EDMA Presentation 2007 European Diagnostic Manufacturers Association JeanFranois








- Slides: 11

EDMA Presentation 2007 European Diagnostic Manufacturers Association Jean-François de Lavison President of EDMA Mérieux Alliance Geneva, Retooling Task Force January, 2008

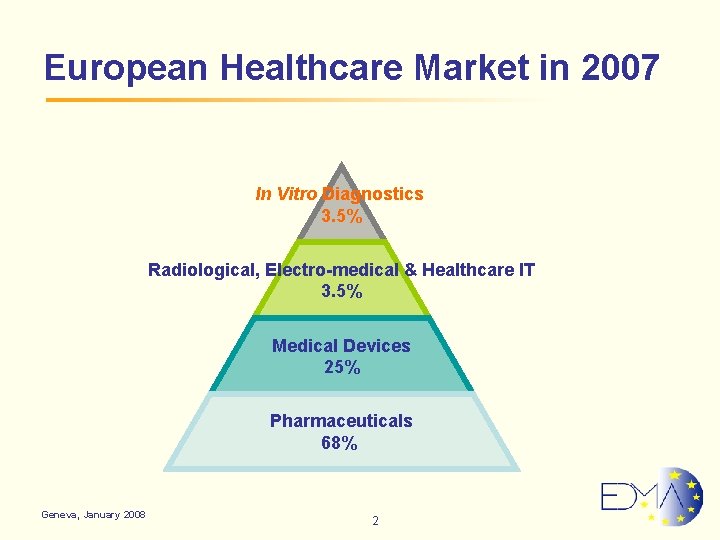
European Healthcare Market in 2007 In Vitro Diagnostics 3. 5% Radiological, Electro-medical & Healthcare IT 3. 5% Medical Devices 25% Pharmaceuticals 68% Geneva, January 2008 2

EDMA Members 30 Corporate Associated Members From 15 to 30 2006 Cellestis Chiron Genzyme Tosoh 2007 Biosystems FIND Diagnostics Innogenetics Luminex Mallinkrodt MLT Orion Randox Siemens Diagnostics STAGO TECAN Philips Geneva, January 2008 3

EDMA Members 21 National Association Members Working to develop new IVD associations From 20 to 21 2007 Turkey Geneva, January 2008 4

EDMA Mission § To raise awareness of the added-value that diagnostic information can provide to health, § To support an appropriate regulatory system, § To work towards a realistic economic environment for healthcare, and § To be an effective voice in globalisation. Geneva, January 2008 5

Advocating the value of IVDs European Diagnostic Manufacturers Association

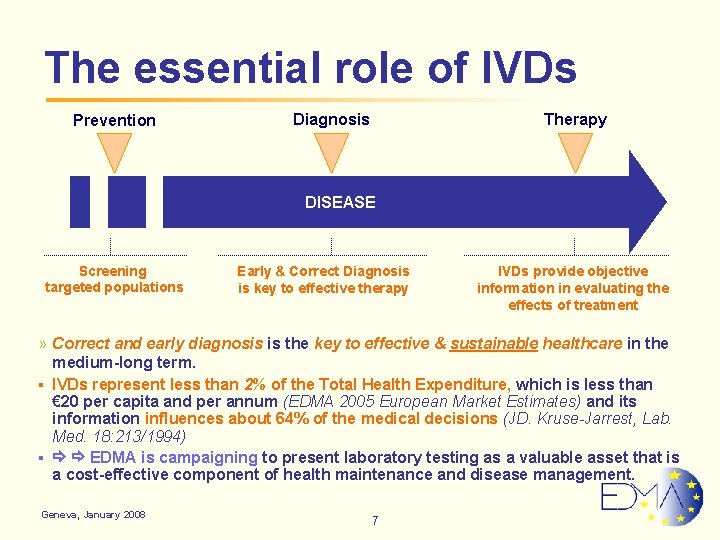
The essential role of IVDs Prevention Therapy Diagnosis DISEASE Screening targeted populations Early & Correct Diagnosis is key to effective therapy IVDs provide objective information in evaluating the effects of treatment » Correct and early diagnosis is the key to effective & sustainable healthcare in the medium-long term. § IVDs represent less than 2% of the Total Health Expenditure, which is less than € 20 per capita and per annum (EDMA 2005 European Market Estimates) and its information influences about 64% of the medical decisions (JD. Kruse-Jarrest, Lab. Med. 18: 213/1994) § EDMA is campaigning to present laboratory testing as a valuable asset that is a cost-effective component of health maintenance and disease management. Geneva, January 2008 7

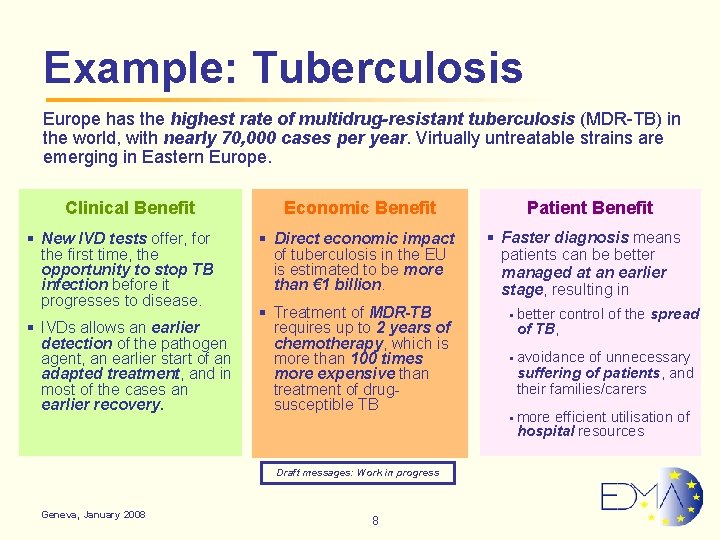
Example: Tuberculosis Europe has the highest rate of multidrug-resistant tuberculosis (MDR-TB) in the world, with nearly 70, 000 cases per year. Virtually untreatable strains are emerging in Eastern Europe. Clinical Benefit § New IVD tests offer, for the first time, the opportunity to stop TB infection before it progresses to disease. § IVDs allows an earlier detection of the pathogen agent, an earlier start of an adapted treatment, and in most of the cases an earlier recovery. Economic Benefit § Direct economic impact of tuberculosis in the EU is estimated to be more than € 1 billion. § Treatment of MDR-TB requires up to 2 years of chemotherapy, which is more than 100 times more expensive than treatment of drugsusceptible TB Draft messages: Work in progress Geneva, January 2008 8 Patient Benefit § Faster diagnosis means patients can be better managed at an earlier stage, resulting in § better of TB, control of the spread § avoidance of unnecessary suffering of patients, and their families/carers § more efficient utilisation of hospital resources

TB Advocacy Actions § Mar 07, Brussels: ECDC Scientific Seminar » EDMA represented IVD sector interests » Contacts established with WHO, Stop TB Partnership, ECDC, etc. § Follow-up Meetings with all stakeholders » Objective: Discuss common actions to define and implement appropriate measures to fight TB § 27 -28 Sep 07, Lisbon: Portuguese Presidency Conference on ‘Health & Migration’ - EDMA to attend and propose participation § 22 October 2007, Berlin: High Level Ministerial Forum “TB is a regional emergency” » Organised by WHO-Europe, in cooperation with the EU, ECDC, Stop TB Partnership, among other stakeholders - EDMA to attend and propose a speaker - Provide input for the future WHO TB Action Plan for Europe Geneva, January 2008 9

Lab Tests Online Soon available in Italian 18 -19 June, Turin Spain: www. Lab. Tests. Online. es Poland: www. Lab. Tests. Online. pl in Hungarian September Combined Traffic: Over 35, 000 unique visitors in May Also available in the future in Portuguese and Czech Germany: www. Lab. Tests. Online. de Geneva, January 2008 + Global access to all sites 10

European Diagnostic Manufacturers Association Place des Maïeurs 2 1150 Brussels Belgium Tel +32 2 772 22 25 Fax +32 2 772 23 29 edma@edma-ivd. be EDMA represents the In Vitro Diagnostics Industry in Europe Visit our website: www. edma-ivd. be
 European diagnostic manufacturers association
European diagnostic manufacturers association Edma memory
Edma memory Lapratory
Lapratory Fiche edma sncf
Fiche edma sncf Edma ivd
Edma ivd Builders hardware manufacturers association
Builders hardware manufacturers association Type ac cable
Type ac cable Plastics machinery manufacturers association of india
Plastics machinery manufacturers association of india Indian pharma association
Indian pharma association Astro foil r value
Astro foil r value European diy retail association
European diy retail association European university association
European university association