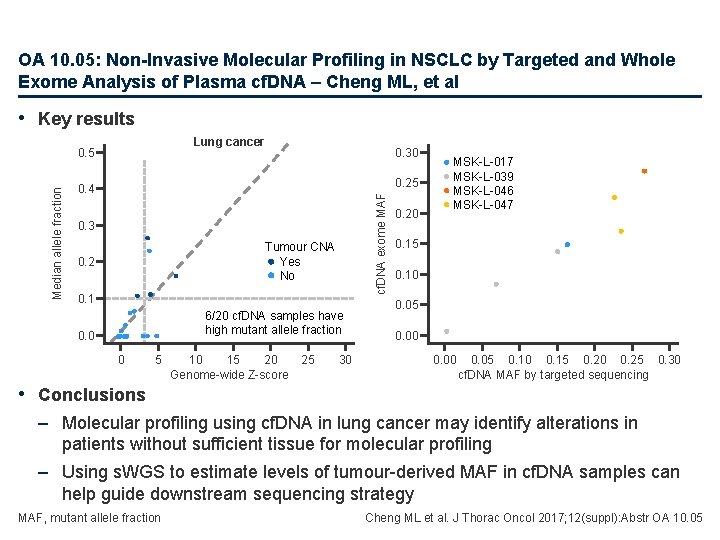
OA 10. 05: Non-Invasive Molecular Profiling in NSCLC by Targeted and Whole Exome Analysis of Plasma cf. DNA – Cheng ML, et al • Key results Lung cancer 0. 30 0. 25 0. 4 cf. DNA exome MAF Median allele fraction 0. 5 0. 3 Tumour CNA Yes No 0. 2 0. 1 6/20 cf. DNA samples have high mutant allele fraction 0. 0 0 5 10 15 20 Genome-wide Z-score 25 30 0. 20 MSK-L-017 MSK-L-039 MSK-L-046 MSK-L-047 0. 15 0. 10 0. 05 0. 00 0. 05 0. 10 0. 15 0. 20 0. 25 0. 30 cf. DNA MAF by targeted sequencing • Conclusions – Molecular profiling using cf. DNA in lung cancer may identify alterations in patients without sufficient tissue for molecular profiling – Using s. WGS to estimate levels of tumour-derived MAF in cf. DNA samples can help guide downstream sequencing strategy MAF, mutant allele fraction Cheng ML et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 10. 05

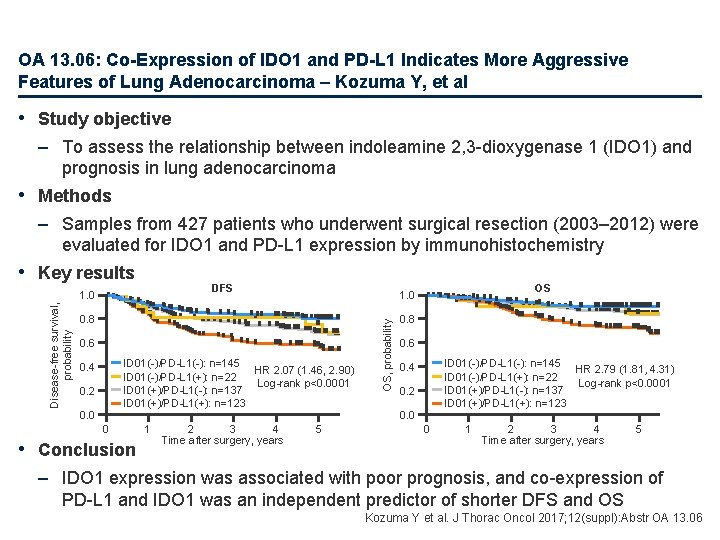
OA 13. 06: Co-Expression of IDO 1 and PD-L 1 Indicates More Aggressive Features of Lung Adenocarcinoma – Kozuma Y, et al • Study objective – To assess the relationship between indoleamine 2, 3 -dioxygenase 1 (IDO 1) and prognosis in lung adenocarcinoma • Methods – Samples from 427 patients who underwent surgical resection (2003– 2012) were evaluated for IDO 1 and PD-L 1 expression by immunohistochemistry • Key results DFS 0. 8 0. 6 ID 01(-)/PD-L 1(-): n=145 HR 2. 07 (1. 46, 2. 90) ID 01(-)/PD-L 1(+): n=22 Log-rank p<0. 0001 ID 01(+)/PD-L 1(-): n=137 ID 01(+)/PD-L 1(+): n=123 0. 4 0. 2 0. 0 0 • Conclusion 1 2 3 4 Time after surgery, years OS 1. 0 5 OS, probability Disease-free survival, probability 1. 0 0. 8 0. 6 ID 01(-)/PD-L 1(-): n=145 HR 2. 79 (1. 81, 4. 31) ID 01(-)/PD-L 1(+): n=22 Log-rank p<0. 0001 ID 01(+)/PD-L 1(-): n=137 ID 01(+)/PD-L 1(+): n=123 0. 4 0. 2 0. 0 0 1 2 3 4 Time after surgery, years 5 – IDO 1 expression was associated with poor prognosis, and co-expression of PD-L 1 and IDO 1 was an independent predictor of shorter DFS and OS Kozuma Y et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 13. 06

Early and locally advanced NSCLC Stages I, II and III

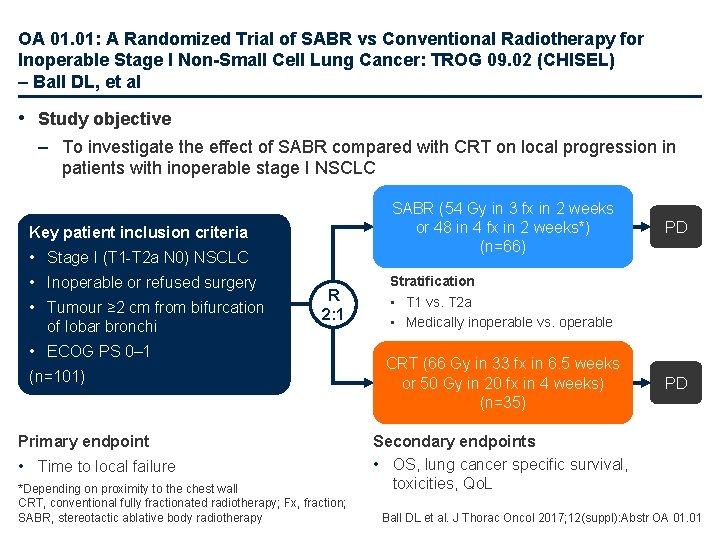
OA 01. 01: A Randomized Trial of SABR vs Conventional Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: TROG 09. 02 (CHISEL) – Ball DL, et al • Study objective – To investigate the effect of SABR compared with CRT on local progression in patients with inoperable stage I NSCLC SABR (54 Gy in 3 fx in 2 weeks or 48 in 4 fx in 2 weeks*) (n=66) Key patient inclusion criteria • Stage I (T 1 -T 2 a N 0) NSCLC • Inoperable or refused surgery • Tumour ≥ 2 cm from bifurcation of lobar bronchi R 2: 1 • ECOG PS 0– 1 (n=101) Primary endpoint • Time to local failure *Depending on proximity to the chest wall CRT, conventional fully fractionated radiotherapy; Fx, fraction; SABR, stereotactic ablative body radiotherapy PD Stratification • T 1 vs. T 2 a • Medically inoperable vs. operable CRT (66 Gy in 33 fx in 6. 5 weeks or 50 Gy in 20 fx in 4 weeks) (n=35) PD Secondary endpoints • OS, lung cancer specific survival, toxicities, Qo. L Ball DL et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 01. 01

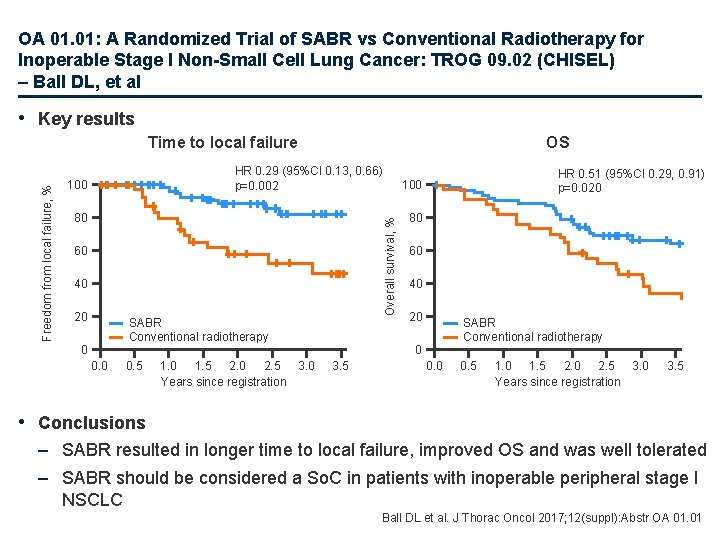
OA 01. 01: A Randomized Trial of SABR vs Conventional Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: TROG 09. 02 (CHISEL) – Ball DL, et al • Key results OS HR 0. 29 (95%CI 0. 13, 0. 66) p=0. 002 100 80 60 40 20 SABR Conventional radiotherapy 0 0. 5 1. 0 1. 5 2. 0 2. 5 3. 0 Years since registration HR 0. 51 (95%CI 0. 29, 0. 91) p=0. 020 100 Overall survival, % Freedom from local failure, % Time to local failure 80 60 40 20 SABR Conventional radiotherapy 0 3. 5 0. 0 0. 5 1. 0 1. 5 2. 0 2. 5 3. 0 Years since registration 3. 5 • Conclusions – SABR resulted in longer time to local failure, improved OS and was well tolerated – SABR should be considered a So. C in patients with inoperable peripheral stage I NSCLC Ball DL et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 01. 01

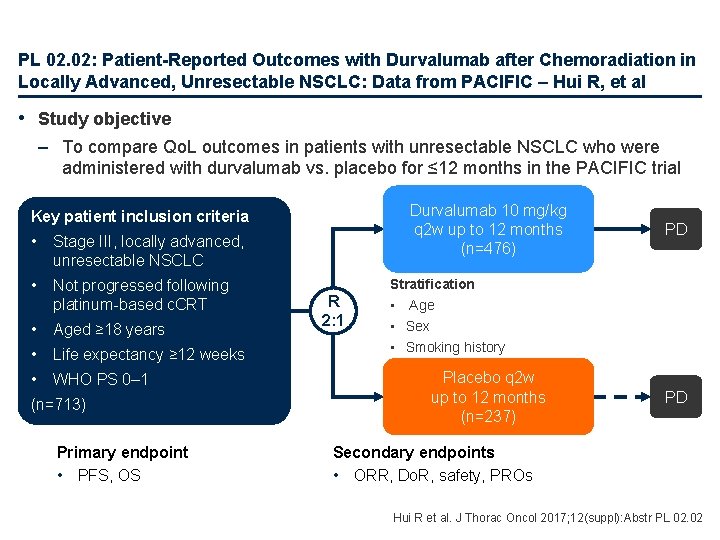
PL 02. 02: Patient-Reported Outcomes with Durvalumab after Chemoradiation in Locally Advanced, Unresectable NSCLC: Data from PACIFIC – Hui R, et al • Study objective – To compare Qo. L outcomes in patients with unresectable NSCLC who were administered with durvalumab vs. placebo for ≤ 12 months in the PACIFIC trial Durvalumab 10 mg/kg q 2 w up to 12 months (n=476) Key patient inclusion criteria • Stage III, locally advanced, unresectable NSCLC • Not progressed following platinum-based c. CRT • Aged ≥ 18 years • Life expectancy ≥ 12 weeks • WHO PS 0– 1 (n=713) Primary endpoint • PFS, OS R 2: 1 PD Stratification • Age • Sex • Smoking history Placebo q 2 w up to 12 months (n=237) PD Secondary endpoints • ORR, Do. R, safety, PROs Hui R et al. J Thorac Oncol 2017; 12(suppl): Abstr PL 02. 02

PL 02. 02: Patient-Reported Outcomes with Durvalumab after Chemoradiation in Locally Advanced, Unresectable NSCLC: Data from PACIFIC – Hui R, et al • Key results – There were no notable differences between treatment arms for global health status/Qo. L, key symptoms or physical function at baseline • Key symptom scores remained stable throughout the study in both treatment groups, with no significant changes from baseline – Clinically relevant improvements from baseline in dysphagia and alopecia were observed at week 48, regardless of treatment – The odds of improvement in ‘appetite loss’ in the durvalumab group was greater than observed in the placebo group (OR 1. 72; 95%CI 1. 04, 2. 85) • Conclusions – Treatment with durvalumab following chemoradiation was associated with maintained function and global health status/Qo. L in patients with locally advanced, unresectable NSCLC – The satisfactory efficacy and safety profile of durvalumab, in addition to the Qo. L results observed in the PACIFIC trial supports its clinical value in this setting Hui R et al. J Thorac Oncol 2017; 12(suppl): Abstr PL 02. 02

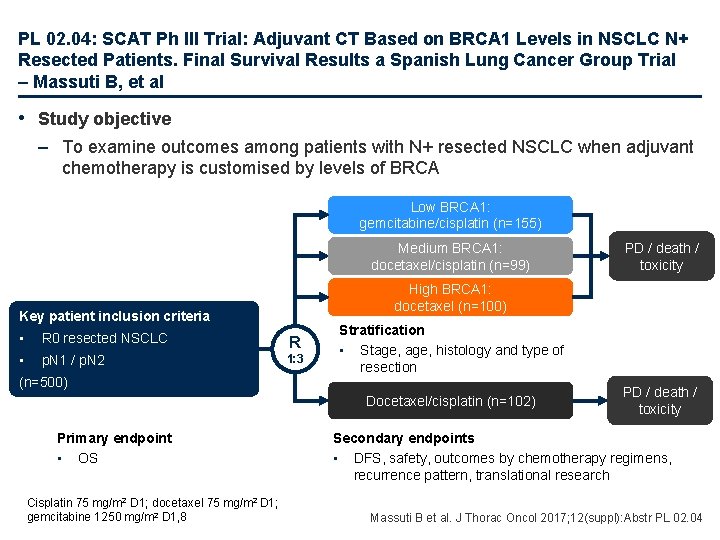
PL 02. 04: SCAT Ph III Trial: Adjuvant CT Based on BRCA 1 Levels in NSCLC N+ Resected Patients. Final Survival Results a Spanish Lung Cancer Group Trial – Massuti B, et al • Study objective – To examine outcomes among patients with N+ resected NSCLC when adjuvant chemotherapy is customised by levels of BRCA Low BRCA 1: gemcitabine/cisplatin (n=155) Medium BRCA 1: docetaxel/cisplatin (n=99) High BRCA 1: docetaxel (n=100) Key patient inclusion criteria • R 0 resected NSCLC R • p. N 1 / p. N 2 1: 3 (n=500) Stratification • Stage, histology and type of resection Docetaxel/cisplatin (n=102) Primary endpoint • OS Cisplatin 75 mg/m 2 D 1; docetaxel 75 mg/m 2 D 1; gemcitabine 1250 mg/m 2 D 1, 8 PD / death / toxicity Secondary endpoints • DFS, safety, outcomes by chemotherapy regimens, recurrence pattern, translational research Massuti B et al. J Thorac Oncol 2017; 12(suppl): Abstr PL 02. 04

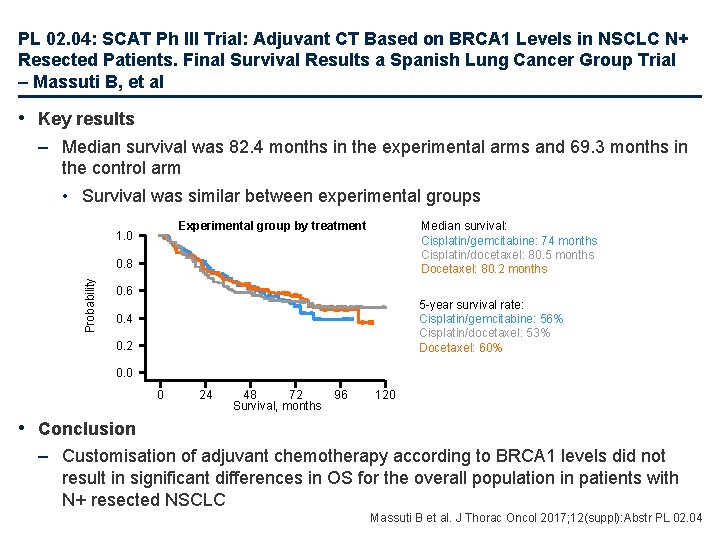
PL 02. 04: SCAT Ph III Trial: Adjuvant CT Based on BRCA 1 Levels in NSCLC N+ Resected Patients. Final Survival Results a Spanish Lung Cancer Group Trial – Massuti B, et al • Key results – Median survival was 82. 4 months in the experimental arms and 69. 3 months in the control arm • Survival was similar between experimental groups Experimental group by treatment 1. 0 Median survival: Cisplatin/gemcitabine: 74 months Cisplatin/docetaxel: 80. 5 months Docetaxel: 80. 2 months Probability 0. 8 0. 6 5 -year survival rate: Cisplatin/gemcitabine: 56% Cisplatin/docetaxel: 53% Docetaxel: 60% 0. 4 0. 2 0. 0 0 24 48 72 Survival, months 96 120 • Conclusion – Customisation of adjuvant chemotherapy according to BRCA 1 levels did not result in significant differences in OS for the overall population in patients with N+ resected NSCLC Massuti B et al. J Thorac Oncol 2017; 12(suppl): Abstr PL 02. 04

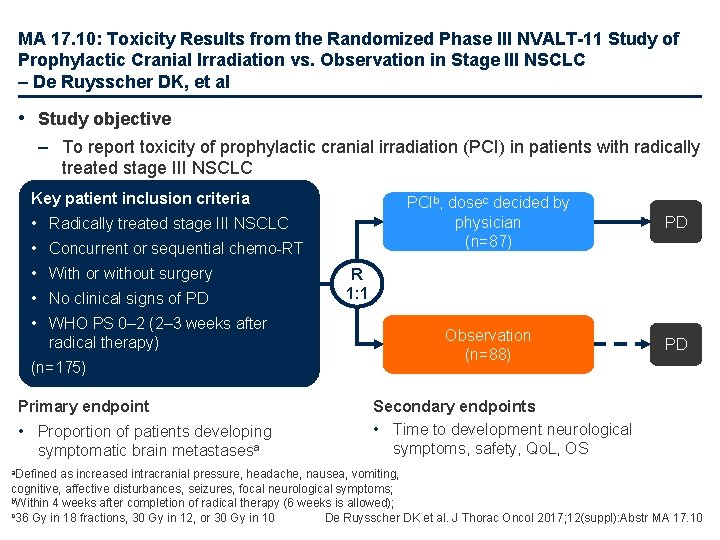
MA 17. 10: Toxicity Results from the Randomized Phase III NVALT-11 Study of Prophylactic Cranial Irradiation vs. Observation in Stage III NSCLC – De Ruysscher DK, et al • Study objective – To report toxicity of prophylactic cranial irradiation (PCI) in patients with radically treated stage III NSCLC Key patient inclusion criteria • Radically treated stage III NSCLC • Concurrent or sequential chemo-RT • With or without surgery • No clinical signs of PD • WHO PS 0– 2 (2– 3 weeks after radical therapy) (n=175) Primary endpoint • Proportion of patients developing symptomatic brain metastasesa a. Defined PCIb, dosec decided by physician (n=87) PD Observation (n=88) PD R 1: 1 Secondary endpoints • Time to development neurological symptoms, safety, Qo. L, OS as increased intracranial pressure, headache, nausea, vomiting, cognitive, affective disturbances, seizures, focal neurological symptoms; b. Within 4 weeks after completion of radical therapy (6 weeks is allowed); c 36 Gy in 18 fractions, 30 Gy in 12, or 30 Gy in 10 De Ruysscher DK et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 17. 10

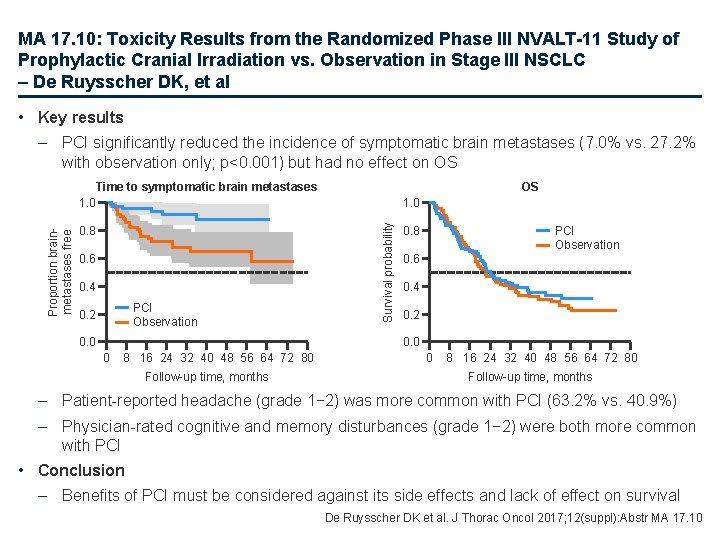
MA 17. 10: Toxicity Results from the Randomized Phase III NVALT-11 Study of Prophylactic Cranial Irradiation vs. Observation in Stage III NSCLC – De Ruysscher DK, et al • Key results – PCI significantly reduced the incidence of symptomatic brain metastases (7. 0% vs. 27. 2% with observation only; p<0. 001) but had no effect on OS 0. 8 0. 6 0. 4 PCI Observation 0. 2 0. 0 OS 1. 0 Survival probability Proportion brainmetastases free Time to symptomatic brain metastases 1. 0 PCI Observation 0. 8 0. 6 0. 4 0. 2 0. 0 0 8 16 24 32 40 48 56 64 72 80 Follow-up time, months – Patient-reported headache (grade 1− 2) was more common with PCI (63. 2% vs. 40. 9%) – Physician-rated cognitive and memory disturbances (grade 1− 2) were both more common with PCI • Conclusion – Benefits of PCI must be considered against its side effects and lack of effect on survival De Ruysscher DK et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 17. 10

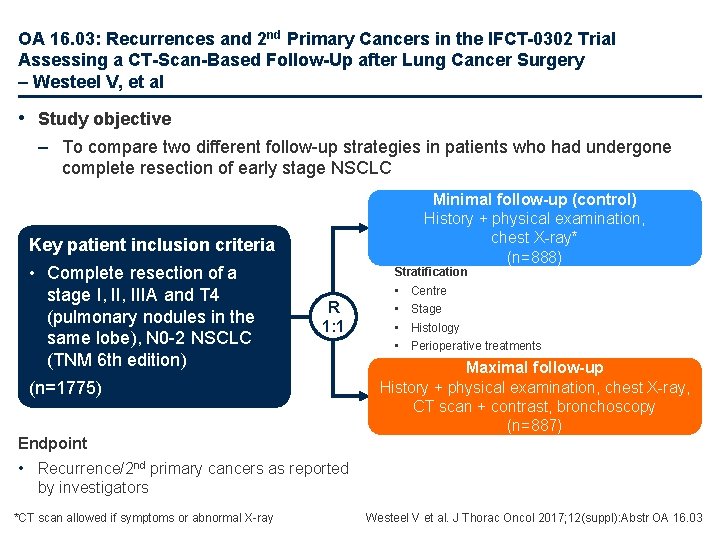
OA 16. 03: Recurrences and 2 nd Primary Cancers in the IFCT-0302 Trial Assessing a CT-Scan-Based Follow-Up after Lung Cancer Surgery – Westeel V, et al • Study objective – To compare two different follow-up strategies in patients who had undergone complete resection of early stage NSCLC Minimal follow-up (control) History + physical examination, chest X-ray* (n=888) Key patient inclusion criteria • Complete resection of a stage I, IIIA and T 4 (pulmonary nodules in the same lobe), N 0 -2 NSCLC (TNM 6 th edition) Stratification R 1: 1 (n=1775) Endpoint • • Centre Stage Histology Perioperative treatments Maximal follow-up History + physical examination, chest X-ray, CT scan + contrast, bronchoscopy (n=887) • Recurrence/2 nd primary cancers as reported by investigators *CT scan allowed if symptoms or abnormal X-ray Westeel V et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 16. 03

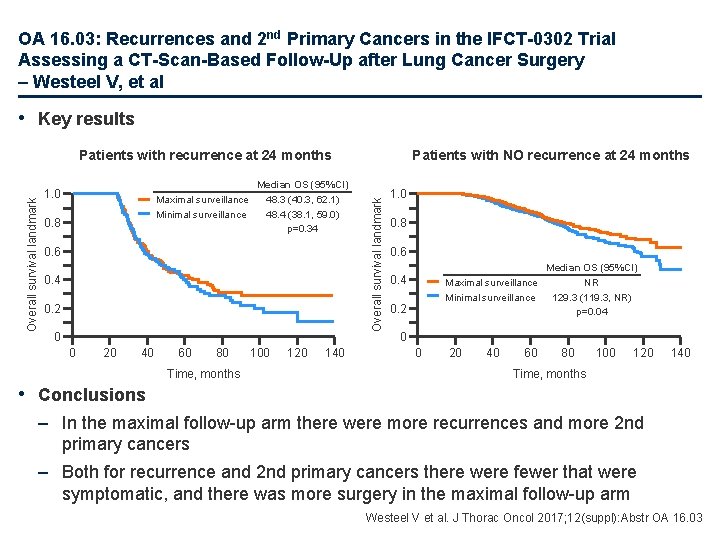
OA 16. 03: Recurrences and 2 nd Primary Cancers in the IFCT-0302 Trial Assessing a CT-Scan-Based Follow-Up after Lung Cancer Surgery – Westeel V, et al • Key results 1. 0 Maximal surveillance Minimal surveillance 0. 8 Median OS (95%CI) 48. 3 (40. 3, 62. 1) 48. 4 (38. 1, 59. 0) p=0. 34 0. 6 0. 4 0. 2 0 0 20 40 60 80 Time, months 100 120 140 Patients with NO recurrence at 24 months Overall survival landmark Patients with recurrence at 24 months 1. 0 0. 8 0. 6 Median OS (95%CI) Maximal surveillance NR Minimal surveillance 129. 3 (119. 3, NR) p=0. 04 0. 2 0 0 20 40 60 80 100 120 140 Time, months • Conclusions – In the maximal follow-up arm there were more recurrences and more 2 nd primary cancers – Both for recurrence and 2 nd primary cancers there were fewer that were symptomatic, and there was more surgery in the maximal follow-up arm Westeel V et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 16. 03

OA 16. 04: Efficacy and Safety of Erlotinib vs Vinorelbine/Cisplatin as Adjuvant Therapy for Stage IIIA EGFR Mutant NSCLC Patients – Yue D, et al • Study objective – To evaluate the efficacy and safety of adjuvant erlotinib vs. vinorelbine + cisplatin in treatment naïve patients with completely resected stage IIIA EGFR mutation positive NSCLC • Methods – Randomized controlled trial of 102 patients who received erlotinib 150 mg/day for 2 years (n=51) or vinorelbine 25 mg/m 2 D 1, 8 + cisplatin 75 mg/m 2 D 1, 21 for 4 cycles (n=51) – Primary endpoint was 2 -year DFS rate; secondary endpoints DFS, OS, safety, Qo. L and biomarkers • Key results – In the erlotinib group, 2 -year DFS rate was significantly higher (81. 35%) than chemotherapy (44. 62%; p<0. 001) and the median DFS was longer (42. 41 months) than chemotherapy (20. 96 months; p<0. 001) – Erlotinib had a more favourable safety profile than chemotherapy • Conclusions – In patients with R 0 resected stage IIIA EGFR-mutant NSCLC erlotinib demonstrates interesting activity – Longer follow-up in this curative setting is needed and the role of chemotherapy in that context will need to be defined Yue D et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 16. 04

Advanced NSCLC Not radically treatable stage III and stage IV First line

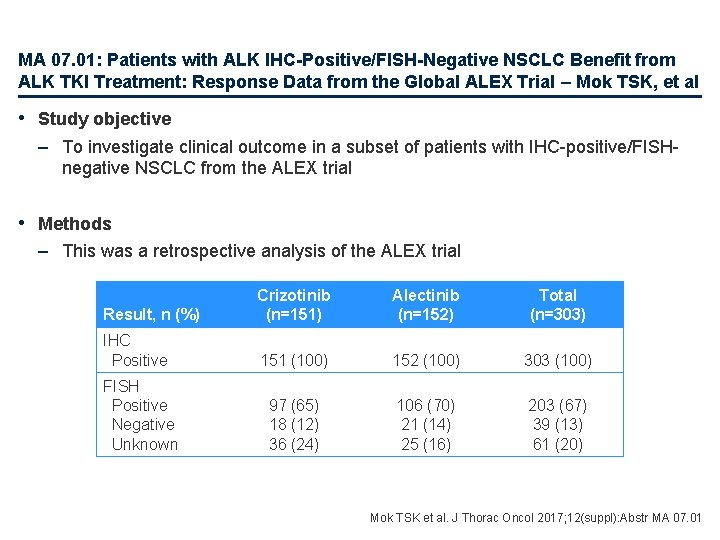
MA 07. 01: Patients with ALK IHC-Positive/FISH-Negative NSCLC Benefit from ALK TKI Treatment: Response Data from the Global ALEX Trial – Mok TSK, et al • Study objective – To investigate clinical outcome in a subset of patients with IHC-positive/FISHnegative NSCLC from the ALEX trial • Methods – This was a retrospective analysis of the ALEX trial Result, n (%) Crizotinib (n=151) Alectinib (n=152) Total (n=303) IHC Positive 151 (100) 152 (100) 303 (100) 97 (65) 18 (12) 36 (24) 106 (70) 21 (14) 25 (16) 203 (67) 39 (13) 61 (20) FISH Positive Negative Unknown Mok TSK et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 07. 01

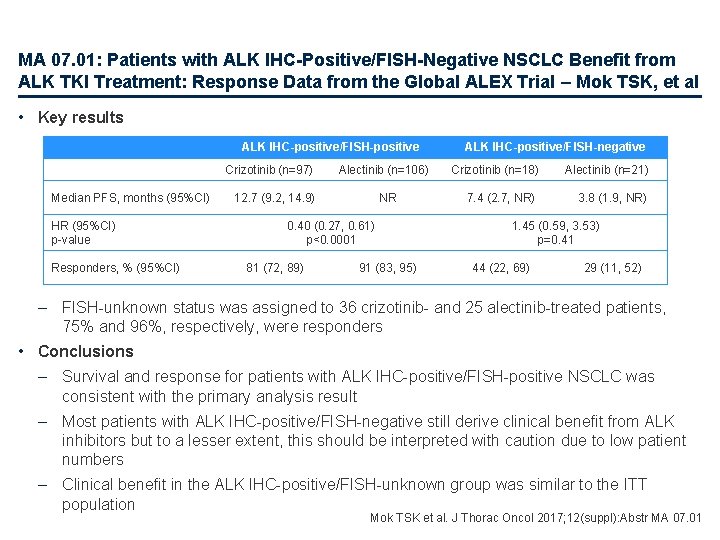
MA 07. 01: Patients with ALK IHC-Positive/FISH-Negative NSCLC Benefit from ALK TKI Treatment: Response Data from the Global ALEX Trial – Mok TSK, et al • Key results ALK IHC-positive/FISH-positive Crizotinib (n=97) Median PFS, months (95%CI) HR (95%CI) p-value Responders, % (95%CI) Alectinib (n=106) 12. 7 (9. 2, 14. 9) NR 0. 40 (0. 27, 0. 61) p<0. 0001 81 (72, 89) 91 (83, 95) ALK IHC-positive/FISH-negative Crizotinib (n=18) 7. 4 (2. 7, NR) Alectinib (n=21) 3. 8 (1. 9, NR) 1. 45 (0. 59, 3. 53) p=0. 41 44 (22, 69) 29 (11, 52) – FISH-unknown status was assigned to 36 crizotinib- and 25 alectinib-treated patients, 75% and 96%, respectively, were responders • Conclusions – Survival and response for patients with ALK IHC-positive/FISH-positive NSCLC was consistent with the primary analysis result – Most patients with ALK IHC-positive/FISH-negative still derive clinical benefit from ALK inhibitors but to a lesser extent, this should be interpreted with caution due to low patient numbers – Clinical benefit in the ALK IHC-positive/FISH-unknown group was similar to the ITT population Mok TSK et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 07. 01

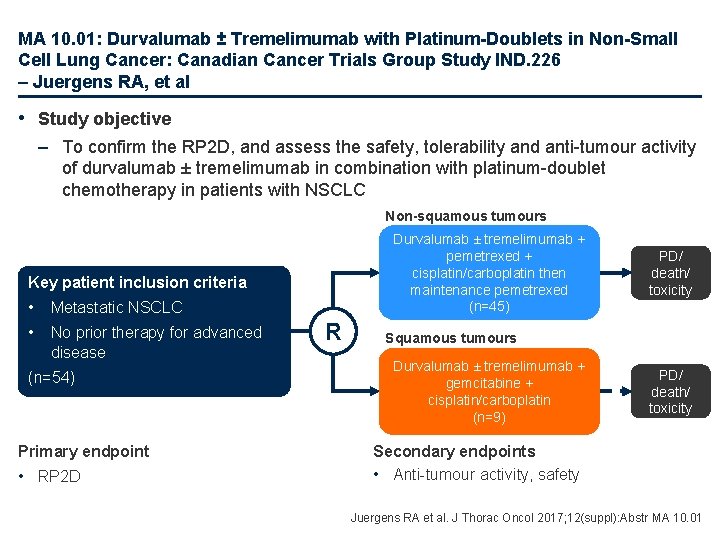
MA 10. 01: Durvalumab ± Tremelimumab with Platinum-Doublets in Non-Small Cell Lung Cancer: Canadian Cancer Trials Group Study IND. 226 – Juergens RA, et al • Study objective – To confirm the RP 2 D, and assess the safety, tolerability and anti-tumour activity of durvalumab ± tremelimumab in combination with platinum-doublet chemotherapy in patients with NSCLC Non-squamous tumours Durvalumab ± tremelimumab + pemetrexed + cisplatin/carboplatin then maintenance pemetrexed (n=45) Key patient inclusion criteria • Metastatic NSCLC • No prior therapy for advanced disease (n=54) Primary endpoint • RP 2 D R PD/ death/ toxicity Squamous tumours Durvalumab ± tremelimumab + gemcitabine + cisplatin/carboplatin (n=9) PD/ death/ toxicity Secondary endpoints • Anti-tumour activity, safety Juergens RA et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 10. 01

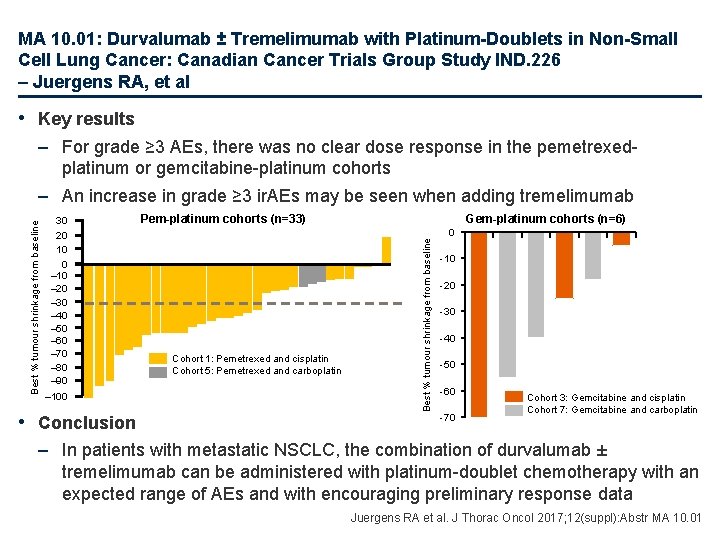
MA 10. 01: Durvalumab ± Tremelimumab with Platinum-Doublets in Non-Small Cell Lung Cancer: Canadian Cancer Trials Group Study IND. 226 – Juergens RA, et al • Key results 30 20 10 0 – 10 – 20 – 30 – 40 – 50 – 60 – 70 – 80 – 90 – 100 • Conclusion Pem-platinum cohorts (n=33) Cohort 1: Pemetrexed and cisplatin Cohort 5: Pemetrexed and carboplatin Gem-platinum cohorts (n=6) Best % tumour shrinkage from baseline – For grade ≥ 3 AEs, there was no clear dose response in the pemetrexedplatinum or gemcitabine-platinum cohorts – An increase in grade ≥ 3 ir. AEs may be seen when adding tremelimumab 0 -10 -20 -30 -40 -50 -60 -70 Cohort 3: Gemcitabine and cisplatin Cohort 7: Gemcitabine and carboplatin – In patients with metastatic NSCLC, the combination of durvalumab ± tremelimumab can be administered with platinum-doublet chemotherapy with an expected range of AEs and with encouraging preliminary response data Juergens RA et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 10. 01

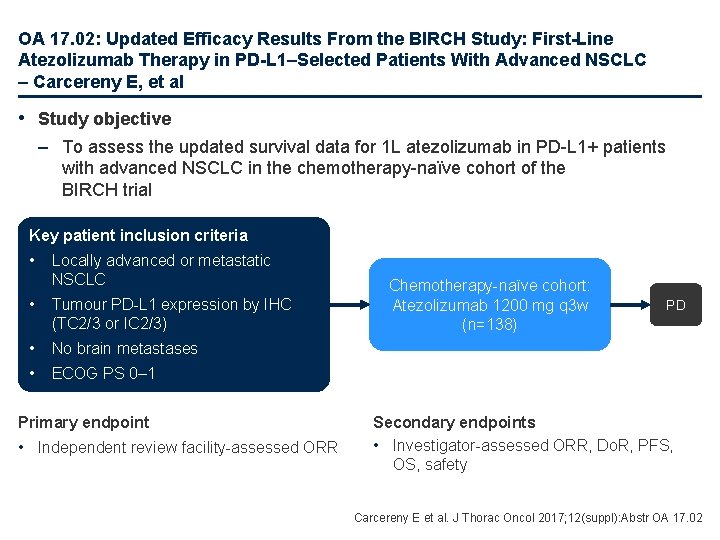
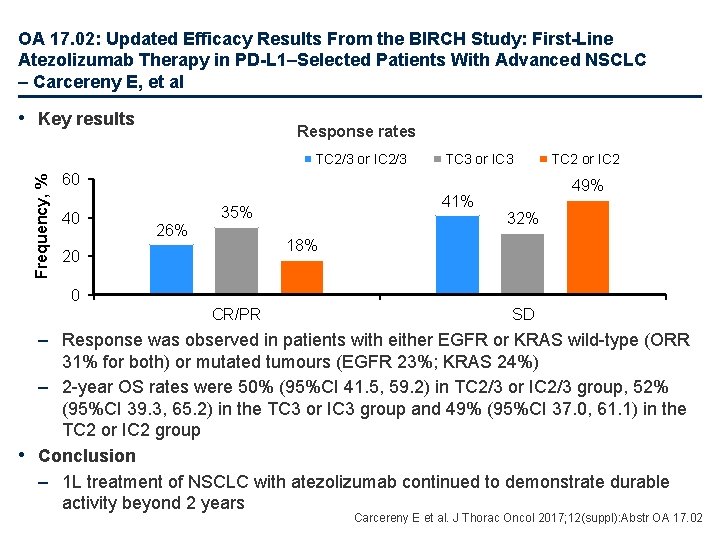
OA 17. 02: Updated Efficacy Results From the BIRCH Study: First-Line Atezolizumab Therapy in PD-L 1–Selected Patients With Advanced NSCLC – Carcereny E, et al • Study objective – To assess the updated survival data for 1 L atezolizumab in PD-L 1+ patients with advanced NSCLC in the chemotherapy-naïve cohort of the BIRCH trial Key patient inclusion criteria • Locally advanced or metastatic NSCLC • Tumour PD-L 1 expression by IHC (TC 2/3 or IC 2/3) • No brain metastases • ECOG PS 0– 1 Primary endpoint • Independent review facility-assessed ORR Chemotherapy-naïve cohort: Atezolizumab 1200 mg q 3 w (n=138) PD Secondary endpoints • Investigator-assessed ORR, Do. R, PFS, OS, safety Carcereny E et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 02

OA 17. 02: Updated Efficacy Results From the BIRCH Study: First-Line Atezolizumab Therapy in PD-L 1–Selected Patients With Advanced NSCLC – Carcereny E, et al • Key results Response rates Frequency, % TC 2/3 or IC 2/3 TC 3 or IC 3 60 40 26% 41% 35% TC 2 or IC 2 49% 32% 18% 20 0 CR/PR SD – Response was observed in patients with either EGFR or KRAS wild-type (ORR 31% for both) or mutated tumours (EGFR 23%; KRAS 24%) – 2 -year OS rates were 50% (95%CI 41. 5, 59. 2) in TC 2/3 or IC 2/3 group, 52% (95%CI 39. 3, 65. 2) in the TC 3 or IC 3 group and 49% (95%CI 37. 0, 61. 1) in the TC 2 or IC 2 group • Conclusion – 1 L treatment of NSCLC with atezolizumab continued to demonstrate durable activity beyond 2 years Carcereny E et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 02

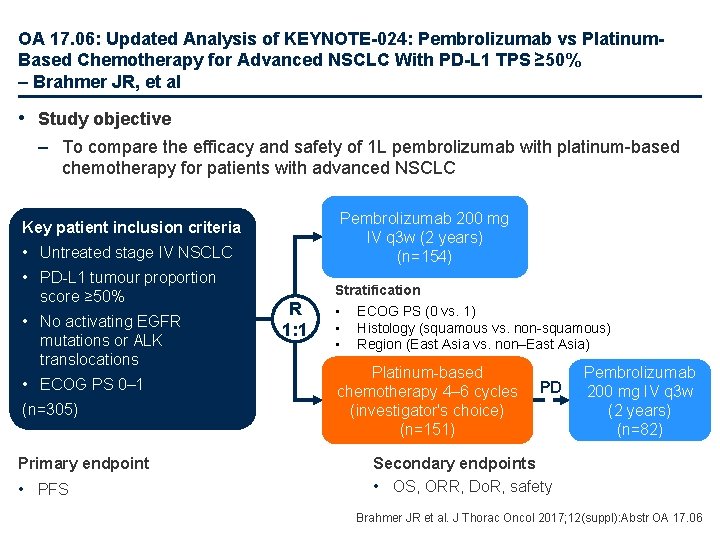
OA 17. 06: Updated Analysis of KEYNOTE-024: Pembrolizumab vs Platinum. Based Chemotherapy for Advanced NSCLC With PD-L 1 TPS ≥ 50% – Brahmer JR, et al • Study objective – To compare the efficacy and safety of 1 L pembrolizumab with platinum-based chemotherapy for patients with advanced NSCLC Pembrolizumab 200 mg IV q 3 w (2 years) (n=154) Key patient inclusion criteria • Untreated stage IV NSCLC • PD-L 1 tumour proportion score ≥ 50% • No activating EGFR mutations or ALK translocations • ECOG PS 0– 1 (n=305) Primary endpoint • PFS R 1: 1 Stratification • ECOG PS (0 vs. 1) • Histology (squamous vs. non-squamous) • Region (East Asia vs. non–East Asia) Platinum-based chemotherapy 4– 6 cycles (investigator's choice) (n=151) PD Pembrolizumab 200 mg IV q 3 w (2 years) (n=82) Secondary endpoints • OS, ORR, Do. R, safety Brahmer JR et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 06

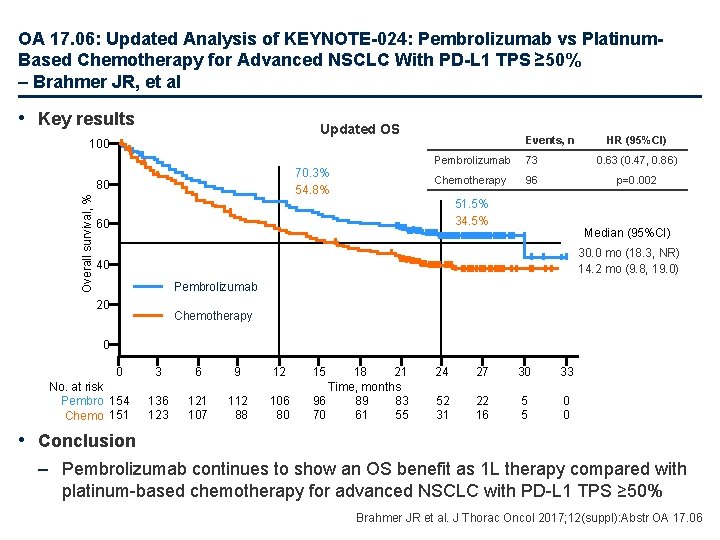
OA 17. 06: Updated Analysis of KEYNOTE-024: Pembrolizumab vs Platinum. Based Chemotherapy for Advanced NSCLC With PD-L 1 TPS ≥ 50% – Brahmer JR, et al • Key results Updated OS 100 70. 3% 54. 8% Overall survival, % 80 Events, n HR (95%CI) Pembrolizumab 73 0. 63 (0. 47, 0. 86) Chemotherapy 96 p=0. 002 51. 5% 34. 5% 60 Median (95%CI) 30. 0 mo (18. 3, NR) 14. 2 mo (9. 8, 19. 0) 40 Pembrolizumab 20 Chemotherapy 0 0 No. at risk Pembro 154 Chemo 151 3 6 9 12 136 123 121 107 112 88 106 80 15 18 21 Time, months 96 89 83 70 61 55 24 27 30 33 52 31 22 16 5 5 0 0 • Conclusion – Pembrolizumab continues to show an OS benefit as 1 L therapy compared with platinum-based chemotherapy for advanced NSCLC with PD-L 1 TPS ≥ 50% Brahmer JR et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 06

Advanced NSCLC Not radically treatable stage III and stage IV Later lines

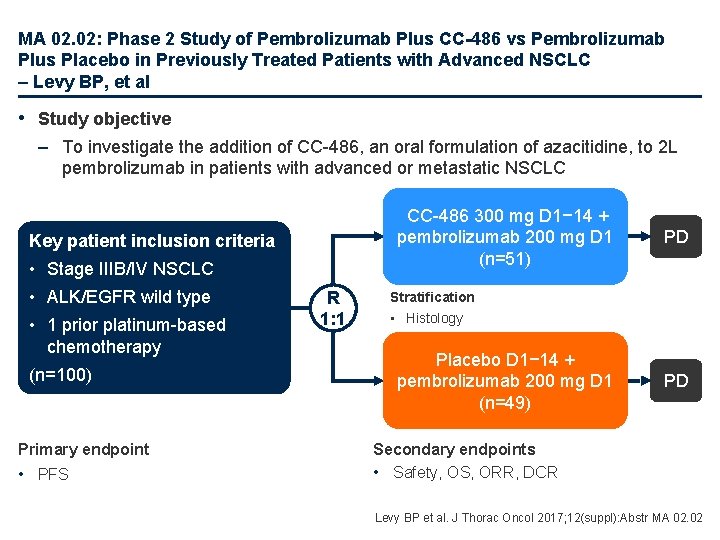
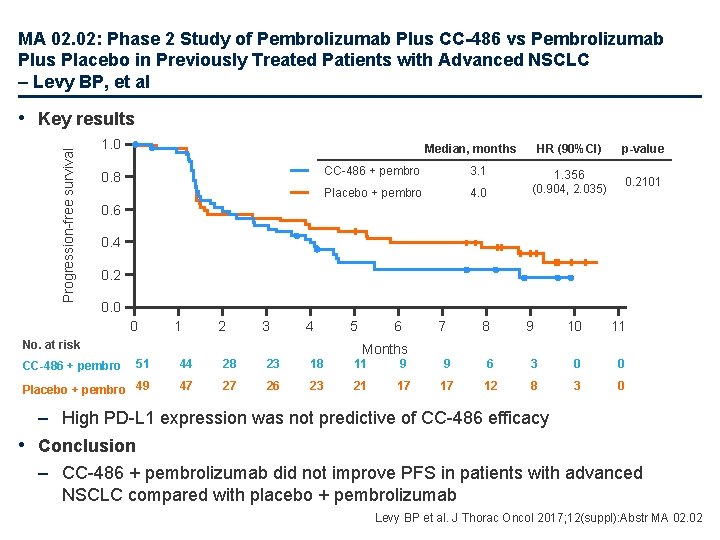
MA 02. 02: Phase 2 Study of Pembrolizumab Plus CC-486 vs Pembrolizumab Plus Placebo in Previously Treated Patients with Advanced NSCLC – Levy BP, et al • Study objective – To investigate the addition of CC-486, an oral formulation of azacitidine, to 2 L pembrolizumab in patients with advanced or metastatic NSCLC CC-486 300 mg D 1− 14 + pembrolizumab 200 mg D 1 (n=51) Key patient inclusion criteria • Stage IIIB/IV NSCLC • ALK/EGFR wild type • 1 prior platinum-based chemotherapy (n=100) Primary endpoint • PFS R 1: 1 PD Stratification • Histology Placebo D 1− 14 + pembrolizumab 200 mg D 1 (n=49) PD Secondary endpoints • Safety, OS, ORR, DCR Levy BP et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 02. 02

MA 02. 02: Phase 2 Study of Pembrolizumab Plus CC-486 vs Pembrolizumab Plus Placebo in Previously Treated Patients with Advanced NSCLC – Levy BP, et al Progression-free survival • Key results 1. 0 Median, months 0. 8 CC-486 + pembro 3. 1 Placebo + pembro 4. 0 HR (90%CI) p-value 1. 356 (0. 904, 2. 035) 0. 2101 0. 6 0. 4 0. 2 0. 0 0 1 2 3 4 No. at risk 5 6 7 8 9 10 11 Months 51 44 28 23 18 11 9 9 6 3 0 0 Placebo + pembro 49 47 27 26 23 21 17 17 12 8 3 0 CC-486 + pembro – High PD-L 1 expression was not predictive of CC-486 efficacy • Conclusion – CC-486 + pembrolizumab did not improve PFS in patients with advanced NSCLC compared with placebo + pembrolizumab Levy BP et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 02. 02

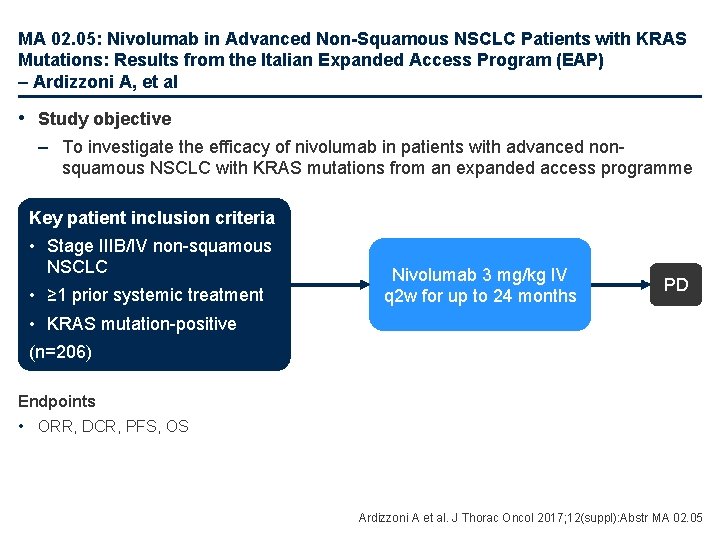
MA 02. 05: Nivolumab in Advanced Non-Squamous NSCLC Patients with KRAS Mutations: Results from the Italian Expanded Access Program (EAP) – Ardizzoni A, et al • Study objective – To investigate the efficacy of nivolumab in patients with advanced nonsquamous NSCLC with KRAS mutations from an expanded access programme Key patient inclusion criteria • Stage IIIB/IV non-squamous NSCLC • ≥ 1 prior systemic treatment Nivolumab 3 mg/kg IV q 2 w for up to 24 months PD • KRAS mutation-positive (n=206) Endpoints • ORR, DCR, PFS, OS Ardizzoni A et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 02. 05

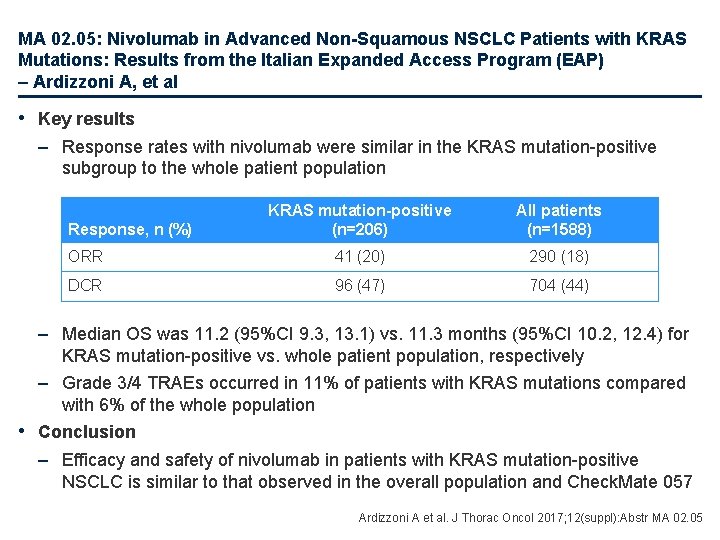
MA 02. 05: Nivolumab in Advanced Non-Squamous NSCLC Patients with KRAS Mutations: Results from the Italian Expanded Access Program (EAP) – Ardizzoni A, et al • Key results – Response rates with nivolumab were similar in the KRAS mutation-positive subgroup to the whole patient population KRAS mutation-positive (n=206) All patients (n=1588) ORR 41 (20) 290 (18) DCR 96 (47) 704 (44) Response, n (%) – Median OS was 11. 2 (95%CI 9. 3, 13. 1) vs. 11. 3 months (95%CI 10. 2, 12. 4) for KRAS mutation-positive vs. whole patient population, respectively – Grade 3/4 TRAEs occurred in 11% of patients with KRAS mutations compared with 6% of the whole population • Conclusion – Efficacy and safety of nivolumab in patients with KRAS mutation-positive NSCLC is similar to that observed in the overall population and Check. Mate 057 Ardizzoni A et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 02. 05

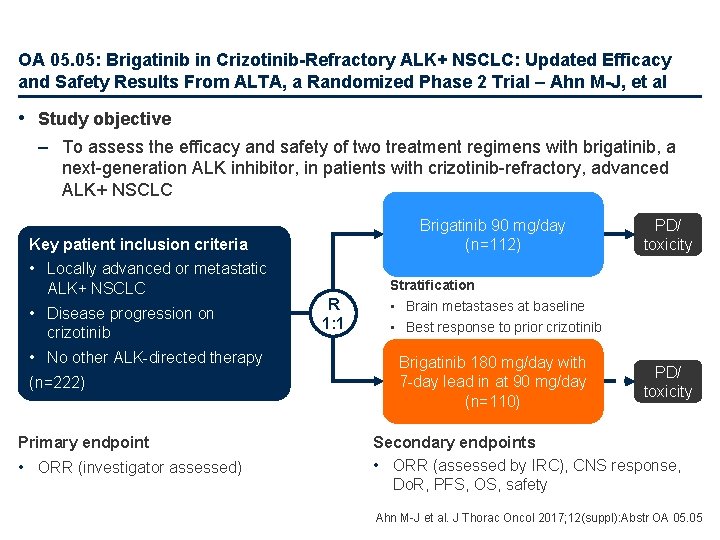
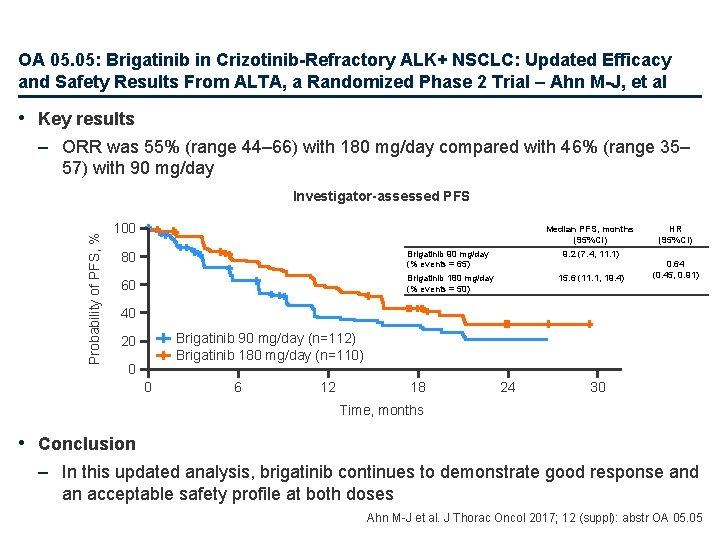
OA 05. 05: Brigatinib in Crizotinib-Refractory ALK+ NSCLC: Updated Efficacy and Safety Results From ALTA, a Randomized Phase 2 Trial – Ahn M-J, et al • Study objective – To assess the efficacy and safety of two treatment regimens with brigatinib, a next-generation ALK inhibitor, in patients with crizotinib-refractory, advanced ALK+ NSCLC Brigatinib 90 mg/day (n=112) Key patient inclusion criteria • Locally advanced or metastatic ALK+ NSCLC • Disease progression on crizotinib • No other ALK-directed therapy (n=222) Primary endpoint • ORR (investigator assessed) R 1: 1 PD/ toxicity Stratification • Brain metastases at baseline • Best response to prior crizotinib Brigatinib 180 mg/day with 7 -day lead in at 90 mg/day (n=110) PD/ toxicity Secondary endpoints • ORR (assessed by IRC), CNS response, Do. R, PFS, OS, safety Ahn M-J et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 05. 05

OA 05. 05: Brigatinib in Crizotinib-Refractory ALK+ NSCLC: Updated Efficacy and Safety Results From ALTA, a Randomized Phase 2 Trial – Ahn M-J, et al • Key results – ORR was 55% (range 44– 66) with 180 mg/day compared with 46% (range 35– 57) with 90 mg/day Probability of PFS, % Investigator-assessed PFS 100 Median PFS, months (95%CI) 80 Brigatinib 90 mg/day (% events = 65) 9. 2 (7. 4, 11. 1) 60 Brigatinib 180 mg/day (% events = 50) 15. 6 (11. 1, 19. 4) HR (95%CI) 0. 64 (0. 45, 0. 91) 40 Brigatinib 90 mg/day (n=112) Brigatinib 180 mg/day (n=110) 20 0 0 6 12 18 24 30 Time, months • Conclusion – In this updated analysis, brigatinib continues to demonstrate good response and an acceptable safety profile at both doses Ahn M-J et al. J Thorac Oncol 2017; 12 (suppl): abstr OA 05. 05

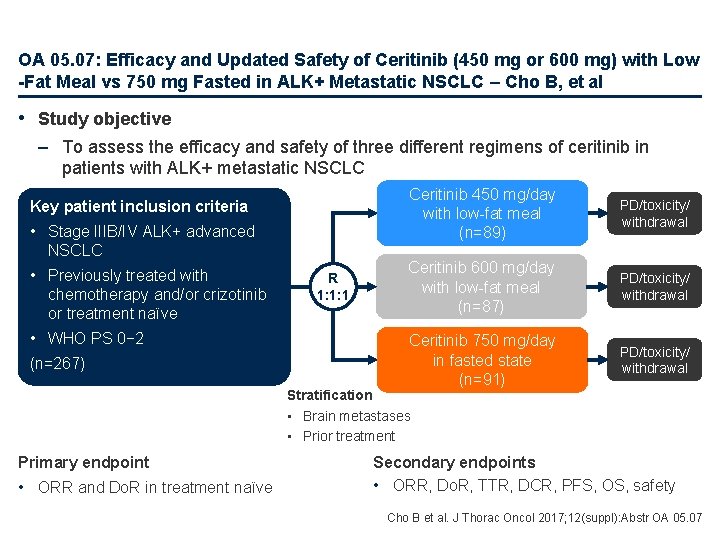
OA 05. 07: Efficacy and Updated Safety of Ceritinib (450 mg or 600 mg) with Low -Fat Meal vs 750 mg Fasted in ALK+ Metastatic NSCLC – Cho B, et al • Study objective – To assess the efficacy and safety of three different regimens of ceritinib in patients with ALK+ metastatic NSCLC Key patient inclusion criteria • Stage IIIB/IV ALK+ advanced NSCLC • Previously treated with chemotherapy and/or crizotinib or treatment naïve • WHO PS 0− 2 (n=267) R 1: 1: 1 Ceritinib 450 mg/day with low-fat meal (n=89) PD/toxicity/ withdrawal Ceritinib 600 mg/day with low-fat meal (n=87) PD/toxicity/ withdrawal Ceritinib 750 mg/day in fasted state (n=91) PD/toxicity/ withdrawal Stratification • Brain metastases • Prior treatment Primary endpoint • ORR and Do. R in treatment naïve Secondary endpoints • ORR, Do. R, TTR, DCR, PFS, OS, safety Cho B et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 05. 07

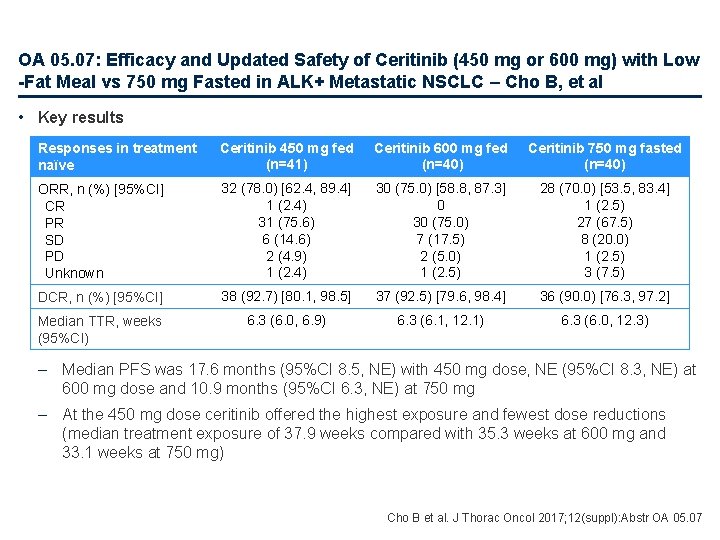
OA 05. 07: Efficacy and Updated Safety of Ceritinib (450 mg or 600 mg) with Low -Fat Meal vs 750 mg Fasted in ALK+ Metastatic NSCLC – Cho B, et al • Key results Responses in treatment naïve Ceritinib 450 mg fed (n=41) Ceritinib 600 mg fed (n=40) Ceritinib 750 mg fasted (n=40) ORR, n (%) [95%CI] CR PR SD PD Unknown 32 (78. 0) [62. 4, 89. 4] 1 (2. 4) 31 (75. 6) 6 (14. 6) 2 (4. 9) 1 (2. 4) 30 (75. 0) [58. 8, 87. 3] 0 30 (75. 0) 7 (17. 5) 2 (5. 0) 1 (2. 5) 28 (70. 0) [53. 5, 83. 4] 1 (2. 5) 27 (67. 5) 8 (20. 0) 1 (2. 5) 3 (7. 5) DCR, n (%) [95%CI] 38 (92. 7) [80. 1, 98. 5] 37 (92. 5) [79. 6, 98. 4] 36 (90. 0) [76. 3, 97. 2] Median TTR, weeks (95%CI) 6. 3 (6. 0, 6. 9) 6. 3 (6. 1, 12. 1) 6. 3 (6. 0, 12. 3) – Median PFS was 17. 6 months (95%CI 8. 5, NE) with 450 mg dose, NE (95%CI 8. 3, NE) at 600 mg dose and 10. 9 months (95%CI 6. 3, NE) at 750 mg – At the 450 mg dose ceritinib offered the highest exposure and fewest dose reductions (median treatment exposure of 37. 9 weeks compared with 35. 3 weeks at 600 mg and 33. 1 weeks at 750 mg) Cho B et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 05. 07

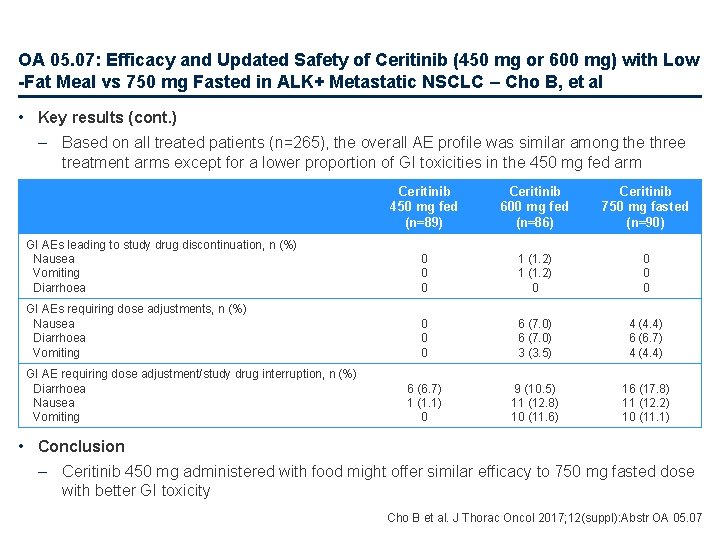
OA 05. 07: Efficacy and Updated Safety of Ceritinib (450 mg or 600 mg) with Low -Fat Meal vs 750 mg Fasted in ALK+ Metastatic NSCLC – Cho B, et al • Key results (cont. ) – Based on all treated patients (n=265), the overall AE profile was similar among the three treatment arms except for a lower proportion of GI toxicities in the 450 mg fed arm Ceritinib 450 mg fed (n=89) Ceritinib 600 mg fed (n=86) Ceritinib 750 mg fasted (n=90) GI AEs leading to study drug discontinuation, n (%) Nausea Vomiting Diarrhoea 0 0 0 1 (1. 2) 0 0 GI AEs requiring dose adjustments, n (%) Nausea Diarrhoea Vomiting 0 0 0 6 (7. 0) 3 (3. 5) 4 (4. 4) 6 (6. 7) 1 (1. 1) 0 9 (10. 5) 11 (12. 8) 10 (11. 6) 16 (17. 8) 11 (12. 2) 10 (11. 1) GI AE requiring dose adjustment/study drug interruption, n (%) Diarrhoea Nausea Vomiting • Conclusion – Ceritinib 450 mg administered with food might offer similar efficacy to 750 mg fasted dose with better GI toxicity Cho B et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 05. 07

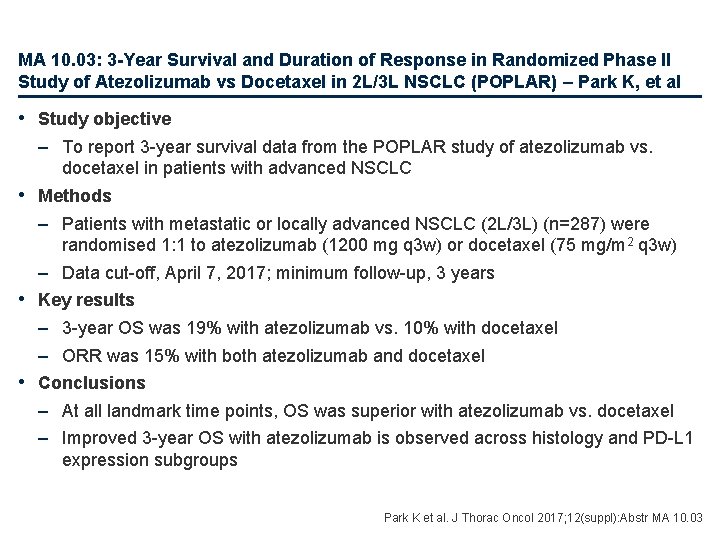
MA 10. 03: 3 -Year Survival and Duration of Response in Randomized Phase II Study of Atezolizumab vs Docetaxel in 2 L/3 L NSCLC (POPLAR) – Park K, et al • Study objective – To report 3 -year survival data from the POPLAR study of atezolizumab vs. docetaxel in patients with advanced NSCLC • Methods – Patients with metastatic or locally advanced NSCLC (2 L/3 L) (n=287) were randomised 1: 1 to atezolizumab (1200 mg q 3 w) or docetaxel (75 mg/m 2 q 3 w) – Data cut-off, April 7, 2017; minimum follow-up, 3 years • Key results – 3 -year OS was 19% with atezolizumab vs. 10% with docetaxel – ORR was 15% with both atezolizumab and docetaxel • Conclusions – At all landmark time points, OS was superior with atezolizumab vs. docetaxel – Improved 3 -year OS with atezolizumab is observed across histology and PD-L 1 expression subgroups Park K et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 10. 03

MA 10. 11: Hyperprogressive Disease (HPD) Is Frequent in Non-Small Cell Lung Cancer (NSCLC) Patients (Pts) Treated with Anti PD 1/PD-L 1 Agents (IO) – Ferrara R, et al • Study objective – To explore the rate and prognostic value of hyperprogressive disease (HPD) in a large cohort of patients with advanced NSCLC treated with anti-PD-1/PD-L 1 agents or chemotherapy • Methods – Multicentre retrospective analysis of patients with advanced NSCLC treated with anti-PD-1/PD-L 1 agents (n=406) or single agent chemotherapy (n=59) – Tumour growth rate (TGR) was calculated before and during treatment – HPD was defined as a ≥ 2 -fold increase in TGR vs. before treatment and PD at first CT scan during treatment Ferrara R et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 10. 11

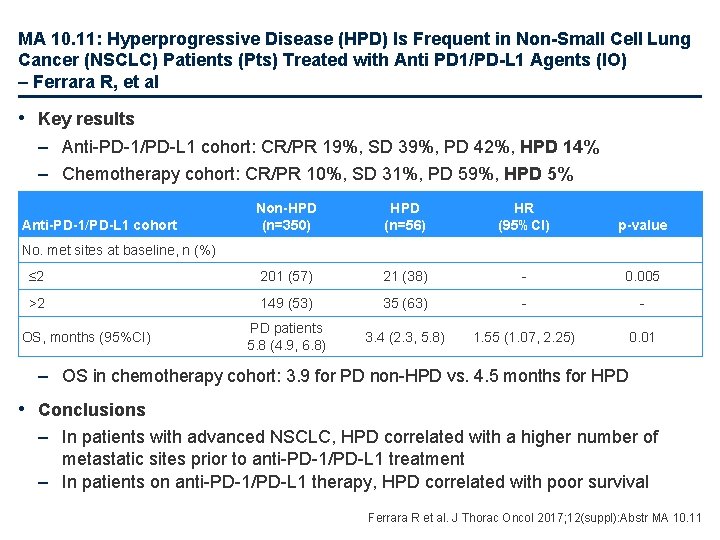
MA 10. 11: Hyperprogressive Disease (HPD) Is Frequent in Non-Small Cell Lung Cancer (NSCLC) Patients (Pts) Treated with Anti PD 1/PD-L 1 Agents (IO) – Ferrara R, et al • Key results – Anti-PD-1/PD-L 1 cohort: CR/PR 19%, SD 39%, PD 42%, HPD 14% – Chemotherapy cohort: CR/PR 10%, SD 31%, PD 59%, HPD 5% Non-HPD (n=350) HPD (n=56) HR (95%CI) p-value ≤ 2 201 (57) 21 (38) - 0. 005 >2 149 (53) 35 (63) - - PD patients 5. 8 (4. 9, 6. 8) 3. 4 (2. 3, 5. 8) 1. 55 (1. 07, 2. 25) 0. 01 Anti-PD-1/PD-L 1 cohort No. met sites at baseline, n (%) OS, months (95%CI) – OS in chemotherapy cohort: 3. 9 for PD non-HPD vs. 4. 5 months for HPD • Conclusions – In patients with advanced NSCLC, HPD correlated with a higher number of metastatic sites prior to anti-PD-1/PD-L 1 treatment – In patients on anti-PD-1/PD-L 1 therapy, HPD correlated with poor survival Ferrara R et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 10. 11

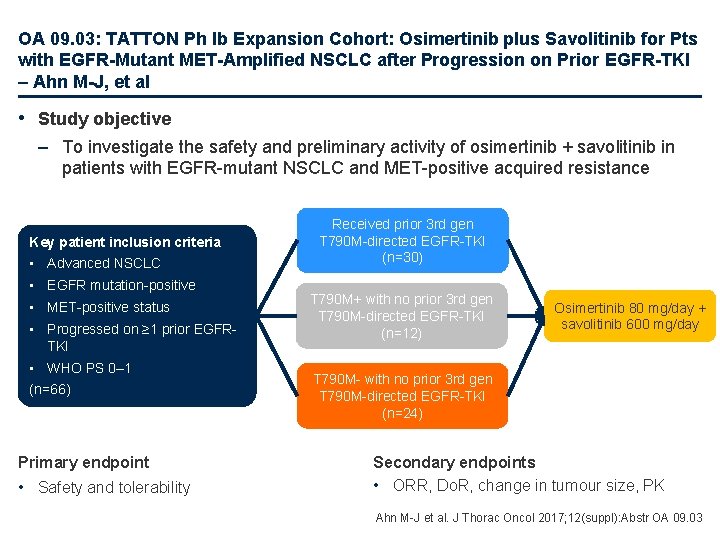
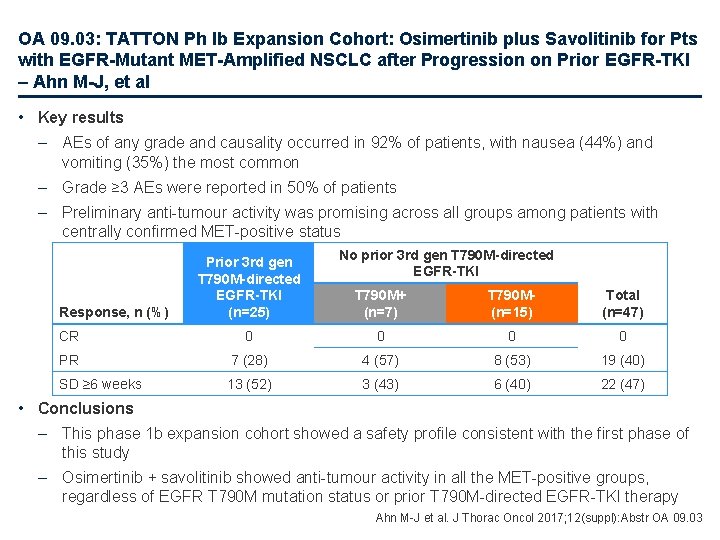
OA 09. 03: TATTON Ph Ib Expansion Cohort: Osimertinib plus Savolitinib for Pts with EGFR-Mutant MET-Amplified NSCLC after Progression on Prior EGFR-TKI – Ahn M-J, et al • Study objective – To investigate the safety and preliminary activity of osimertinib + savolitinib in patients with EGFR-mutant NSCLC and MET-positive acquired resistance Key patient inclusion criteria • Advanced NSCLC • EGFR mutation-positive • MET-positive status • Progressed on ≥ 1 prior EGFRTKI • WHO PS 0– 1 (n=66) Primary endpoint • Safety and tolerability Received prior 3 rd gen T 790 M-directed EGFR-TKI (n=30) T 790 M+ with no prior 3 rd gen T 790 M-directed EGFR-TKI (n=12) Osimertinib 80 mg/day + savolitinib 600 mg/day T 790 M- with no prior 3 rd gen T 790 M-directed EGFR-TKI (n=24) Secondary endpoints • ORR, Do. R, change in tumour size, PK Ahn M-J et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 09. 03

OA 09. 03: TATTON Ph Ib Expansion Cohort: Osimertinib plus Savolitinib for Pts with EGFR-Mutant MET-Amplified NSCLC after Progression on Prior EGFR-TKI – Ahn M-J, et al • Key results – AEs of any grade and causality occurred in 92% of patients, with nausea (44%) and vomiting (35%) the most common – Grade ≥ 3 AEs were reported in 50% of patients – Preliminary anti-tumour activity was promising across all groups among patients with centrally confirmed MET-positive status Response, n (%) Prior 3 rd gen T 790 M-directed EGFR-TKI (n=25) No prior 3 rd gen T 790 M-directed EGFR-TKI T 790 M+ (n=7) T 790 M(n=15) Total (n=47) CR 0 0 PR 7 (28) 4 (57) 8 (53) 19 (40) SD ≥ 6 weeks 13 (52) 3 (43) 6 (40) 22 (47) • Conclusions – This phase 1 b expansion cohort showed a safety profile consistent with the first phase of this study – Osimertinib + savolitinib showed anti-tumour activity in all the MET-positive groups, regardless of EGFR T 790 M mutation status or prior T 790 M-directed EGFR-TKI therapy Ahn M-J et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 09. 03

OA 17. 07: Long-Term Survival in Atezolizumab-Treated Patients with 2 L+ NSCLC from Ph III Randomized OAK Study – Satouchi M, et al • Study objective – To evaluate the characteristics of long-term survivors (LTS) with NSCLC receiving cancer immunotherapy in the OAK study • Methods – Phase 3 randomised controlled trial of atezolizumab 1200 mg q 3 w vs. docetaxel 75 mg/m 2 in patients with locally advanced or metastatic NSCLC who had received 1− 2 prior lines of chemotherapy (at least 1 platinum-based) – LTS were patients who lived ≥ 24 months from randomisation • Key results – 2 -year OS was 31% with atezolizumab vs. 21% with docetaxel – Atezolizumab LTS were not limited to radiographic responders; 21% atezolizumab vs. 9% docetaxel LTS had PD as best overall response • Conclusions – Long-term survival of patients with NSCLC is better with atezolizumab than docetaxel – This benefit was consistent across histologies (squamous/non-squamous) and PD-L 1 expression Satouchi M et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 07

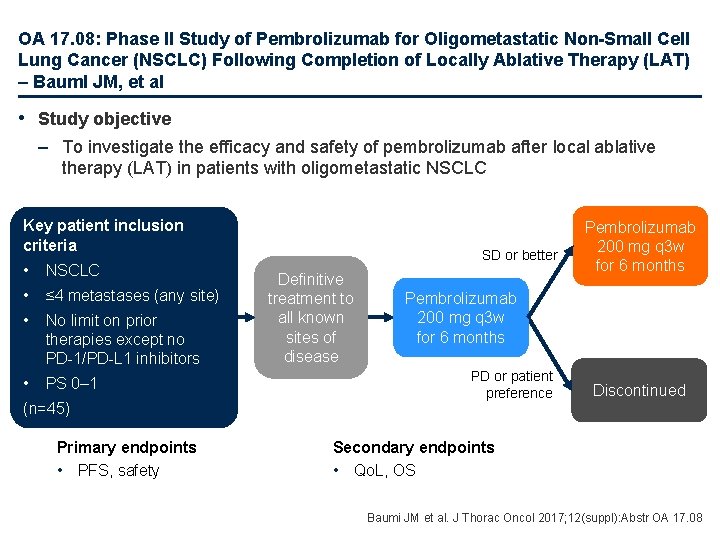
OA 17. 08: Phase II Study of Pembrolizumab for Oligometastatic Non-Small Cell Lung Cancer (NSCLC) Following Completion of Locally Ablative Therapy (LAT) – Bauml JM, et al • Study objective – To investigate the efficacy and safety of pembrolizumab after local ablative therapy (LAT) in patients with oligometastatic NSCLC Key patient inclusion criteria • NSCLC • ≤ 4 metastases (any site) • No limit on prior therapies except no PD-1/PD-L 1 inhibitors • PS 0– 1 (n=45) Primary endpoints • PFS, safety SD or better Definitive treatment to all known sites of disease Pembrolizumab 200 mg q 3 w for 6 months PD or patient preference Discontinued Secondary endpoints • Qo. L, OS Baumi JM et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 08

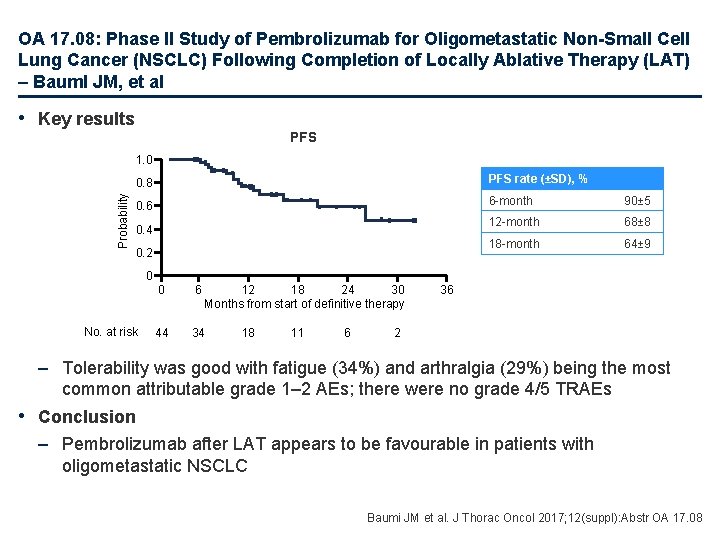
OA 17. 08: Phase II Study of Pembrolizumab for Oligometastatic Non-Small Cell Lung Cancer (NSCLC) Following Completion of Locally Ablative Therapy (LAT) – Bauml JM, et al • Key results PFS Probability 1. 0 0. 8 PFS rate (±SD), % 0. 6 6 -month 90± 5 12 -month 68± 8 18 -month 64± 9 0. 4 0. 2 0 No. at risk 0 6 12 18 24 30 Months from start of definitive therapy 44 34 18 11 6 36 2 – Tolerability was good with fatigue (34%) and arthralgia (29%) being the most common attributable grade 1– 2 AEs; there were no grade 4/5 TRAEs • Conclusion – Pembrolizumab after LAT appears to be favourable in patients with oligometastatic NSCLC Baumi JM et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 17. 08

Other malignancies SCLC, mesothelioma and thymic epithelial tumours

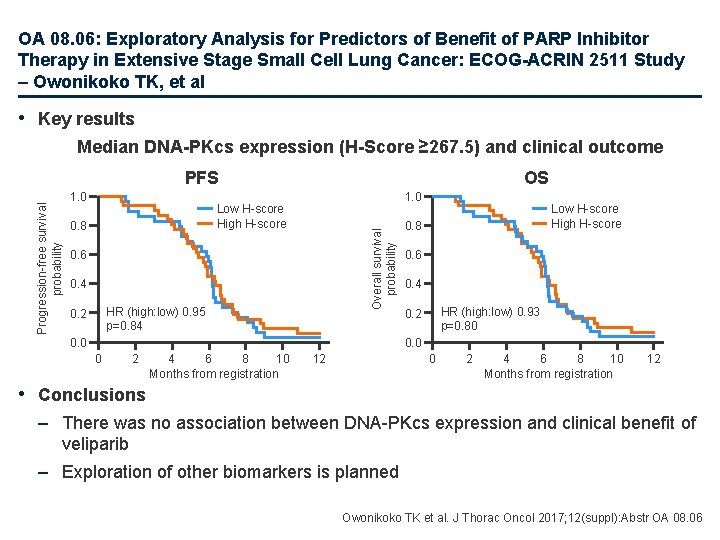
OA 08. 06: Exploratory Analysis for Predictors of Benefit of PARP Inhibitor Therapy in Extensive Stage Small Cell Lung Cancer: ECOG-ACRIN 2511 Study – Owonikoko TK, et al • Study objective – To explore whether tissue expression of DNA-protein kinase (DNA-PKcs) is associated with clinical benefit of veliparib as 1 L therapy in patients with extensive stage SCLC • Methods – Post-hoc analysis of archival tissue samples from the E 2511 study, which included patients with previously untreated, extensive stage SCLC • 63/147 (51%) cases had available samples and were included in the analysis – Immunohistochemistry was used to determine DNA-PKcs expression, and association with clinical outcomes, PFS and OS, was assessed – The study had 82% power to detect a PFS HR of 0. 5 comparing biomarker positive to biomarker negative patients at a one-sided 0. 05 level log-rank test Owonikoko TK et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 08. 06

OA 08. 06: Exploratory Analysis for Predictors of Benefit of PARP Inhibitor Therapy in Extensive Stage Small Cell Lung Cancer: ECOG-ACRIN 2511 Study – Owonikoko TK, et al • Key results Median DNA-PKcs expression (H-Score ≥ 267. 5) and clinical outcome 1. 0 OS 1. 0 Low H-score High H-score 0. 8 Overall survival probability Progression-free survival probability PFS 0. 6 0. 4 HR (high: low) 0. 95 p=0. 84 0. 2 0. 0 Low H-score High H-score 0. 8 0. 6 0. 4 HR (high: low) 0. 93 p=0. 80 0. 2 0. 0 0 2 4 6 8 10 Months from registration 12 • Conclusions – There was no association between DNA-PKcs expression and clinical benefit of veliparib – Exploration of other biomarkers is planned Owonikoko TK et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 08. 06

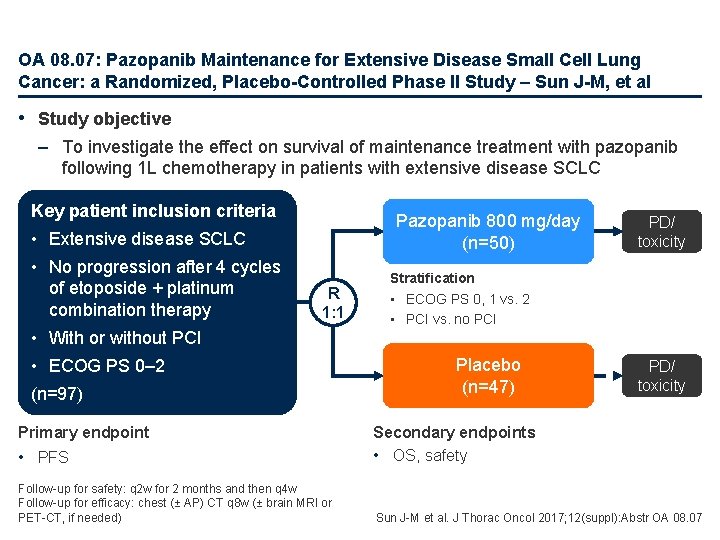
OA 08. 07: Pazopanib Maintenance for Extensive Disease Small Cell Lung Cancer: a Randomized, Placebo-Controlled Phase II Study – Sun J-M, et al • Study objective – To investigate the effect on survival of maintenance treatment with pazopanib following 1 L chemotherapy in patients with extensive disease SCLC Key patient inclusion criteria Pazopanib 800 mg/day (n=50) • Extensive disease SCLC • No progression after 4 cycles of etoposide + platinum combination therapy R 1: 1 • With or without PCI • ECOG PS 0– 2 (n=97) Primary endpoint PD/ toxicity Stratification • ECOG PS 0, 1 vs. 2 • PCI vs. no PCI Placebo (n=47) PD/ toxicity • PFS Secondary endpoints • OS, safety Follow-up for safety: q 2 w for 2 months and then q 4 w Follow-up for efficacy: chest (± AP) CT q 8 w (± brain MRI or PET-CT, if needed) Sun J-M et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 08. 07

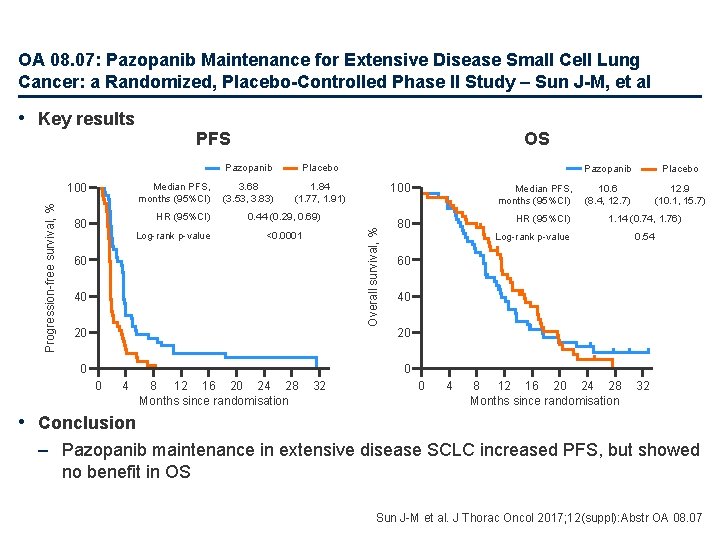
OA 08. 07: Pazopanib Maintenance for Extensive Disease Small Cell Lung Cancer: a Randomized, Placebo-Controlled Phase II Study – Sun J-M, et al Median PFS, months (95%CI) 100 Progression-free survival, % PFS HR (95%CI) 80 Log-rank p-value OS Pazopanib Placebo 3. 68 (3. 53, 3. 83) 1. 84 (1. 77, 1. 91) 0. 44 (0. 29, 0. 69) Overall survival, % • Key results <0. 0001 60 40 20 0 100 Median PFS, months (95%CI) 80 HR (95%CI) Pazopanib Placebo 10. 6 (8. 4, 12. 7) 12. 9 (10. 1, 15. 7) 1. 14 (0. 74, 1. 76) Log-rank p-value 0. 54 60 40 20 0 0 4 8 12 16 20 24 28 Months since randomisation 32 • Conclusion – Pazopanib maintenance in extensive disease SCLC increased PFS, but showed no benefit in OS Sun J-M et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 08. 07

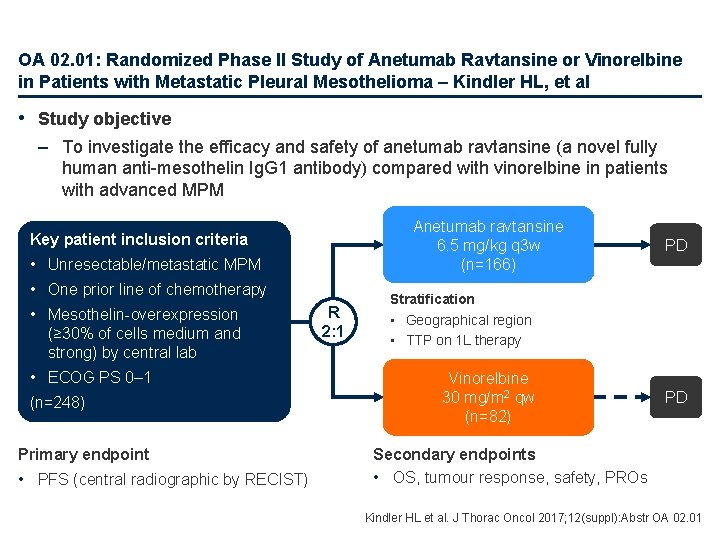
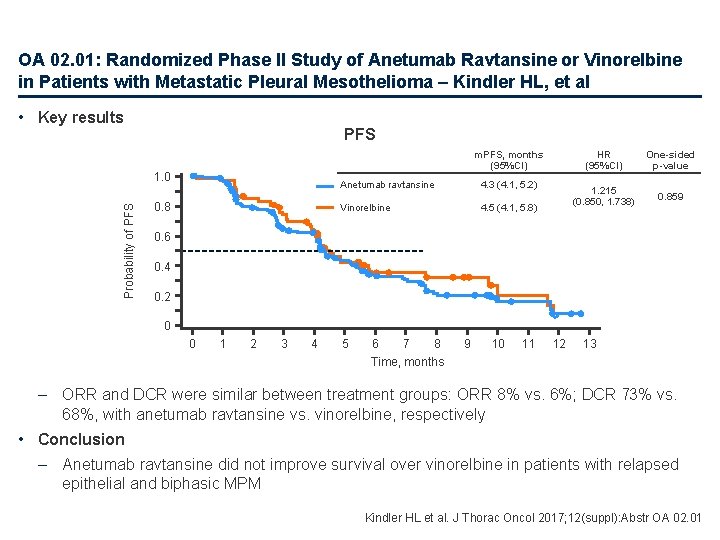
OA 02. 01: Randomized Phase II Study of Anetumab Ravtansine or Vinorelbine in Patients with Metastatic Pleural Mesothelioma – Kindler HL, et al • Study objective – To investigate the efficacy and safety of anetumab ravtansine (a novel fully human anti-mesothelin Ig. G 1 antibody) compared with vinorelbine in patients with advanced MPM Anetumab ravtansine 6. 5 mg/kg q 3 w (n=166) Key patient inclusion criteria • Unresectable/metastatic MPM • One prior line of chemotherapy • Mesothelin-overexpression (≥ 30% of cells medium and strong) by central lab • ECOG PS 0– 1 (n=248) Primary endpoint • PFS (central radiographic by RECIST) R 2: 1 PD Stratification • Geographical region • TTP on 1 L therapy Vinorelbine 30 mg/m 2 qw (n=82) PD Secondary endpoints • OS, tumour response, safety, PROs Kindler HL et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 02. 01

OA 02. 01: Randomized Phase II Study of Anetumab Ravtansine or Vinorelbine in Patients with Metastatic Pleural Mesothelioma – Kindler HL, et al • Key results PFS m. PFS, months (95%CI) Probability of PFS 1. 0 0. 8 Anetumab ravtansine 4. 3 (4. 1, 5. 2) Vinorelbine 4. 5 (4. 1, 5. 8) HR (95%CI) One-sided p-value 1. 215 (0. 850, 1. 738) 0. 859 0. 6 0. 4 0. 2 0 0 1 2 3 4 5 6 7 8 Time, months 9 10 11 12 13 – ORR and DCR were similar between treatment groups: ORR 8% vs. 6%; DCR 73% vs. 68%, with anetumab ravtansine vs. vinorelbine, respectively • Conclusion – Anetumab ravtansine did not improve survival over vinorelbine in patients with relapsed epithelial and biphasic MPM Kindler HL et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 02. 01

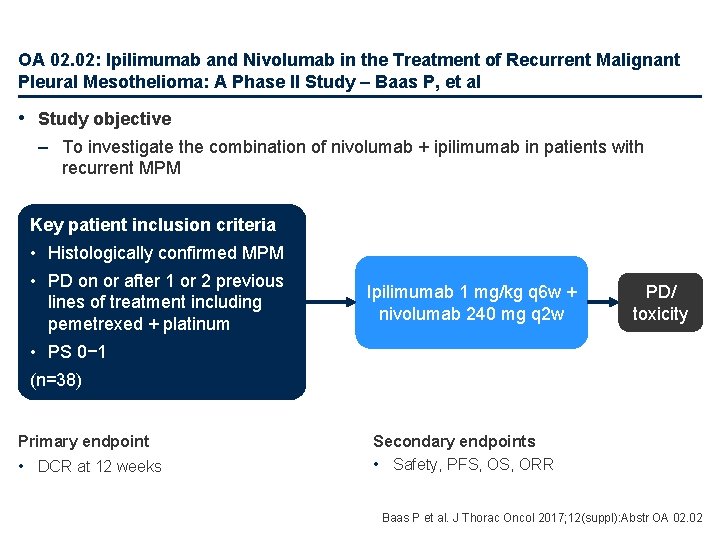
OA 02. 02: Ipilimumab and Nivolumab in the Treatment of Recurrent Malignant Pleural Mesothelioma: A Phase II Study – Baas P, et al • Study objective – To investigate the combination of nivolumab + ipilimumab in patients with recurrent MPM Key patient inclusion criteria • Histologically confirmed MPM • PD on or after 1 or 2 previous lines of treatment including pemetrexed + platinum Ipilimumab 1 mg/kg q 6 w + nivolumab 240 mg q 2 w PD/ toxicity • PS 0− 1 (n=38) Primary endpoint • DCR at 12 weeks Secondary endpoints • Safety, PFS, ORR Baas P et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 02. 02

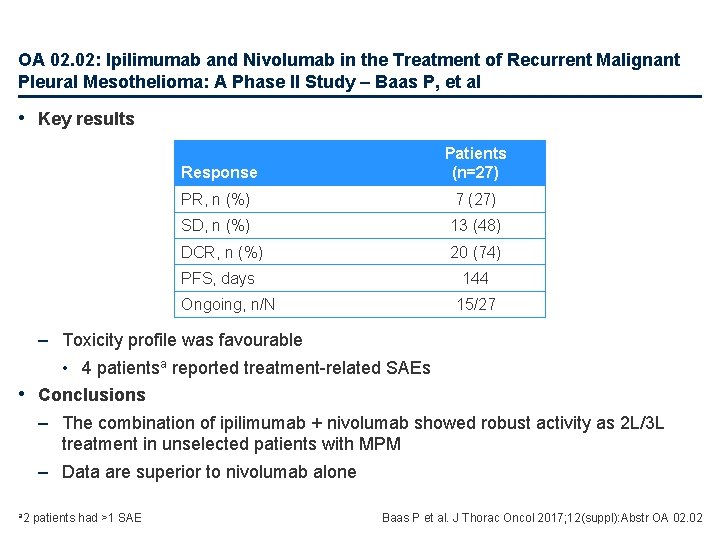
OA 02. 02: Ipilimumab and Nivolumab in the Treatment of Recurrent Malignant Pleural Mesothelioma: A Phase II Study – Baas P, et al • Key results Response Patients (n=27) PR, n (%) 7 (27) SD, n (%) 13 (48) DCR, n (%) 20 (74) PFS, days 144 Ongoing, n/N 15/27 – Toxicity profile was favourable • 4 patientsa reported treatment-related SAEs • Conclusions – The combination of ipilimumab + nivolumab showed robust activity as 2 L/3 L treatment in unselected patients with MPM – Data are superior to nivolumab alone a 2 patients had >1 SAE Baas P et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 02. 02

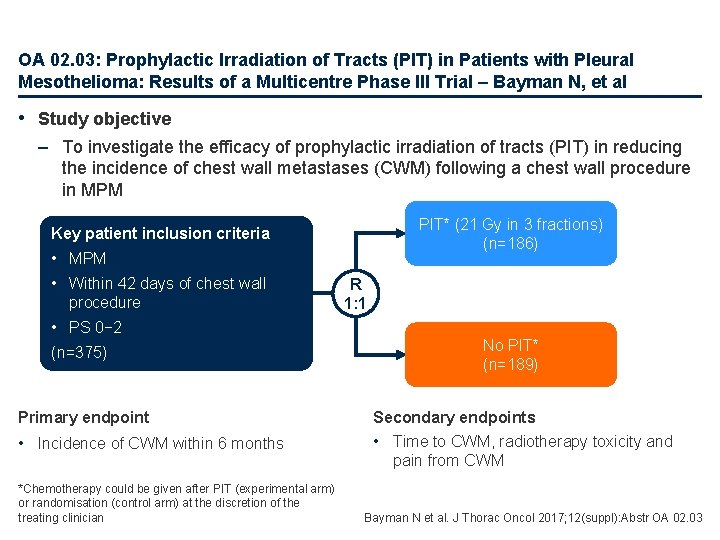
OA 02. 03: Prophylactic Irradiation of Tracts (PIT) in Patients with Pleural Mesothelioma: Results of a Multicentre Phase III Trial – Bayman N, et al • Study objective – To investigate the efficacy of prophylactic irradiation of tracts (PIT) in reducing the incidence of chest wall metastases (CWM) following a chest wall procedure in MPM PIT* (21 Gy in 3 fractions) (n=186) Key patient inclusion criteria • MPM • Within 42 days of chest wall procedure • PS 0− 2 (n=375) Primary endpoint • Incidence of CWM within 6 months *Chemotherapy could be given after PIT (experimental arm) or randomisation (control arm) at the discretion of the treating clinician R 1: 1 No PIT* (n=189) Secondary endpoints • Time to CWM, radiotherapy toxicity and pain from CWM Bayman N et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 02. 03

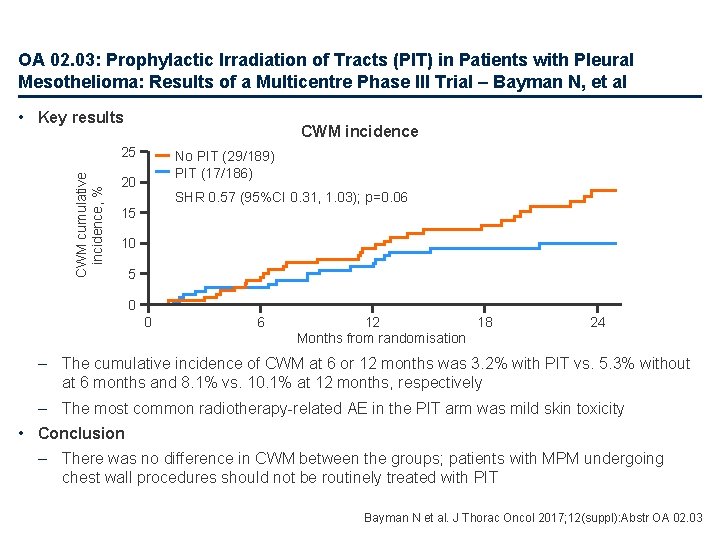
OA 02. 03: Prophylactic Irradiation of Tracts (PIT) in Patients with Pleural Mesothelioma: Results of a Multicentre Phase III Trial – Bayman N, et al • Key results CWM incidence CWM cumulative incidence, % 25 No PIT (29/189) PIT (17/186) 20 SHR 0. 57 (95%CI 0. 31, 1. 03); p=0. 06 15 10 5 0 0 6 12 18 Months from randomisation 24 – The cumulative incidence of CWM at 6 or 12 months was 3. 2% with PIT vs. 5. 3% without at 6 months and 8. 1% vs. 10. 1% at 12 months, respectively – The most common radiotherapy-related AE in the PIT arm was mild skin toxicity • Conclusion – There was no difference in CWM between the groups; patients with MPM undergoing chest wall procedures should not be routinely treated with PIT Bayman N et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 02. 03

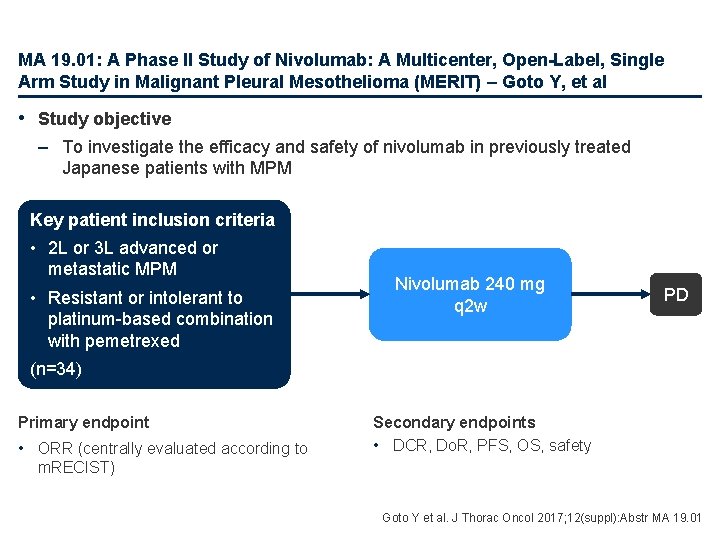
MA 19. 01: A Phase II Study of Nivolumab: A Multicenter, Open-Label, Single Arm Study in Malignant Pleural Mesothelioma (MERIT) – Goto Y, et al • Study objective – To investigate the efficacy and safety of nivolumab in previously treated Japanese patients with MPM Key patient inclusion criteria • 2 L or 3 L advanced or metastatic MPM • Resistant or intolerant to platinum-based combination with pemetrexed Nivolumab 240 mg q 2 w PD (n=34) Primary endpoint • ORR (centrally evaluated according to Secondary endpoints • DCR, Do. R, PFS, OS, safety m. RECIST) Goto Y et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 19. 01

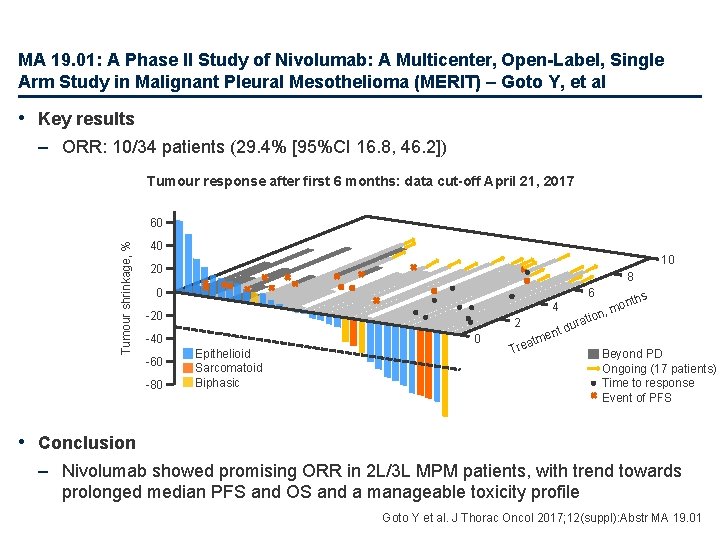
MA 19. 01: A Phase II Study of Nivolumab: A Multicenter, Open-Label, Single Arm Study in Malignant Pleural Mesothelioma (MERIT) – Goto Y, et al • Key results – ORR: 10/34 patients (29. 4% [95%CI 16. 8, 46. 2]) Tumour response after first 6 months: data cut-off April 21, 2017 Tumour shrinkage, % 60 40 10 20 8 0 6 4 -20 2 -40 -60 -80 0 Epithelioid Sarcomatoid Biphasic atm Tre o rati du ent hs ont m n, Beyond PD Ongoing (17 patients) Time to response Event of PFS • Conclusion – Nivolumab showed promising ORR in 2 L/3 L MPM patients, with trend towards prolonged median PFS and OS and a manageable toxicity profile Goto Y et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 19. 01

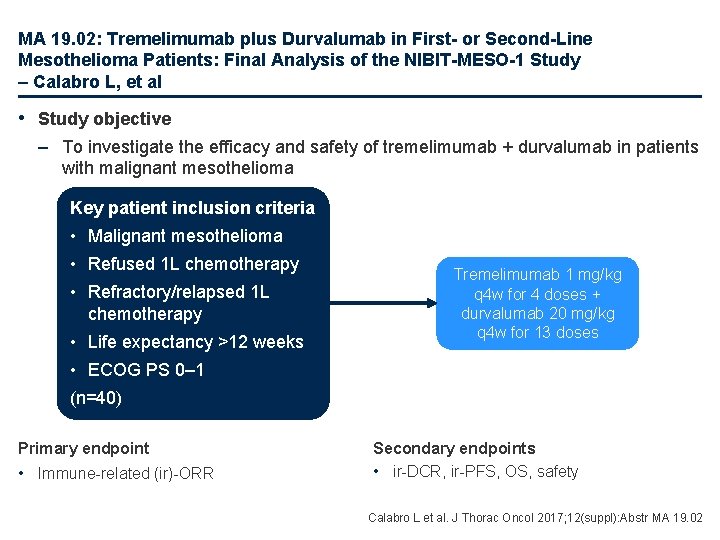
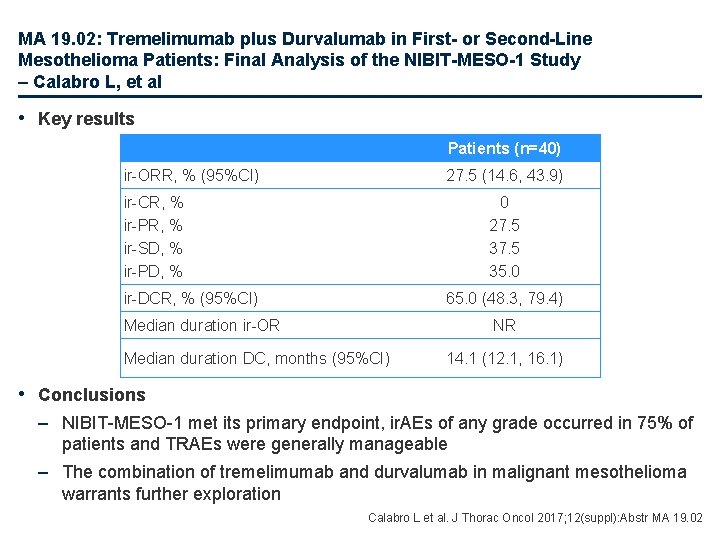
MA 19. 02: Tremelimumab plus Durvalumab in First- or Second-Line Mesothelioma Patients: Final Analysis of the NIBIT-MESO-1 Study – Calabro L, et al • Study objective – To investigate the efficacy and safety of tremelimumab + durvalumab in patients with malignant mesothelioma Key patient inclusion criteria • Malignant mesothelioma • Refused 1 L chemotherapy • Refractory/relapsed 1 L chemotherapy • Life expectancy >12 weeks Tremelimumab 1 mg/kg q 4 w for 4 doses + durvalumab 20 mg/kg q 4 w for 13 doses • ECOG PS 0– 1 (n=40) Primary endpoint • Immune-related (ir)-ORR Secondary endpoints • ir-DCR, ir-PFS, OS, safety Calabro L et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 19. 02

MA 19. 02: Tremelimumab plus Durvalumab in First- or Second-Line Mesothelioma Patients: Final Analysis of the NIBIT-MESO-1 Study – Calabro L, et al • Key results Patients (n=40) ir-ORR, % (95%CI) 27. 5 (14. 6, 43. 9) ir-CR, % ir-PR, % ir-SD, % ir-PD, % 0 27. 5 35. 0 ir-DCR, % (95%CI) 65. 0 (48. 3, 79. 4) Median duration ir-OR NR Median duration DC, months (95%CI) 14. 1 (12. 1, 16. 1) • Conclusions – NIBIT-MESO-1 met its primary endpoint, ir. AEs of any grade occurred in 75% of patients and TRAEs were generally manageable – The combination of tremelimumab and durvalumab in malignant mesothelioma warrants further exploration Calabro L et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 19. 02

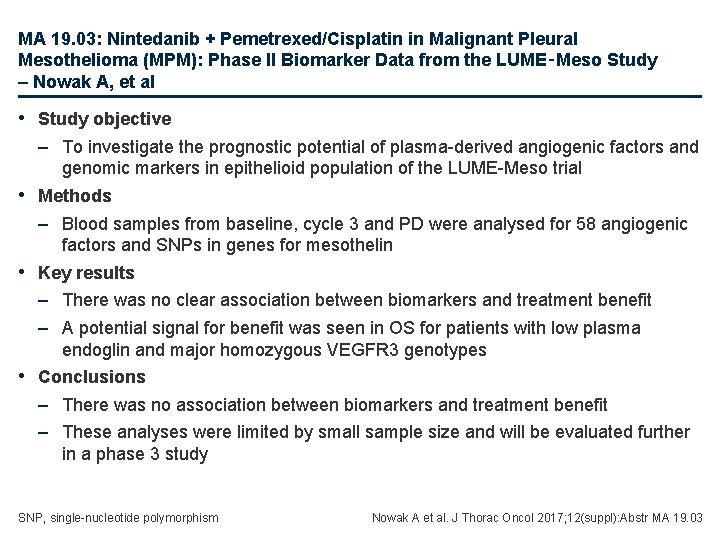
MA 19. 03: Nintedanib + Pemetrexed/Cisplatin in Malignant Pleural Mesothelioma (MPM): Phase II Biomarker Data from the LUME‑Meso Study – Nowak A, et al • Study objective – To investigate the prognostic potential of plasma-derived angiogenic factors and genomic markers in epithelioid population of the LUME-Meso trial • Methods – Blood samples from baseline, cycle 3 and PD were analysed for 58 angiogenic factors and SNPs in genes for mesothelin • Key results – There was no clear association between biomarkers and treatment benefit – A potential signal for benefit was seen in OS for patients with low plasma endoglin and major homozygous VEGFR 3 genotypes • Conclusions – There was no association between biomarkers and treatment benefit – These analyses were limited by small sample size and will be evaluated further in a phase 3 study SNP, single-nucleotide polymorphism Nowak A et al. J Thorac Oncol 2017; 12(suppl): Abstr MA 19. 03

OA 03. 02: Comprehensive Characterization of Thymic Epithelial Tumour Subtypes Through an Analysis of Somatic Mutations and Copy Number Alterations – Gennatas S, et al • Study objective – To identify mutations in thymic epithelial tumours (TETs) beyond the only known recurrent mutation that occurs in GTF 21 • Methods – Sanger sequencing was used to determine the frequency of the GTF 2 I mutation on DNA from 144 TETs of all subtypes – Whole exome sequencing was performed on 17 TETs of different subtypes (2 AB, 1 B 1, 3 B 2, 2 B 3, 6 CA and 3 NETT) and matched normal tissue – SNP genotyping was performed on 100 TETs of all subtypes with matched normal tissue to detect copy number alterations Gennatas S et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 03. 02

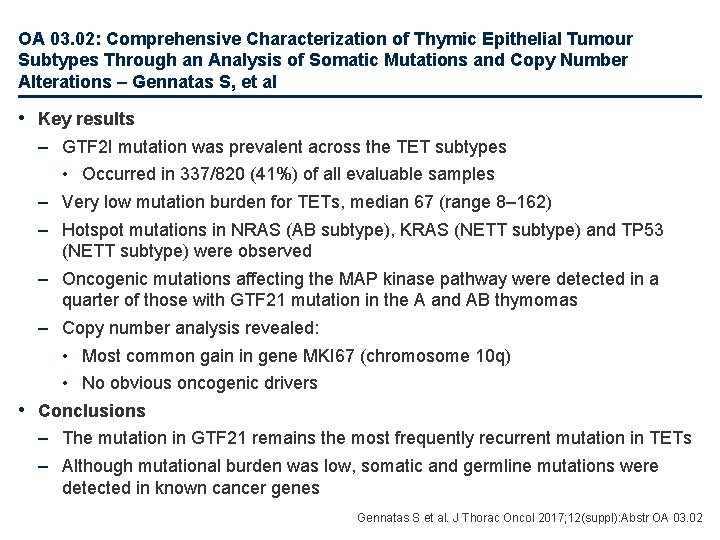
OA 03. 02: Comprehensive Characterization of Thymic Epithelial Tumour Subtypes Through an Analysis of Somatic Mutations and Copy Number Alterations – Gennatas S, et al • Key results – GTF 2 I mutation was prevalent across the TET subtypes • Occurred in 337/820 (41%) of all evaluable samples – Very low mutation burden for TETs, median 67 (range 8– 162) – Hotspot mutations in NRAS (AB subtype), KRAS (NETT subtype) and TP 53 (NETT subtype) were observed – Oncogenic mutations affecting the MAP kinase pathway were detected in a quarter of those with GTF 21 mutation in the A and AB thymomas – Copy number analysis revealed: • Most common gain in gene MKI 67 (chromosome 10 q) • No obvious oncogenic drivers • Conclusions – The mutation in GTF 21 remains the most frequently recurrent mutation in TETs – Although mutational burden was low, somatic and germline mutations were detected in known cancer genes Gennatas S et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 03. 02

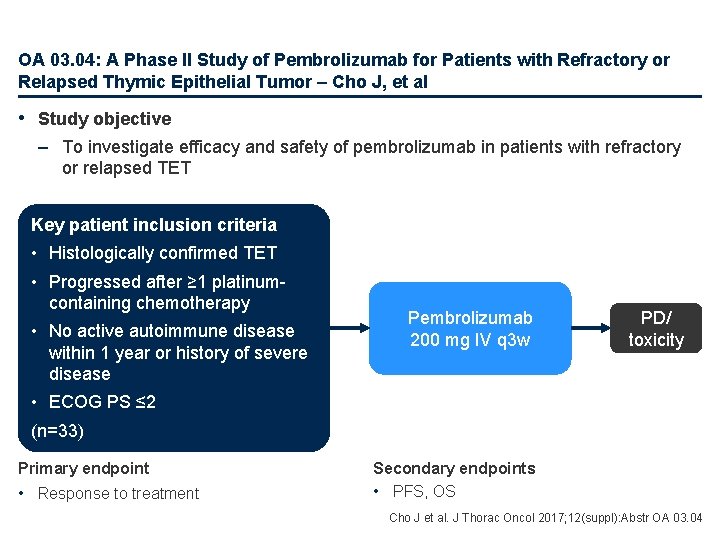
OA 03. 04: A Phase II Study of Pembrolizumab for Patients with Refractory or Relapsed Thymic Epithelial Tumor – Cho J, et al • Study objective – To investigate efficacy and safety of pembrolizumab in patients with refractory or relapsed TET Key patient inclusion criteria • Histologically confirmed TET • Progressed after ≥ 1 platinumcontaining chemotherapy • No active autoimmune disease within 1 year or history of severe disease Pembrolizumab 200 mg IV q 3 w PD/ toxicity • ECOG PS ≤ 2 (n=33) Primary endpoint • Response to treatment Secondary endpoints • PFS, OS Cho J et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 03. 04

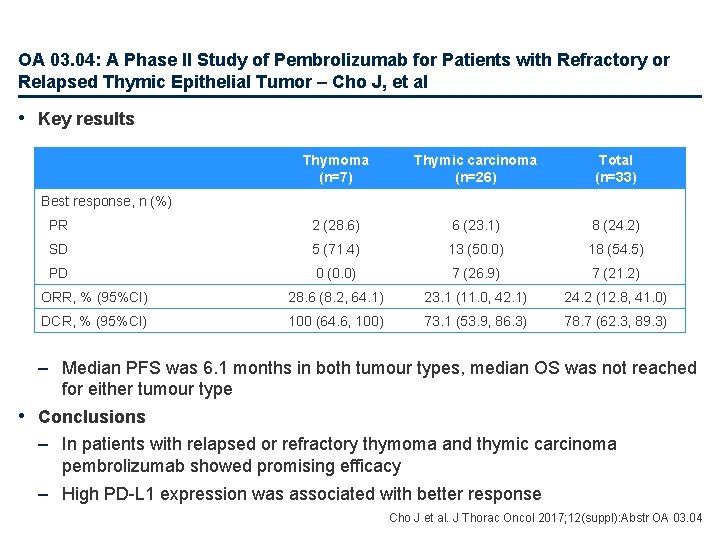
OA 03. 04: A Phase II Study of Pembrolizumab for Patients with Refractory or Relapsed Thymic Epithelial Tumor – Cho J, et al • Key results Thymoma (n=7) Thymic carcinoma (n=26) Total (n=33) PR 2 (28. 6) 6 (23. 1) 8 (24. 2) SD 5 (71. 4) 13 (50. 0) 18 (54. 5) PD 0 (0. 0) 7 (26. 9) 7 (21. 2) ORR, % (95%CI) 28. 6 (8. 2, 64. 1) 23. 1 (11. 0, 42. 1) 24. 2 (12. 8, 41. 0) DCR, % (95%CI) 100 (64. 6, 100) 73. 1 (53. 9, 86. 3) 78. 7 (62. 3, 89. 3) Best response, n (%) – Median PFS was 6. 1 months in both tumour types, median OS was not reached for either tumour type • Conclusions – In patients with relapsed or refractory thymoma and thymic carcinoma pembrolizumab showed promising efficacy – High PD-L 1 expression was associated with better response Cho J et al. J Thorac Oncol 2017; 12(suppl): Abstr OA 03. 04
 European delirium association
European delirium association European diagnostic manufacturers association
European diagnostic manufacturers association European parents association
European parents association European university association
European university association Ema magnetism
Ema magnetism European biomass industry association
European biomass industry association European diy retail association
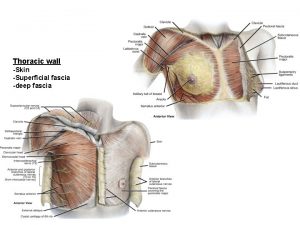
European diy retail association Horizontal fissure of right lung
Horizontal fissure of right lung Jugular notches
Jugular notches Surface anatomy of the breast
Surface anatomy of the breast Thoracic duct
Thoracic duct Median sacral crest
Median sacral crest Neurogeen thoracic outlet syndroom
Neurogeen thoracic outlet syndroom Body planes directions and cavities worksheet
Body planes directions and cavities worksheet Which of the following elevates the ribs?
Which of the following elevates the ribs? Thoracic configuration
Thoracic configuration Thoracic inlet x ray
Thoracic inlet x ray Corpus callosum anatomy radiology
Corpus callosum anatomy radiology Iwbat definition
Iwbat definition Thorax
Thorax Auscultatory triangle of the back
Auscultatory triangle of the back Inguinal lymph nodes
Inguinal lymph nodes Sternal angle of louis
Sternal angle of louis Thoracic aorta supplies blood to
Thoracic aorta supplies blood to Lumbar referral patterns
Lumbar referral patterns Thoracic surgeon
Thoracic surgeon Thoracic duct
Thoracic duct Rajesh shah thoracic surgeon
Rajesh shah thoracic surgeon Thoracic cavity and abdominal cavity
Thoracic cavity and abdominal cavity Superficial fascia of thorax
Superficial fascia of thorax Pouchlike structure at the start of the thoracic duct
Pouchlike structure at the start of the thoracic duct Diaphragm thoracic level
Diaphragm thoracic level Aorta branch
Aorta branch Thoracic surgery
Thoracic surgery False vertebrae
False vertebrae Muscles of thoracic spine
Muscles of thoracic spine Thoracic cage anterior view
Thoracic cage anterior view Middle mediastinum: contents mnemonic
Middle mediastinum: contents mnemonic Dorsal cavity
Dorsal cavity Thoracic membranes
Thoracic membranes Soft, spongy, cone-shaped organs in the thoracic cavity.
Soft, spongy, cone-shaped organs in the thoracic cavity. Pig thoracic cavity diagram
Pig thoracic cavity diagram Thoracic cavity
Thoracic cavity Costal facet
Costal facet Chest wall anatomy
Chest wall anatomy Thorax anatomy
Thorax anatomy Thoracic vertebrae not labeled
Thoracic vertebrae not labeled Thoracic kyphosis
Thoracic kyphosis Thoracic nerves
Thoracic nerves Venous drainage of thoracic wall
Venous drainage of thoracic wall Lateral thoracic vessels
Lateral thoracic vessels Thoracic cavity labeled
Thoracic cavity labeled Volume of thoracic cavity
Volume of thoracic cavity Sacral counterstrain points
Sacral counterstrain points Thoracic outlet wedge pillow
Thoracic outlet wedge pillow Scheuermann’s disease
Scheuermann’s disease Venturi mask uses
Venturi mask uses Atheromatous thoracic aorta
Atheromatous thoracic aorta Lumbar vertebrae characteristics
Lumbar vertebrae characteristics Thoracic inlet anatomy
Thoracic inlet anatomy Lateral raphe of thoracolumbar fascia
Lateral raphe of thoracolumbar fascia Rib tubercle
Rib tubercle Chest cavity
Chest cavity Thalasemia
Thalasemia Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Dot
Dot Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Phản ứng thế ankan
Phản ứng thế ankan Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan điện thế nghỉ
điện thế nghỉ Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Lp html
Lp html Hệ hô hấp
Hệ hô hấp Các số nguyên tố
Các số nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Glasgow thang điểm
Glasgow thang điểm ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết