Developed in association with the European Thoracic Oncology












![ORAL 02. 05: Safety and Efficacy of First-Line Nivolumab (NIVO; Anti. Programmed Death-1 [PD-1]) ORAL 02. 05: Safety and Efficacy of First-Line Nivolumab (NIVO; Anti. Programmed Death-1 [PD-1])](https://slidetodoc.com/presentation_image_h2/531de7db6f58a53e88c014b528ad16ad/image-59.jpg)





- Slides: 100

Developed in association with the European Thoracic Oncology Platform 16 th World Conference on Lung Cancer (WCLC) 6– 9 September 2015 | Denver, Colorado, USA Supported by Eli Lilly and Company has not influenced the content of this publication

Letter from Prof Rolf Stahel Dear Colleagues It is my pleasure to present this ETOP slide set which has been designed to highlight and summarise key findings in thoracic cancers from the major congresses in 2015. This slide set specifically focuses on the IASLC 16 th World Conference on Lung Cancer and is available in 4 languages – English, French, Italian and Japanese. The area of clinical research in oncology is a challenging and ever changing environment. Within this environment we all value access to scientific data and research which helps to educate and inspire further advancements in our roles as scientists, clinicians and educators. I hope you find this review of the latest developments in thoracic cancers of benefit to you in your practice. If you would like to share your thoughts with us we would welcome your comments. Please send any correspondence to etop@etop. eu-org. I would like to thank our ETOP members Drs Solange Peters and Martin Reck for their roles as Editors – for prioritising abstracts and reviewing slide content – also Dr Serena Ricciardi for overseeing translation to Italian. The slide set you see before you would not be possible without their commitment and hard work. And finally, we are also very grateful to Lilly Oncology for their financial, administerial and logistical support in the realisation of this complex yet rewarding activity. Yours sincerely, Rolf Stahel President, ETOP Foundation Council

ETOP Medical Oncology Slide Deck Editors 2015 Focus: Advanced NSCLC (not radically treatable stage III & stage IV) and related biomarker data Dr Solange Peters Multidisciplinary Oncology Center, Lausanne Cancer Center, Lausanne, Switzerland Focus: Early and locally advanced NSCLC (stage I–III) and related biomarker data / other malignancies Dr Martin Reck Department of Thoracic Oncology, Hospital Grosshansdorf, Germany

Contents • Biomarkers and screening • Early stage and locally advanced NSCLC – Stages I, II and III • Advanced NSCLC – Not radically treatable stage III and stage IV – 1 st line – Later lines • Other malignancies – SCLC and mesothelioma – Rare tumours – Brain metastases

Biomarkers and screening

ORAL 06. 03: Genome-Wide Gene Copy Number Analysis by Onco. Scan. TM FFPE Assay in 976 Resected NSCLC From LACE-Bio 2 – Tsao M et al • Study objectives – To identify novel prognostic/predictive markers using genome-wide copy number profiling and to investigate the associations between copy number aberrations and survival using the LACE-Bio bio-bank • Study design – DNA was extracted from FFPE tumour samples (n=1, 013) and profiled using Affymetrix Onco. Scan. TM arrays with >300, 000 probes (primary analysis n=976) • Key results – OS, DFS & LCSS were similar in patients with/without gene copy number alterations – 195 aberrant MCRs were significantly different between ADC and SCC • More gains in SCC in 3 q, 22 q and 12; more losses in SCC in 3 p, 4, 5 q • Conclusions – Chromosomal MCRs were identified that harbour candidate copy number aberrant genes in patients with NSCLC – Strong copy number differences were observed between ADC and SCC and, therefore, copy number aberrations may be histology specific FFPE, formalin-fixed paraffin embedded; LCSS, lung cancer specific survival; MCR, minimum common region Tsao et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 06. 03

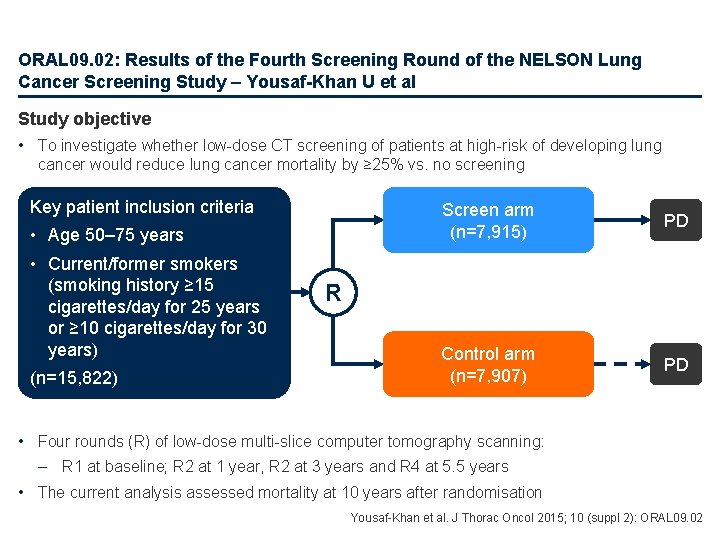
ORAL 09. 02: Results of the Fourth Screening Round of the NELSON Lung Cancer Screening Study – Yousaf-Khan U et al Study objective • To investigate whether low-dose CT screening of patients at high-risk of developing lung cancer would reduce lung cancer mortality by ≥ 25% vs. no screening Key patient inclusion criteria • Age 50– 75 years • Current/former smokers (smoking history ≥ 15 cigarettes/day for 25 years or ≥ 10 cigarettes/day for 30 years) (n=15, 822) Screen arm (n=7, 915) PD Control arm (n=7, 907) PD R • Four rounds (R) of low-dose multi-slice computer tomography scanning: – R 1 at baseline; R 2 at 1 year, R 2 at 3 years and R 4 at 5. 5 years • The current analysis assessed mortality at 10 years after randomisation Yousaf-Khan et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 09. 02

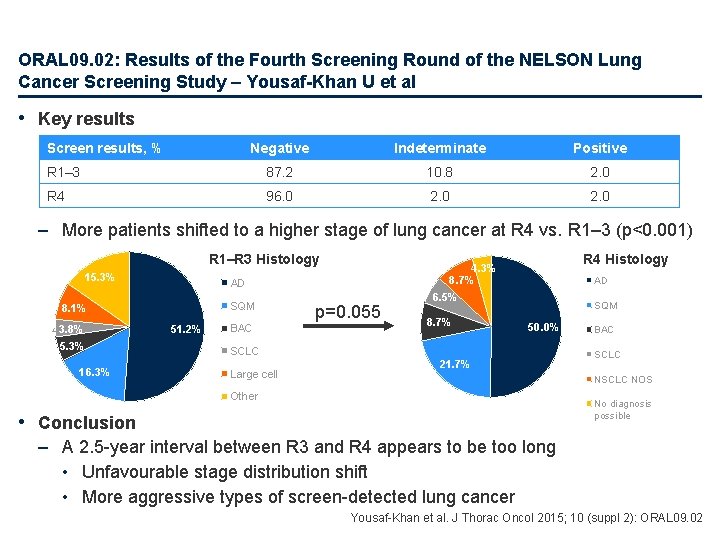
ORAL 09. 02: Results of the Fourth Screening Round of the NELSON Lung Cancer Screening Study – Yousaf-Khan U et al • Key results Screen results, % Negative Indeterminate Positive R 1– 3 87. 2 10. 8 2. 0 R 4 96. 0 2. 0 – More patients shifted to a higher stage of lung cancer at R 4 vs. R 1– 3 (p<0. 001) R 1–R 3 Histology 15. 3% AD SQM 8. 1% 3. 8% 5. 3% 16. 3% 51. 2% BAC R 4 Histology 4. 3% 8. 7% p=0. 055 AD 6. 5% 8. 7% SQM 50. 0% SCLC Large cell 21. 7% BAC SCLC NOS Other No diagnosis possible • Conclusion – A 2. 5 -year interval between R 3 and R 4 appears to be too long • Unfavourable stage distribution shift • More aggressive types of screen-detected lung cancer Yousaf-Khan et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 09. 02

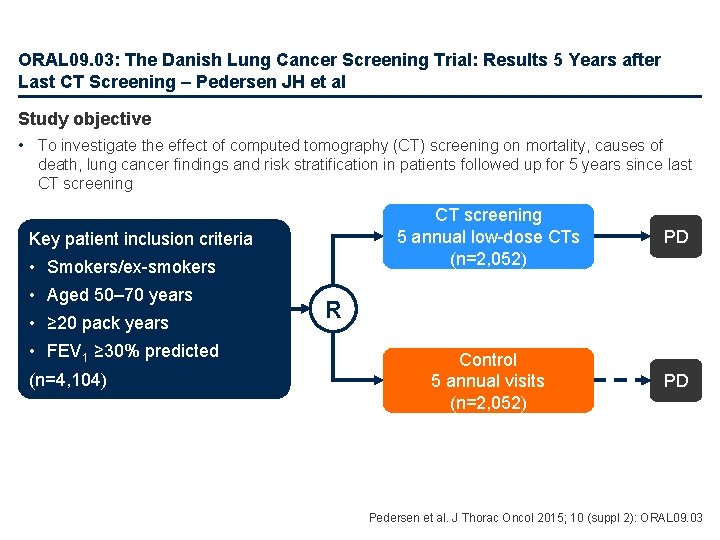
ORAL 09. 03: The Danish Lung Cancer Screening Trial: Results 5 Years after Last CT Screening – Pedersen JH et al Study objective • To investigate the effect of computed tomography (CT) screening on mortality, causes of death, lung cancer findings and risk stratification in patients followed up for 5 years since last CT screening Key patient inclusion criteria • Smokers/ex-smokers • Aged 50– 70 years • ≥ 20 pack years • FEV 1 ≥ 30% predicted (n=4, 104) CT screening 5 annual low-dose CTs (n=2, 052) PD Control 5 annual visits (n=2, 052) PD R Pedersen et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 09. 03

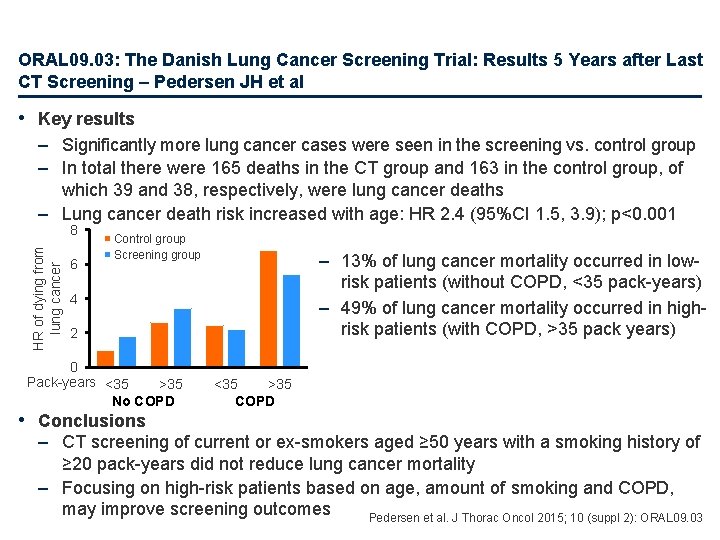
ORAL 09. 03: The Danish Lung Cancer Screening Trial: Results 5 Years after Last CT Screening – Pedersen JH et al • Key results – Significantly more lung cancer cases were seen in the screening vs. control group – In total there were 165 deaths in the CT group and 163 in the control group, of which 39 and 38, respectively, were lung cancer deaths – Lung cancer death risk increased with age: HR 2. 4 (95%CI 1. 5, 3. 9); p<0. 001 HR of dying from lung cancer 8 6 Control group Screening group – 13% of lung cancer mortality occurred in lowrisk patients (without COPD, <35 pack-years) – 49% of lung cancer mortality occurred in highrisk patients (with COPD, >35 pack years) 4 2 0 Pack-years <35 >35 No COPD • Conclusions <35 >35 COPD – CT screening of current or ex-smokers aged ≥ 50 years with a smoking history of ≥ 20 pack-years did not reduce lung cancer mortality – Focusing on high-risk patients based on age, amount of smoking and COPD, may improve screening outcomes Pedersen et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 09. 03

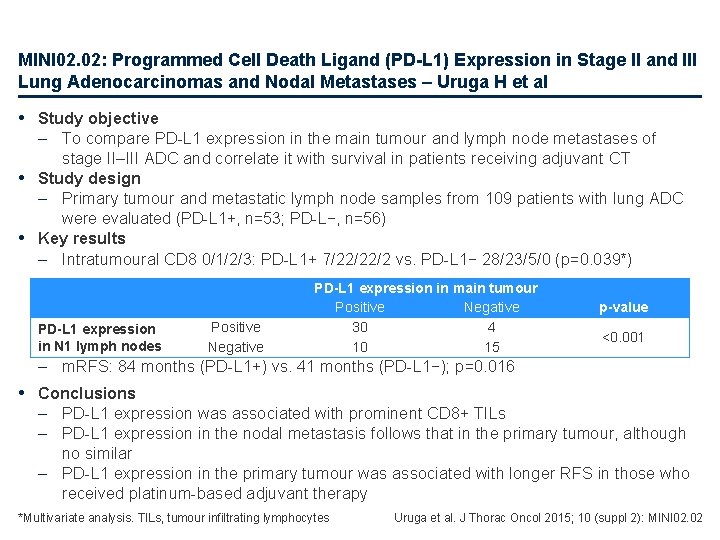
MINI 02. 02: Programmed Cell Death Ligand (PD-L 1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases – Uruga H et al • Study objective – To compare PD-L 1 expression in the main tumour and lymph node metastases of stage II–III ADC and correlate it with survival in patients receiving adjuvant CT • Study design – Primary tumour and metastatic lymph node samples from 109 patients with lung ADC were evaluated (PD-L 1+, n=53; PD-L−, n=56) • Key results – Intratumoural CD 8 0/1/2/3: PD-L 1+ 7/22/22/2 vs. PD-L 1− 28/23/5/0 (p=0. 039*) PD-L 1 expression in N 1 lymph nodes Positive Negative PD-L 1 expression in main tumour Positive Negative 30 4 10 15 p-value <0. 001 – m. RFS: 84 months (PD-L 1+) vs. 41 months (PD-L 1−); p=0. 016 • Conclusions – PD-L 1 expression was associated with prominent CD 8+ TILs – PD-L 1 expression in the nodal metastasis follows that in the primary tumour, although no similar – PD-L 1 expression in the primary tumour was associated with longer RFS in those who received platinum-based adjuvant therapy *Multivariate analysis. TILs, tumour infiltrating lymphocytes Uruga et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 02. 02

ORAL 13. 02: Characterization of PD-L 1 Expression Related to Unique Genes in NSCLC Tissue Samples – Garon EB et al • Study objective – • • • To assess PD-L 1 expression in resected tumour specimens that could potentially guide the design of adjuvant approaches based on immune checkpoint inhibitors Study design – Microarray analyses were performed to assess gene expression for 320 NSCLC and 15 normal lung resection specimens profiled on the Agilent Whole Human Genome 4 x 44 K 2 -colour platform – The Rosetta Similarity Tool (ROAST) was used to find genes correlated to PD-L 1 expression – Survival analyses based on PD-L 1 expression were performed using the Kaplan-Meier method and compared using the log-rank test Key results – PD-L 1 expression was higher in NSCLC than breast or endometrial tumours (p<0. 01) – Within the 320 NSCLC tissue samples, 174 unique genes are highly correlated with PD-L 1 expression (r range= 0. 692– 0. 904) – 80 (25%) tissue samples had a PD-L 1 log ratio >0, and 63 tissue samples had large sets of highly correlated genes known to be involved in immune and inflammatory response – No significant difference in OS was noted (p=0. 661), but increased PD-L 1 expression was clearly not associated with better outcomes Conclusion – Within the NSCLC cohort, there is a group of patients with high expression for PD-L 1 and related genes; this group does not have a better prognosis vs. those with typical or decreased PD-L 1 expression Garon et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 13. 02

ORAL 16. 07: Intratumor Heterogeneity of EGFR Activating Mutations Analyzed in Single Cancer Cells in Advanced NSCLC Patients – Guo LH* et al • Study objective – To explore the intratumour heterogeneity of EGFR activating mutations in patients with NSCLC • Study design – Part 1 investigated the feasibility of single-cell analysis for EGFR exon 21 – Part 2 treated 6 NSCLC patients who had EGFR exon 21 mutation identified by direct sequencing with gefitinib; single-cell analysis of H 1975 cells was used to test EGFR exon 21 status and compare the amplification rate of nested PCR and EGFR mutational rate according to PFS >14 months (Group A, n=3) and <6 months (Group B, n=3) • Key results – Analysis of 104 single H 1975 cells detected EGFR exon mutational status with an amplification rate of 96. 2% and an allele drop out rate of 7. 0% – 135 tumour cells were captured from the samples from the group of 6 patients • There was no difference between the groups in amplification rate (84. 3% in Group A; 93. 8% in Group B; p=0. 077) • The total mutational rate was higher in Group A than Group B (86. 4% vs. 68. 9%; p=0. 021) • Conclusions – It is feasible to perform EGFR mutation detection in single cancer cells and intratumour heterogeneity does exist based on this analysis technique – These data suggest that the abundance of EGFR activating mutation is relevant to the benefit from EGFR-TKI treatment *Presented by Zhang Q Guo et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 16. 07

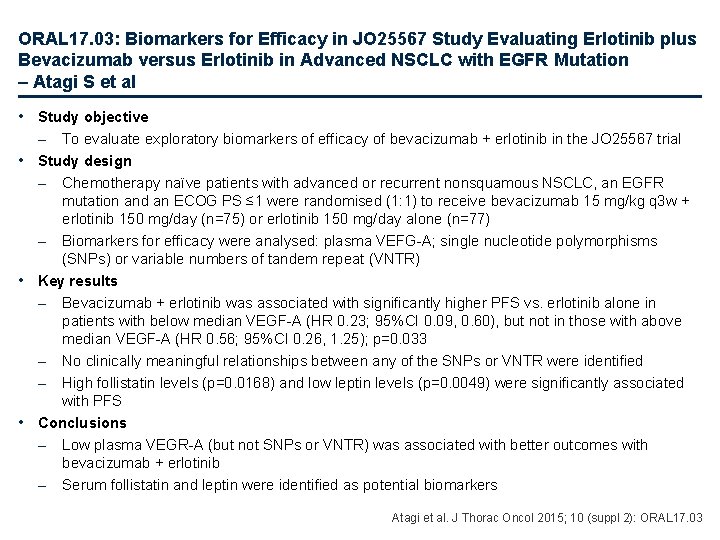
ORAL 17. 03: Biomarkers for Efficacy in JO 25567 Study Evaluating Erlotinib plus Bevacizumab versus Erlotinib in Advanced NSCLC with EGFR Mutation – Atagi S et al • Study objective – To evaluate exploratory biomarkers of efficacy of bevacizumab + erlotinib in the JO 25567 trial • Study design – Chemotherapy naïve patients with advanced or recurrent nonsquamous NSCLC, an EGFR mutation and an ECOG PS ≤ 1 were randomised (1: 1) to receive bevacizumab 15 mg/kg q 3 w + erlotinib 150 mg/day (n=75) or erlotinib 150 mg/day alone (n=77) – Biomarkers for efficacy were analysed: plasma VEFG-A; single nucleotide polymorphisms (SNPs) or variable numbers of tandem repeat (VNTR) • Key results – Bevacizumab + erlotinib was associated with significantly higher PFS vs. erlotinib alone in patients with below median VEGF-A (HR 0. 23; 95%CI 0. 09, 0. 60), but not in those with above median VEGF-A (HR 0. 56; 95%CI 0. 26, 1. 25); p=0. 033 – No clinically meaningful relationships between any of the SNPs or VNTR were identified – High follistatin levels (p=0. 0168) and low leptin levels (p=0. 0049) were significantly associated with PFS • Conclusions – Low plasma VEGR-A (but not SNPs or VNTR) was associated with better outcomes with bevacizumab + erlotinib – Serum follistatin and leptin were identified as potential biomarkers Atagi et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 17. 03

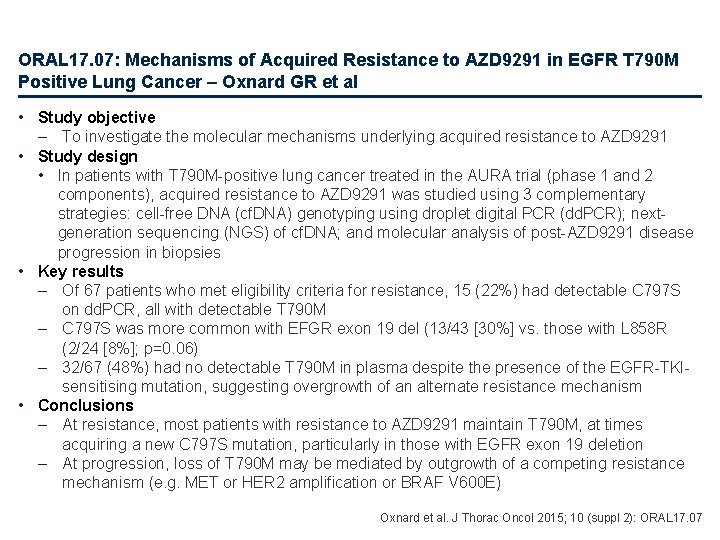
ORAL 17. 07: Mechanisms of Acquired Resistance to AZD 9291 in EGFR T 790 M Positive Lung Cancer – Oxnard GR et al • Study objective – To investigate the molecular mechanisms underlying acquired resistance to AZD 9291 • Study design • In patients with T 790 M-positive lung cancer treated in the AURA trial (phase 1 and 2 components), acquired resistance to AZD 9291 was studied using 3 complementary strategies: cell-free DNA (cf. DNA) genotyping using droplet digital PCR (dd. PCR); nextgeneration sequencing (NGS) of cf. DNA; and molecular analysis of post-AZD 9291 disease progression in biopsies • Key results – Of 67 patients who met eligibility criteria for resistance, 15 (22%) had detectable C 797 S on dd. PCR, all with detectable T 790 M – C 797 S was more common with EFGR exon 19 del (13/43 [30%] vs. those with L 858 R (2/24 [8%]; p=0. 06) – 32/67 (48%) had no detectable T 790 M in plasma despite the presence of the EGFR-TKIsensitising mutation, suggesting overgrowth of an alternate resistance mechanism • Conclusions – At resistance, most patients with resistance to AZD 9291 maintain T 790 M, at times acquiring a new C 797 S mutation, particularly in those with EGFR exon 19 deletion – At progression, loss of T 790 M may be mediated by outgrowth of a competing resistance mechanism (e. g. MET or HER 2 amplification or BRAF V 600 E) Oxnard et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 17. 07

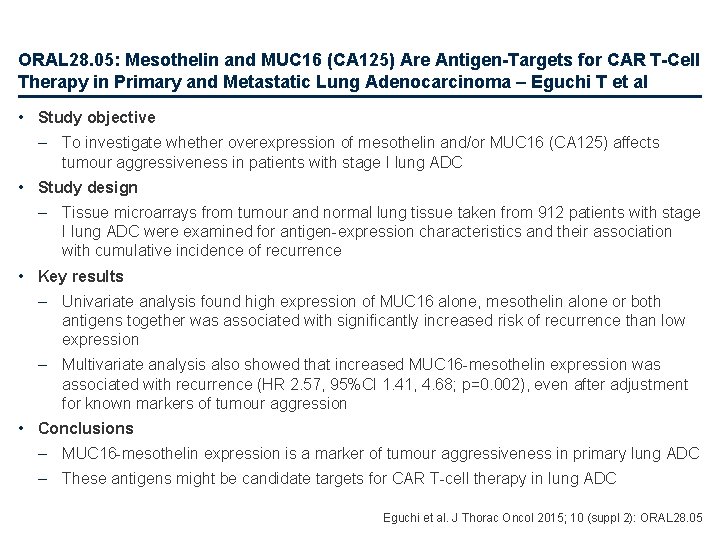
ORAL 28. 05: Mesothelin and MUC 16 (CA 125) Are Antigen-Targets for CAR T-Cell Therapy in Primary and Metastatic Lung Adenocarcinoma – Eguchi T et al • Study objective – To investigate whether overexpression of mesothelin and/or MUC 16 (CA 125) affects tumour aggressiveness in patients with stage I lung ADC • Study design – Tissue microarrays from tumour and normal lung tissue taken from 912 patients with stage I lung ADC were examined for antigen-expression characteristics and their association with cumulative incidence of recurrence • Key results – Univariate analysis found high expression of MUC 16 alone, mesothelin alone or both antigens together was associated with significantly increased risk of recurrence than low expression – Multivariate analysis also showed that increased MUC 16 -mesothelin expression was associated with recurrence (HR 2. 57, 95%CI 1. 41, 4. 68; p=0. 002), even after adjustment for known markers of tumour aggression • Conclusions – MUC 16 -mesothelin expression is a marker of tumour aggressiveness in primary lung ADC – These antigens might be candidate targets for CAR T-cell therapy in lung ADC Eguchi et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 28. 05

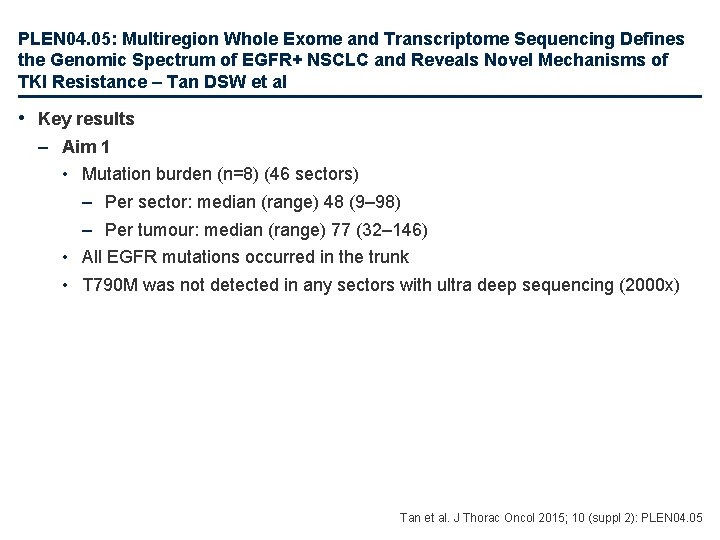
PLEN 04. 05: Multiregion Whole Exome and Transcriptome Sequencing Defines the Genomic Spectrum of EGFR+ NSCLC and Reveals Novel Mechanisms of TKI Resistance – Tan DSW et al Study objective • To determine clonal architecture through multi-sector sequencing (Aim 1) and to elucidate mechanisms of EGFR TKI resistance through integrative omics (Aim 2) Study design • Aim 1 – Multi-sector sequencing on spatially separated regions from resected EGFR M+ lung adenocarcinoma (9 patients from East Asia, 47 sectors) • Aim 2 – 30 EGFR M+ TKI resistant samples from 25 patients • All samples – Paired tumour-normal exome/transcriptome sequencing and SNP array was performed – Genomic alterations were validated with targeted re-sequencing Tan et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 05

PLEN 04. 05: Multiregion Whole Exome and Transcriptome Sequencing Defines the Genomic Spectrum of EGFR+ NSCLC and Reveals Novel Mechanisms of TKI Resistance – Tan DSW et al • Key results – Aim 1 • Mutation burden (n=8) (46 sectors) – Per sector: median (range) 48 (9– 98) – Per tumour: median (range) 77 (32– 146) • All EGFR mutations occurred in the trunk • T 790 M was not detected in any sectors with ultra deep sequencing (2000 x) Tan et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 05

PLEN 04. 05: Multiregion Whole Exome and Transcriptome Sequencing Defines the Genomic Spectrum of EGFR+ NSCLC and Reveals Novel Mechanisms of TKI Resistance – Tan DSW et al • Key results (cont. ) – AIM 2 • Higher mutation burden in TKI-resistant EGFR M+ NSCLC – TKI naïve vs. resistant (48 vs. 83 median SNVs; p=0. 003) • Smoking and APOBEC signature were associated with a high mutation burden • Recurrent genes of potential significance included MED 12 – Modulates EGFR resistance through TGFβR Tan et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 05

PLEN 04. 05: Multiregion Whole Exome and Transcriptome Sequencing Defines the Genomic Spectrum of EGFR+ NSCLC and Reveals Novel Mechanisms of TKI Resistance – Tan DSW et al • Conclusions – Genomic architecture of TKI naïve East Asia EGFR M+ NSCLC • EGFR mutations are truncal events • Low mutation burden • Higher clonal diversity (branch/private>trunk) – Mutation burden is relatively higher in TKI-resistant samples • Association with smoking and APOBEC signature in a subset of EGFR M+ NSCLC – Multiple potential mechanisms of TKI resistance co-exist • For example, MED 12 alterations may be subclonal mediators of resistance • One patient with co-occurring trunk drivers demonstrated primary TKI resistance Tan et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 05

PLEN 04. 07: Stopping Smoking Reduces Mortality in Low-Dose Computed Tomography (LDCT) Screening Volunteers – Pastorino U et al Study objective • To investigate the effect of smoking cessation on the overall mortality of volunteers undergoing low-dose computed tomography (LDCT) screening Study design • Follow-up analysis of heavy smokers aged >50 years (n=3, 381) who had been enrolled into two LDCT screening programmes between 2000 and 2010 • Subjects were divided into three groups: – Current smokers, those who smoked throughout the screening period or stopped <1 year before the end of follow-up or death – Ex-smokers, those who had stopped smoking at the time of accrual / randomisation – Quitters, those who were active smokers at the time of accrual / randomisation but who stopped smoking at least 1 year before the end of follow-up or death • The effect of smoking on mortality was adjusted for gender, age, BMI, lung function (FEV 1 %) and pack-years at baseline Pastorino et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 07

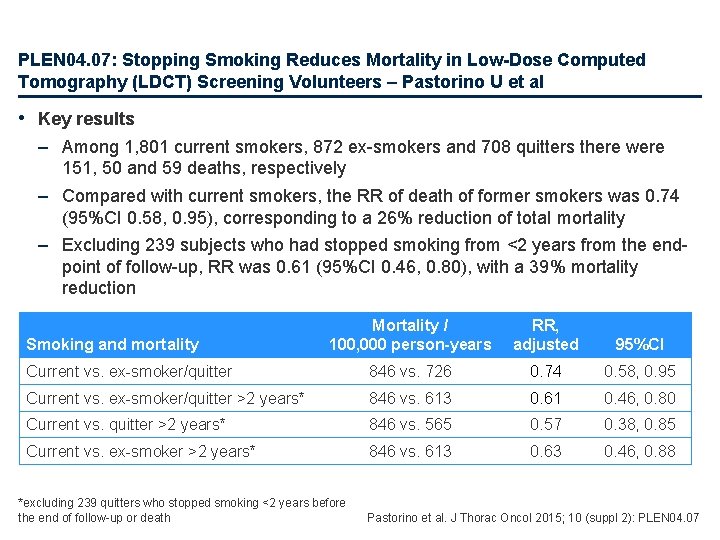
PLEN 04. 07: Stopping Smoking Reduces Mortality in Low-Dose Computed Tomography (LDCT) Screening Volunteers – Pastorino U et al • Key results – Among 1, 801 current smokers, 872 ex-smokers and 708 quitters there were 151, 50 and 59 deaths, respectively – Compared with current smokers, the RR of death of former smokers was 0. 74 (95%CI 0. 58, 0. 95), corresponding to a 26% reduction of total mortality – Excluding 239 subjects who had stopped smoking from <2 years from the endpoint of follow-up, RR was 0. 61 (95%CI 0. 46, 0. 80), with a 39% mortality reduction Mortality / 100, 000 person-years RR, adjusted 95%CI Current vs. ex-smoker/quitter 846 vs. 726 0. 74 0. 58, 0. 95 Current vs. ex-smoker/quitter >2 years* 846 vs. 613 0. 61 0. 46, 0. 80 Current vs. quitter >2 years* 846 vs. 565 0. 57 0. 38, 0. 85 Current vs. ex-smoker >2 years* 846 vs. 613 0. 63 0. 46, 0. 88 Smoking and mortality *excluding 239 quitters who stopped smoking <2 years before the end of follow-up or death Pastorino et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 07

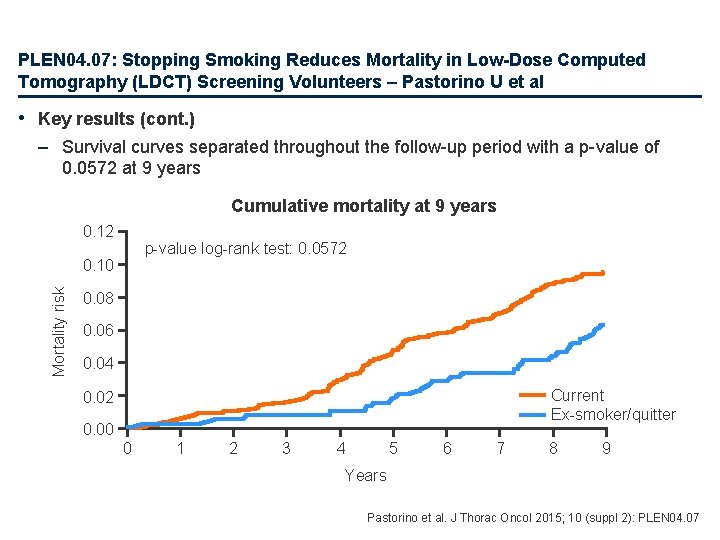
PLEN 04. 07: Stopping Smoking Reduces Mortality in Low-Dose Computed Tomography (LDCT) Screening Volunteers – Pastorino U et al • Key results (cont. ) – Survival curves separated throughout the follow-up period with a p-value of 0. 0572 at 9 years Cumulative mortality at 9 years 0. 12 p-value log-rank test: 0. 0572 Mortality risk 0. 10 0. 08 0. 06 0. 04 Current Ex-smoker/quitter 0. 02 0. 00 0 1 2 3 4 5 6 7 8 9 Years Pastorino et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 07

PLEN 04. 07: Stopping Smoking Reduces Mortality in Low-Dose Computed Tomography (LDCT) Screening Volunteers – Pastorino U et al • Conclusions – Stopping smoking is associated with a significant reduction of the overall mortality of heavy smokers enrolled in LDCT screening programs – The benefit of stopping smoking appears to be 3 - to 5 -fold greater than the one achieved by earlier detection in the NLST trial Pastorino et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 07

Early and locally advanced NSCLC Stages I, II and III

ORAL 04. 01: Final Results of Phase III Trial of Adjuvant Chemo-Immunotherapy in Lung Cancer – Kimura H et al Study objective • To determine whether the addition of immunotherapy to chemotherapy improves survival Key patient inclusion criteria Chemo-immunotherapy* (n=50) PD Chemotherapy (n=51) PD • Stage IB–IV NSCLC • ECOG PS 0, 1 R • Age <76 years (n=556) Primary endpoint • OS (5 -year rate) *Adoptive transfer of autologous activated killer T cells and dendritic cells (AKT-DC) from the patients’ own regional lymph nodes q 8 w for 10– 14 courses Secondary endpoint • RFS Kimura et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 04. 01

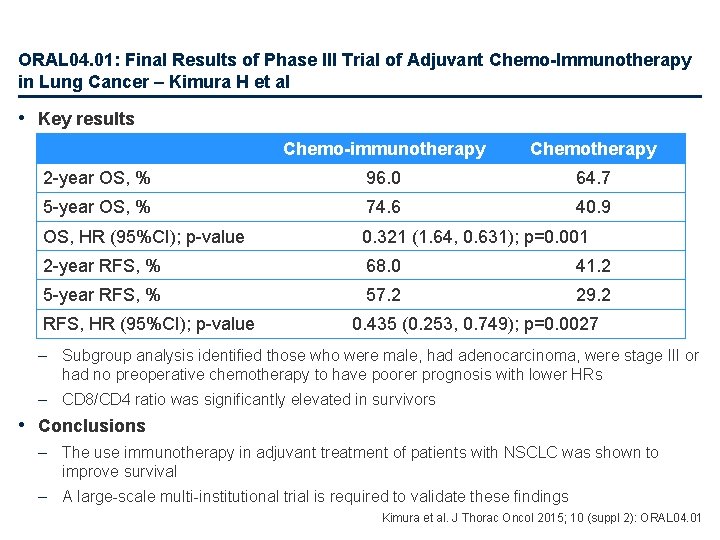
ORAL 04. 01: Final Results of Phase III Trial of Adjuvant Chemo-Immunotherapy in Lung Cancer – Kimura H et al • Key results Chemo-immunotherapy Chemotherapy 2 -year OS, % 96. 0 64. 7 5 -year OS, % 74. 6 40. 9 OS, HR (95%CI); p-value 0. 321 (1. 64, 0. 631); p=0. 001 2 -year RFS, % 68. 0 41. 2 5 -year RFS, % 57. 2 29. 2 RFS, HR (95%CI); p-value 0. 435 (0. 253, 0. 749); p=0. 0027 – Subgroup analysis identified those who were male, had adenocarcinoma, were stage III or had no preoperative chemotherapy to have poorer prognosis with lower HRs – CD 8/CD 4 ratio was significantly elevated in survivors • Conclusions – The use immunotherapy in adjuvant treatment of patients with NSCLC was shown to improve survival – A large-scale multi-institutional trial is required to validate these findings Kimura et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 04. 01

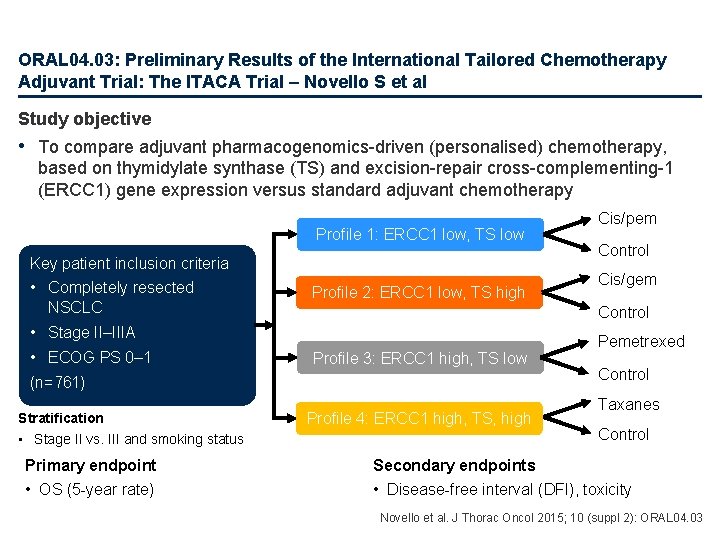
ORAL 04. 03: Preliminary Results of the International Tailored Chemotherapy Adjuvant Trial: The ITACA Trial – Novello S et al Study objective • To compare adjuvant pharmacogenomics-driven (personalised) chemotherapy, based on thymidylate synthase (TS) and excision-repair cross-complementing-1 (ERCC 1) gene expression versus standard adjuvant chemotherapy Profile 1: ERCC 1 low, TS low Key patient inclusion criteria • Completely resected NSCLC Profile 2: ERCC 1 low, TS high Profile 3: ERCC 1 high, TS low (n=761) Stratification • Stage II vs. III and smoking status Primary endpoint • OS (5 -year rate) Control Cis/gem Control • Stage II–IIIA • ECOG PS 0– 1 Cis/pem Profile 4: ERCC 1 high, TS, high Pemetrexed Control Taxanes Control Secondary endpoints • Disease-free interval (DFI), toxicity Novello et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 04. 03

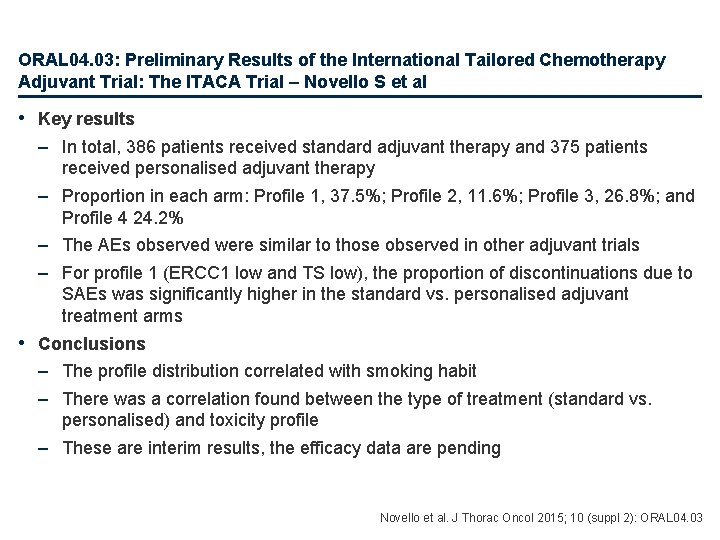
ORAL 04. 03: Preliminary Results of the International Tailored Chemotherapy Adjuvant Trial: The ITACA Trial – Novello S et al • Key results – In total, 386 patients received standard adjuvant therapy and 375 patients received personalised adjuvant therapy – Proportion in each arm: Profile 1, 37. 5%; Profile 2, 11. 6%; Profile 3, 26. 8%; and Profile 4 24. 2% – The AEs observed were similar to those observed in other adjuvant trials – For profile 1 (ERCC 1 low and TS low), the proportion of discontinuations due to SAEs was significantly higher in the standard vs. personalised adjuvant treatment arms • Conclusions – The profile distribution correlated with smoking habit – There was a correlation found between the type of treatment (standard vs. personalised) and toxicity profile – These are interim results, the efficacy data are pending Novello et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 04. 03

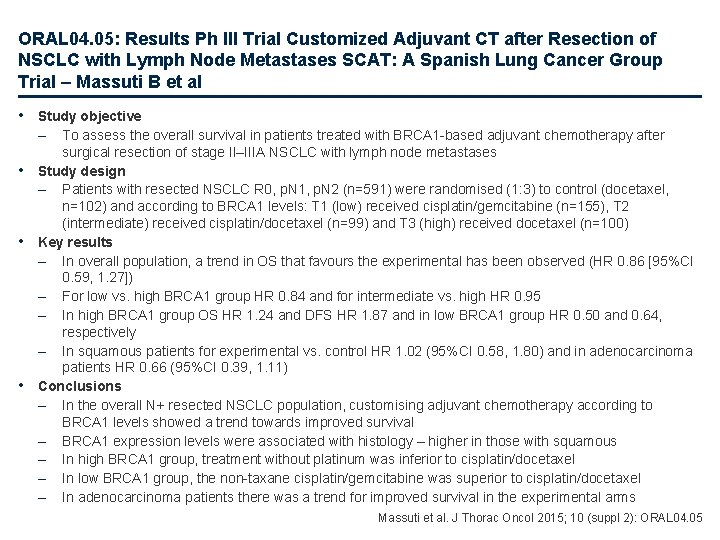
ORAL 04. 05: Results Ph III Trial Customized Adjuvant CT after Resection of NSCLC with Lymph Node Metastases SCAT: A Spanish Lung Cancer Group Trial – Massuti B et al • Study objective – • • • To assess the overall survival in patients treated with BRCA 1 -based adjuvant chemotherapy after surgical resection of stage II–IIIA NSCLC with lymph node metastases Study design – Patients with resected NSCLC R 0, p. N 1, p. N 2 (n=591) were randomised (1: 3) to control (docetaxel, n=102) and according to BRCA 1 levels: T 1 (low) received cisplatin/gemcitabine (n=155), T 2 (intermediate) received cisplatin/docetaxel (n=99) and T 3 (high) received docetaxel (n=100) Key results – In overall population, a trend in OS that favours the experimental has been observed (HR 0. 86 [95%CI 0. 59, 1. 27]) – For low vs. high BRCA 1 group HR 0. 84 and for intermediate vs. high HR 0. 95 – In high BRCA 1 group OS HR 1. 24 and DFS HR 1. 87 and in low BRCA 1 group HR 0. 50 and 0. 64, respectively – In squamous patients for experimental vs. control HR 1. 02 (95%CI 0. 58, 1. 80) and in adenocarcinoma patients HR 0. 66 (95%CI 0. 39, 1. 11) Conclusions – In the overall N+ resected NSCLC population, customising adjuvant chemotherapy according to BRCA 1 levels showed a trend towards improved survival – BRCA 1 expression levels were associated with histology – higher in those with squamous – In high BRCA 1 group, treatment without platinum was inferior to cisplatin/docetaxel – In low BRCA 1 group, the non-taxane cisplatin/gemcitabine was superior to cisplatin/docetaxel – In adenocarcinoma patients there was a trend for improved survival in the experimental arms Massuti et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 04. 05

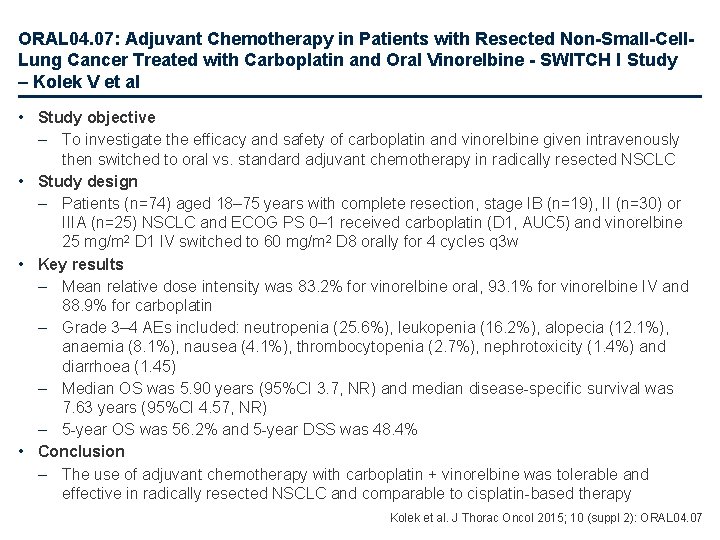
ORAL 04. 07: Adjuvant Chemotherapy in Patients with Resected Non-Small-Cell. Lung Cancer Treated with Carboplatin and Oral Vinorelbine - SWITCH I Study – Kolek V et al • Study objective – To investigate the efficacy and safety of carboplatin and vinorelbine given intravenously then switched to oral vs. standard adjuvant chemotherapy in radically resected NSCLC • Study design – Patients (n=74) aged 18– 75 years with complete resection, stage IB (n=19), II (n=30) or IIIA (n=25) NSCLC and ECOG PS 0– 1 received carboplatin (D 1, AUC 5) and vinorelbine 25 mg/m 2 D 1 IV switched to 60 mg/m 2 D 8 orally for 4 cycles q 3 w • Key results – Mean relative dose intensity was 83. 2% for vinorelbine oral, 93. 1% for vinorelbine IV and 88. 9% for carboplatin – Grade 3– 4 AEs included: neutropenia (25. 6%), leukopenia (16. 2%), alopecia (12. 1%), anaemia (8. 1%), nausea (4. 1%), thrombocytopenia (2. 7%), nephrotoxicity (1. 4%) and diarrhoea (1. 45) – Median OS was 5. 90 years (95%CI 3. 7, NR) and median disease-specific survival was 7. 63 years (95%CI 4. 57, NR) – 5 -year OS was 56. 2% and 5 -year DSS was 48. 4% • Conclusion – The use of adjuvant chemotherapy with carboplatin + vinorelbine was tolerable and effective in radically resected NSCLC and comparable to cisplatin-based therapy Kolek et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 04. 07

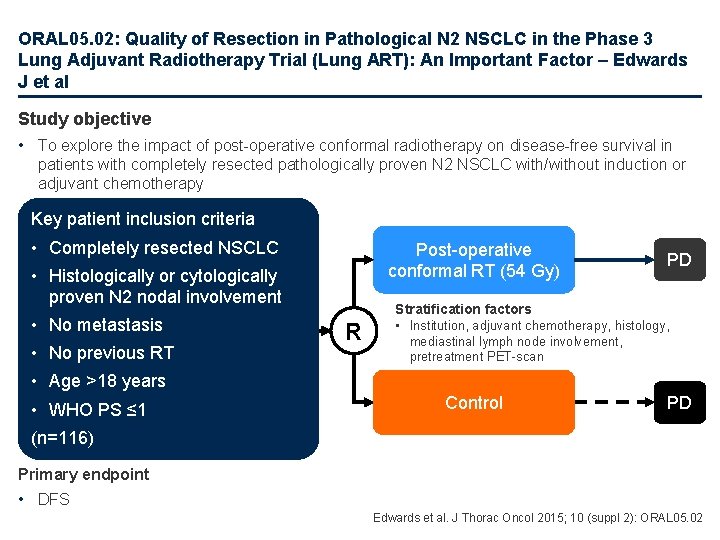
ORAL 05. 02: Quality of Resection in Pathological N 2 NSCLC in the Phase 3 Lung Adjuvant Radiotherapy Trial (Lung ART): An Important Factor – Edwards J et al Study objective • To explore the impact of post-operative conformal radiotherapy on disease-free survival in patients with completely resected pathologically proven N 2 NSCLC with/without induction or adjuvant chemotherapy Key patient inclusion criteria • Completely resected NSCLC Post-operative conformal RT (54 Gy) • Histologically or cytologically proven N 2 nodal involvement • No metastasis • No previous RT R PD Stratification factors • Institution, adjuvant chemotherapy, histology, mediastinal lymph node involvement, pretreatment PET-scan • Age >18 years • WHO PS ≤ 1 Control PD (n=116) Primary endpoint • DFS Edwards et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 05. 02

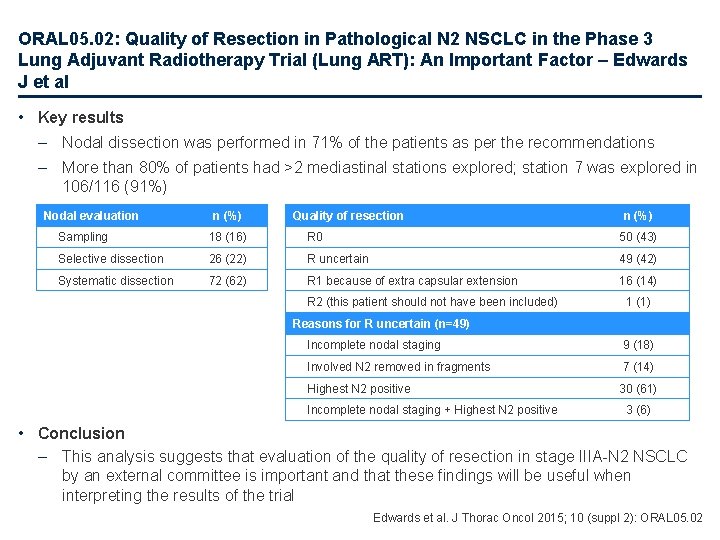
ORAL 05. 02: Quality of Resection in Pathological N 2 NSCLC in the Phase 3 Lung Adjuvant Radiotherapy Trial (Lung ART): An Important Factor – Edwards J et al • Key results – Nodal dissection was performed in 71% of the patients as per the recommendations – More than 80% of patients had >2 mediastinal stations explored; station 7 was explored in 106/116 (91%) Nodal evaluation n (%) Quality of resection n (%) Sampling 18 (16) R 0 50 (43) Selective dissection 26 (22) R uncertain 49 (42) Systematic dissection 72 (62) R 1 because of extra capsular extension 16 (14) R 2 (this patient should not have been included) 1 (1) Reasons for R uncertain (n=49) Incomplete nodal staging 9 (18) Involved N 2 removed in fragments 7 (14) Highest N 2 positive 30 (61) Incomplete nodal staging + Highest N 2 positive 3 (6) • Conclusion – This analysis suggests that evaluation of the quality of resection in stage IIIA-N 2 NSCLC by an external committee is important and that these findings will be useful when interpreting the results of the trial Edwards et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 05. 02

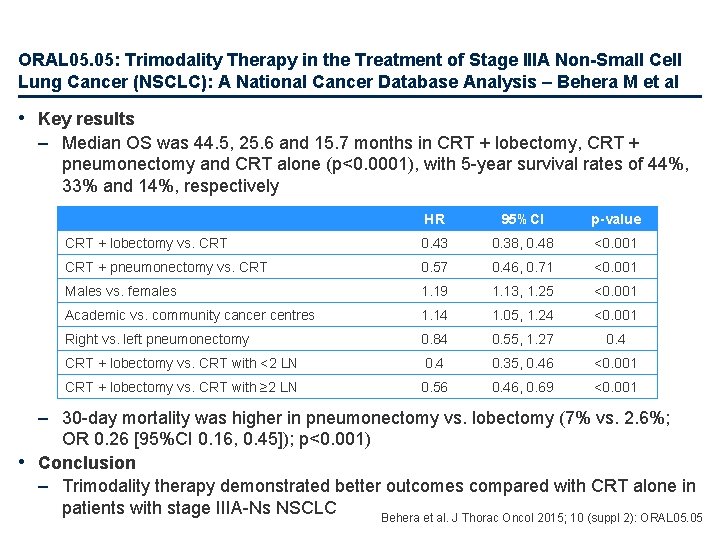
ORAL 05. 05: Trimodality Therapy in the Treatment of Stage IIIA Non-Small Cell Lung Cancer (NSCLC): A National Cancer Database Analysis – Behera M et al Study objective • To compare the outcomes and predictors associated with trimodality therapy vs. chemoradiotherapy alone in patients with NSCLC from the National Cancer Database CRT + lobectomy PD CRT + pneumonectomy PD CRT alone PD Key patient inclusion criteria • Stage IIIA-N 2 (n=29, 584) Primary endpoint • OS Behera et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 05. 05

ORAL 05. 05: Trimodality Therapy in the Treatment of Stage IIIA Non-Small Cell Lung Cancer (NSCLC): A National Cancer Database Analysis – Behera M et al • Key results – Median OS was 44. 5, 25. 6 and 15. 7 months in CRT + lobectomy, CRT + pneumonectomy and CRT alone (p<0. 0001), with 5 -year survival rates of 44%, 33% and 14%, respectively HR 95%CI p-value CRT + lobectomy vs. CRT 0. 43 0. 38, 0. 48 <0. 001 CRT + pneumonectomy vs. CRT 0. 57 0. 46, 0. 71 <0. 001 Males vs. females 1. 19 1. 13, 1. 25 <0. 001 Academic vs. community cancer centres 1. 14 1. 05, 1. 24 <0. 001 Right vs. left pneumonectomy 0. 84 0. 55, 1. 27 0. 4 CRT + lobectomy vs. CRT with <2 LN 0. 4 0. 35, 0. 46 <0. 001 CRT + lobectomy vs. CRT with ≥ 2 LN 0. 56 0. 46, 0. 69 <0. 001 – 30 -day mortality was higher in pneumonectomy vs. lobectomy (7% vs. 2. 6%; OR 0. 26 [95%CI 0. 16, 0. 45]); p<0. 001) • Conclusion – Trimodality therapy demonstrated better outcomes compared with CRT alone in patients with stage IIIA-Ns NSCLC Behera et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 05. 05

ORAL 19. 05: Japanese Multicenter Study of Stereotactic Body Radiotherapy for 661 Medically Operable Patients with Stage I Non-Small Cell Lung Cancer – Komiyama T et al • Study objective – To investigate the outcome of stereotactic body radiotherapy (SBRT) in medically operable patients with stage I NSCLC • Study design – Retrospective analysis of 661 patients from a large, multicentre Japanese database; 486 patients had histologically proven NSCLC – Patients had received a total dose of 32– 70 Gy, administered in 4– 15 Gy fractions – The median calculated biological effective dose was 107 Gy • Key results – Median follow-up for all patients was 35 months – 3 -year OS and disease-specific survival rates were 79% and 89%, respectively – Multivariate analysis showed only the presence of pulmonary interstitial change was associated with worse survival Komiyama et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 19. 05

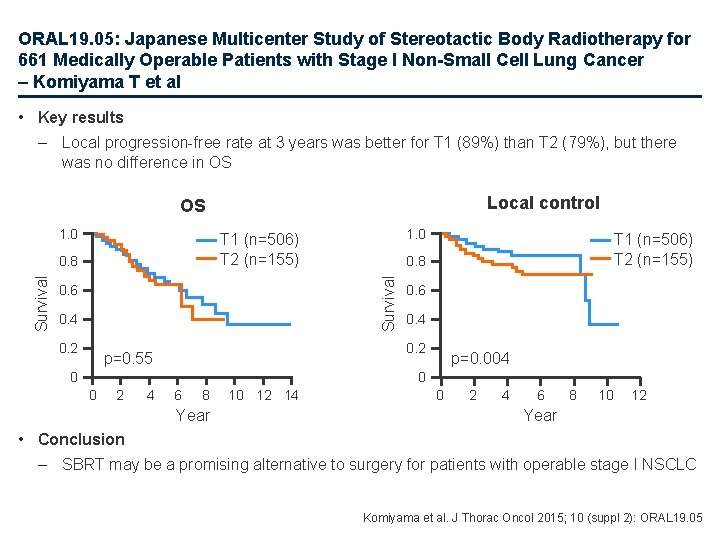
ORAL 19. 05: Japanese Multicenter Study of Stereotactic Body Radiotherapy for 661 Medically Operable Patients with Stage I Non-Small Cell Lung Cancer – Komiyama T et al • Key results – Local progression-free rate at 3 years was better for T 1 (89%) than T 2 (79%), but there was no difference in OS Local control OS 1. 0 0. 6 0. 4 0. 2 T 1 (n=506) T 2 (n=155) 0. 8 Survival 1. 0 T 1 (n=506) T 2 (n=155) 0. 6 0. 4 0. 2 p=0. 55 0 p=0. 004 0 0 2 4 6 8 Year 10 12 14 0 2 4 6 8 10 12 Year • Conclusion – SBRT may be a promising alternative to surgery for patients with operable stage I NSCLC Komiyama et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 19. 05

ORAL 20. 01: A Systematic Review of Carboplatin-Paclitaxel versus Cisplatin. Etoposide Concurrent with Thoracic Radiation for Stage III NSCLC Patients – Steuer C et al Study objective • To assess the efficacy and safety of carboplatin-paclitaxel (CP) vs. cisplatinetoposide (CE) in patients with stage III NSCLC receiving concurrent RT Study design • A systematic review was conducted of trials that compared outcomes and toxicities between CP and CE – Trials were excluded if they were phase 1, had fewer than 10 patients or included surgical resection • From 2, 111 trials identified, 84 trials were included in this analysis: 51 trials evaluating CP (3, 789 patients) and 32 trials evaluating CE (2, 887 patients) Steuer et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 20. 01

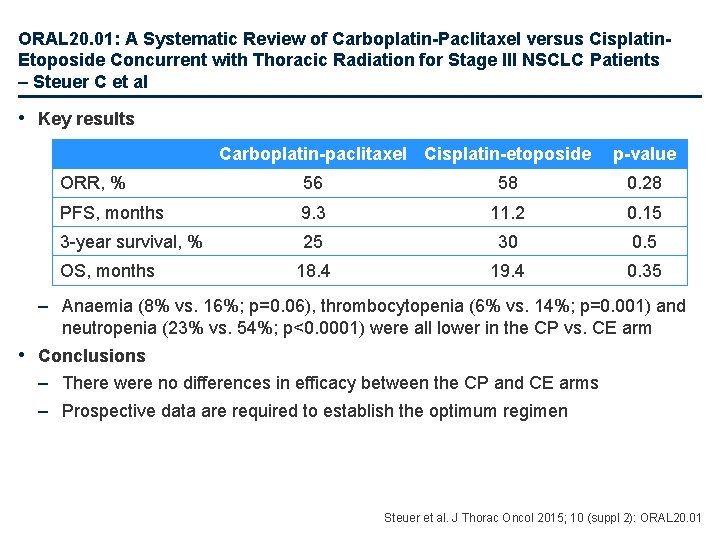
ORAL 20. 01: A Systematic Review of Carboplatin-Paclitaxel versus Cisplatin. Etoposide Concurrent with Thoracic Radiation for Stage III NSCLC Patients – Steuer C et al • Key results Carboplatin-paclitaxel Cisplatin-etoposide p-value ORR, % 56 58 0. 28 PFS, months 9. 3 11. 2 0. 15 3 -year survival, % 25 30 0. 5 18. 4 19. 4 0. 35 OS, months – Anaemia (8% vs. 16%; p=0. 06), thrombocytopenia (6% vs. 14%; p=0. 001) and neutropenia (23% vs. 54%; p<0. 0001) were all lower in the CP vs. CE arm • Conclusions – There were no differences in efficacy between the CP and CE arms – Prospective data are required to establish the optimum regimen Steuer et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 20. 01

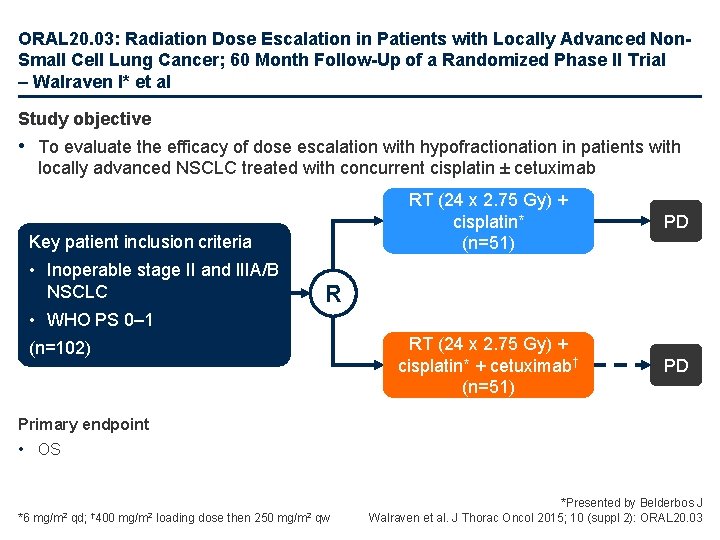
ORAL 20. 03: Radiation Dose Escalation in Patients with Locally Advanced Non. Small Cell Lung Cancer; 60 Month Follow-Up of a Randomized Phase II Trial – Walraven I* et al Study objective • To evaluate the efficacy of dose escalation with hypofractionation in patients with locally advanced NSCLC treated with concurrent cisplatin ± cetuximab Key patient inclusion criteria • Inoperable stage II and IIIA/B NSCLC RT (24 x 2. 75 Gy) + cisplatin* (n=51) PD RT (24 x 2. 75 Gy) + cisplatin* + cetuximab† (n=51) PD R • WHO PS 0– 1 (n=102) Primary endpoint • OS *6 mg/m 2 qd; † 400 mg/m 2 loading dose then 250 mg/m 2 qw *Presented by Belderbos J Walraven et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 20. 03

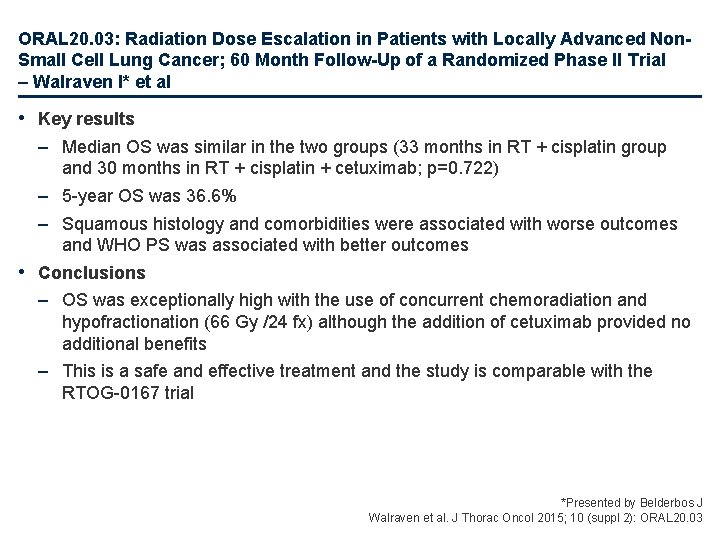
ORAL 20. 03: Radiation Dose Escalation in Patients with Locally Advanced Non. Small Cell Lung Cancer; 60 Month Follow-Up of a Randomized Phase II Trial – Walraven I* et al • Key results – Median OS was similar in the two groups (33 months in RT + cisplatin group and 30 months in RT + cisplatin + cetuximab; p=0. 722) – 5 -year OS was 36. 6% – Squamous histology and comorbidities were associated with worse outcomes and WHO PS was associated with better outcomes • Conclusions – OS was exceptionally high with the use of concurrent chemoradiation and hypofractionation (66 Gy /24 fx) although the addition of cetuximab provided no additional benefits – This is a safe and effective treatment and the study is comparable with the RTOG-0167 trial *Presented by Belderbos J Walraven et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 20. 03

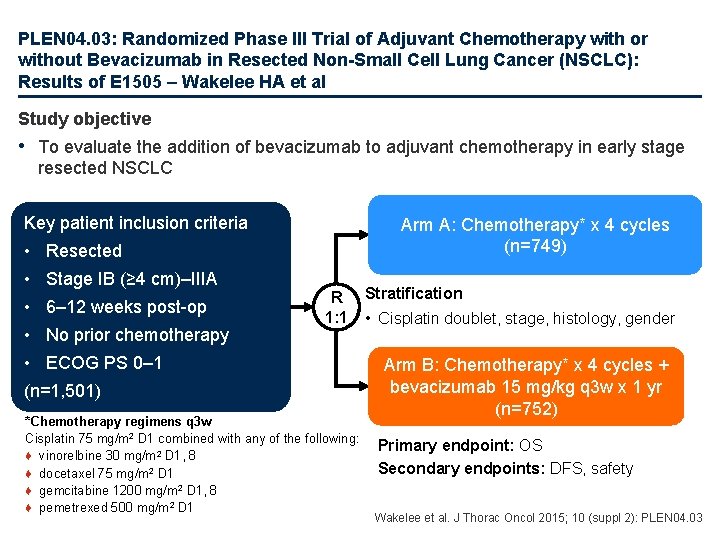
PLEN 04. 03: Randomized Phase III Trial of Adjuvant Chemotherapy with or without Bevacizumab in Resected Non-Small Cell Lung Cancer (NSCLC): Results of E 1505 – Wakelee HA et al Study objective • To evaluate the addition of bevacizumab to adjuvant chemotherapy in early stage resected NSCLC Key patient inclusion criteria Arm A: Chemotherapy* x 4 cycles (n=749) • Resected • Stage IB (≥ 4 cm)–IIIA • 6– 12 weeks post-op • No prior chemotherapy R 1: 1 • ECOG PS 0– 1 (n=1, 501) *Chemotherapy regimens q 3 w Cisplatin 75 mg/m 2 D 1 combined with any of the following: ♦ vinorelbine 30 mg/m 2 D 1, 8 ♦ docetaxel 75 mg/m 2 D 1 ♦ gemcitabine 1200 mg/m 2 D 1, 8 ♦ pemetrexed 500 mg/m 2 D 1 Stratification • Cisplatin doublet, stage, histology, gender Arm B: Chemotherapy* x 4 cycles + bevacizumab 15 mg/kg q 3 w x 1 yr (n=752) Primary endpoint: OS Secondary endpoints: DFS, safety Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 03

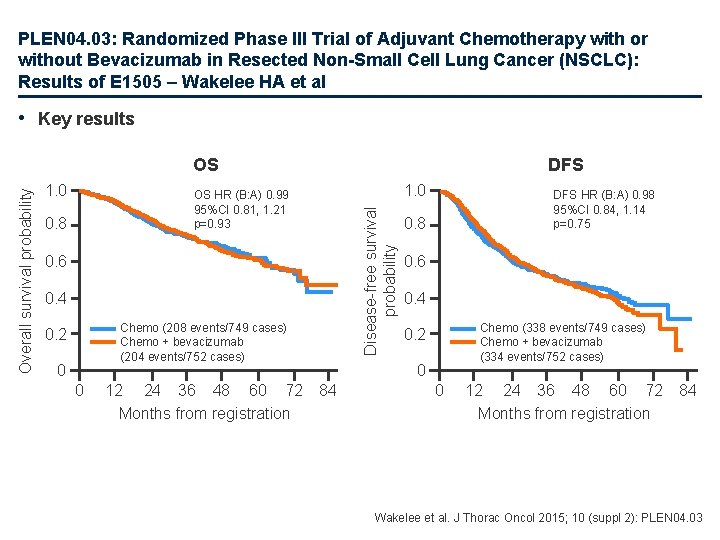
PLEN 04. 03: Randomized Phase III Trial of Adjuvant Chemotherapy with or without Bevacizumab in Resected Non-Small Cell Lung Cancer (NSCLC): Results of E 1505 – Wakelee HA et al • Key results 1. 0 DFS 1. 0 OS HR (B: A) 0. 99 95%CI 0. 81, 1. 21 p=0. 93 0. 8 Disease-free survival probability Overall survival probability OS 0. 6 0. 4 Chemo (208 events/749 cases) Chemo + bevacizumab (204 events/752 cases) 0. 2 0 0 12 24 36 48 60 72 Months from registration DFS HR (B: A) 0. 98 95%CI 0. 84, 1. 14 p=0. 75 0. 8 0. 6 0. 4 Chemo (338 events/749 cases) Chemo + bevacizumab (334 events/752 cases) 0. 2 0 84 0 12 24 36 48 60 72 Months from registration 84 Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 03

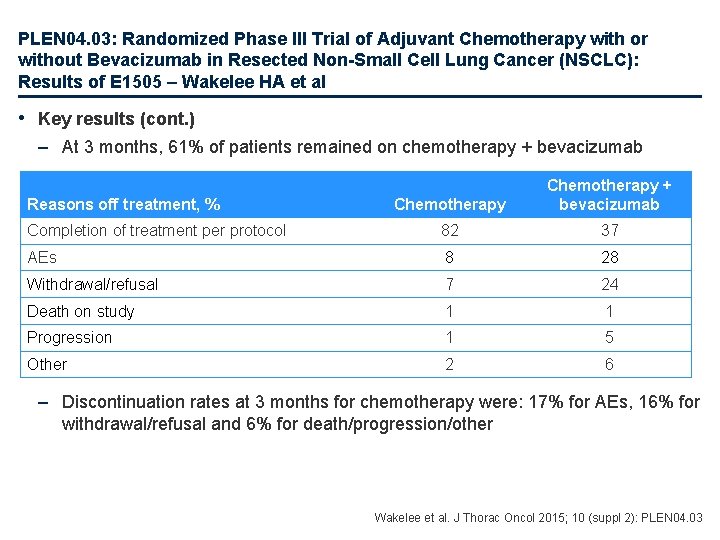
PLEN 04. 03: Randomized Phase III Trial of Adjuvant Chemotherapy with or without Bevacizumab in Resected Non-Small Cell Lung Cancer (NSCLC): Results of E 1505 – Wakelee HA et al • Key results (cont. ) – At 3 months, 61% of patients remained on chemotherapy + bevacizumab Chemotherapy + bevacizumab Completion of treatment per protocol 82 37 AEs 8 28 Withdrawal/refusal 7 24 Death on study 1 1 Progression 1 5 Other 2 6 Reasons off treatment, % – Discontinuation rates at 3 months for chemotherapy were: 17% for AEs, 16% for withdrawal/refusal and 6% for death/progression/other Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 03

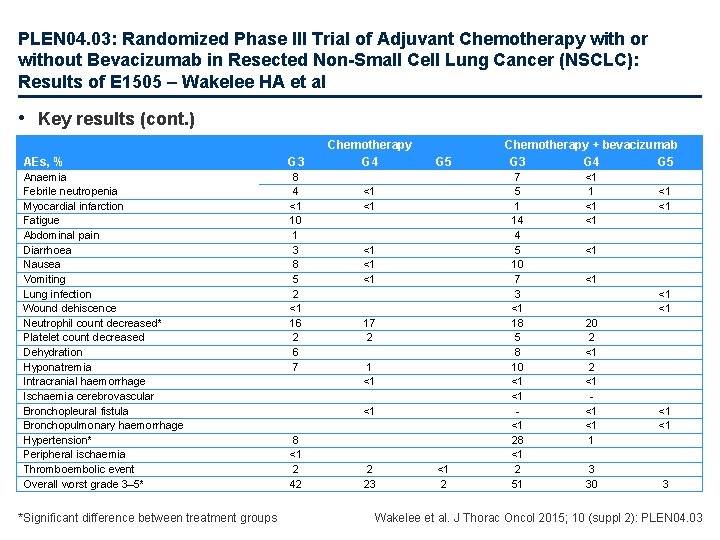
PLEN 04. 03: Randomized Phase III Trial of Adjuvant Chemotherapy with or without Bevacizumab in Resected Non-Small Cell Lung Cancer (NSCLC): Results of E 1505 – Wakelee HA et al • Key results (cont. ) AEs, % G 3 Anaemia Febrile neutropenia Myocardial infarction Fatigue Abdominal pain Diarrhoea Nausea Vomiting Lung infection Wound dehiscence Neutrophil count decreased* Platelet count decreased Dehydration Hyponatremia Intracranial haemorrhage Ischaemia cerebrovascular Bronchopleural fistula Bronchopulmonary haemorrhage Hypertension* Peripheral ischaemia Thromboembolic event Overall worst grade 3– 5* 8 4 <1 10 1 3 8 5 2 <1 16 2 6 7 *Significant difference between treatment groups Chemotherapy G 4 G 5 <1 <1 <1 17 2 1 <1 <1 8 <1 2 42 2 23 <1 2 Chemotherapy + bevacizumab G 3 G 4 G 5 7 5 1 14 4 5 10 7 3 <1 18 5 8 10 <1 <1 <1 28 <1 2 51 <1 <1 <1 20 2 <1 <1 <1 1 3 30 <1 <1 3 Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 03

PLEN 04. 03: Randomized Phase III Trial of Adjuvant Chemotherapy with or without Bevacizumab in Resected Non-Small Cell Lung Cancer (NSCLC): Results of E 1505 – Wakelee HA et al • Conclusion – The addition of bevacizumab to adjuvant chemotherapy failed to improve survival for patients with surgically resected early stage NSCLC Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 03

ORAL 35. 01: Surgical Approach and Disease Recurrence in NSCLC Patients in the MAGRIT Study – Vallieres E et al Study objective • To evaluate surgical approach and recurrence with MAGE-A vs. placebo in patients undergoing R 0 resection for NSCLC Key patient inclusion criteria • Histologically-proven, MAGE -A 3 -positive stage IB–IIIA NSCLC (AJCC 6. 0) • R 0 anatomic resection • ECOG PS 0– 2 (n=2, 272) MAGE-A (n=1, 515) R 2: 1 PD Stratification • Number of chemotherapy cycles; AJCC stage; lymph node sampling; ECOG PS; smoking status Placebo (n=757) PD Descriptive analyses • Use of mediastinal lymph node sampling (MLNS) vs. mediastinal lymph node dissection (MLND) as standard of care • Patterns of recurrence by type of lymphadenectomy • Cox model to assess the prognostic value of lymphadenectomy for DFS and OS Vallieres et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 35. 01

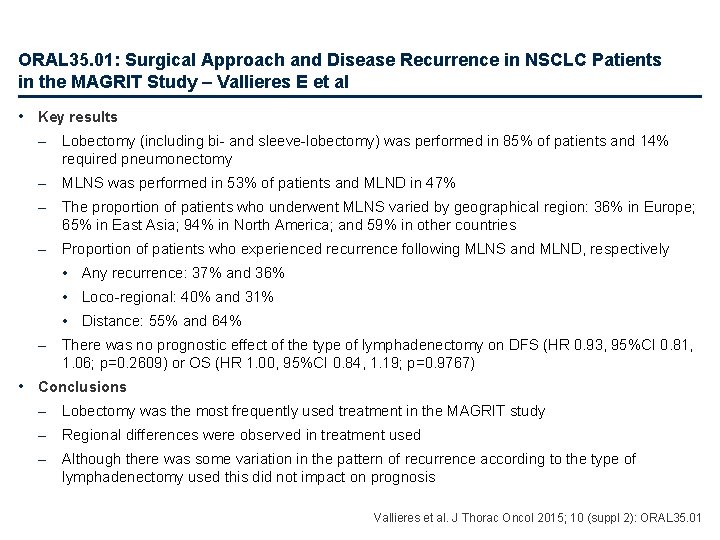
ORAL 35. 01: Surgical Approach and Disease Recurrence in NSCLC Patients in the MAGRIT Study – Vallieres E et al • Key results – Lobectomy (including bi- and sleeve-lobectomy) was performed in 85% of patients and 14% required pneumonectomy – MLNS was performed in 53% of patients and MLND in 47% – The proportion of patients who underwent MLNS varied by geographical region: 36% in Europe; 65% in East Asia; 94% in North America; and 59% in other countries – Proportion of patients who experienced recurrence following MLNS and MLND, respectively • Any recurrence: 37% and 36% • Loco-regional: 40% and 31% • Distance: 55% and 64% – There was no prognostic effect of the type of lymphadenectomy on DFS (HR 0. 93, 95%CI 0. 81, 1. 06; p=0. 2609) or OS (HR 1. 00, 95%CI 0. 84, 1. 19; p=0. 9767) • Conclusions – Lobectomy was the most frequently used treatment in the MAGRIT study – Regional differences were observed in treatment used – Although there was some variation in the pattern of recurrence according to the type of lymphadenectomy used this did not impact on prognosis Vallieres et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 35. 01

ORAL 35. 02: Wedge Resection vs Segmentectomy for Patients with T 1 A N 0 Non-Small Cell Lung Cancer – Yang CFJ DR et al Study objective • To investigate the efficacy and safety of wedge resection vs. segmentectomy in patients with stage T 1 a N 0 NSCLC Key patient inclusion criteria Wedge resection (n=7, 517) PD Segmentectomy (n=1, 268) PD • Stage T 1 a N 0 NSCLC • Undergone wedge resection or segmentectomy 2003– 2011 (n=8, 785) • Patients were identified using the USA National Cancer Data Base • After propensity score matching, baseline covariates were balanced between the wedge resection (n=1, 231) and segmentectomy (n=1, 231) groups Yang et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 35. 02

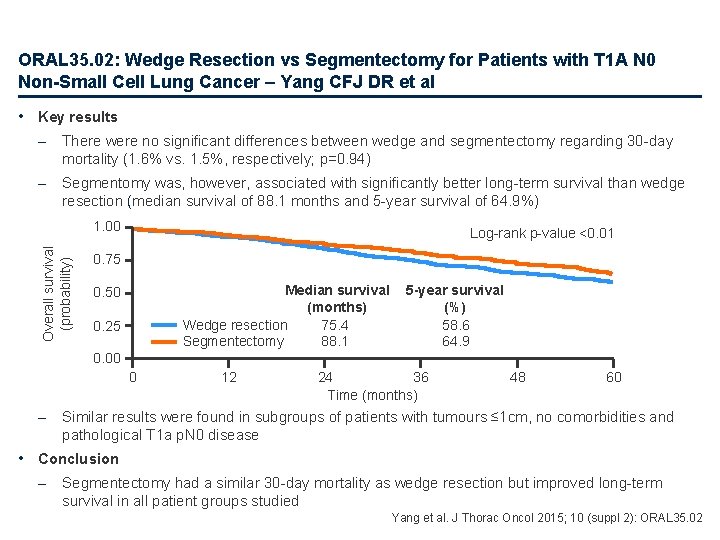
ORAL 35. 02: Wedge Resection vs Segmentectomy for Patients with T 1 A N 0 Non-Small Cell Lung Cancer – Yang CFJ DR et al • Key results – There were no significant differences between wedge and segmentectomy regarding 30 -day mortality (1. 6% vs. 1. 5%, respectively; p=0. 94) – Segmentomy was, however, associated with significantly better long-term survival than wedge resection (median survival of 88. 1 months and 5 -year survival of 64. 9%) Overall survival (probability) 1. 00 Log-rank p-value <0. 01 0. 75 Median survival (months) Wedge resection 75. 4 Segmentectomy 88. 1 0. 50 0. 25 5 -year survival (%) 58. 6 64. 9 0. 00 0 – 12 24 36 Time (months) 48 60 Similar results were found in subgroups of patients with tumours ≤ 1 cm, no comorbidities and pathological T 1 a p. N 0 disease • Conclusion – Segmentectomy had a similar 30 -day mortality as wedge resection but improved long-term survival in all patient groups studied Yang et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 35. 02

Advanced NSCLC Not radically treatable stage III and stage IV 1 st line

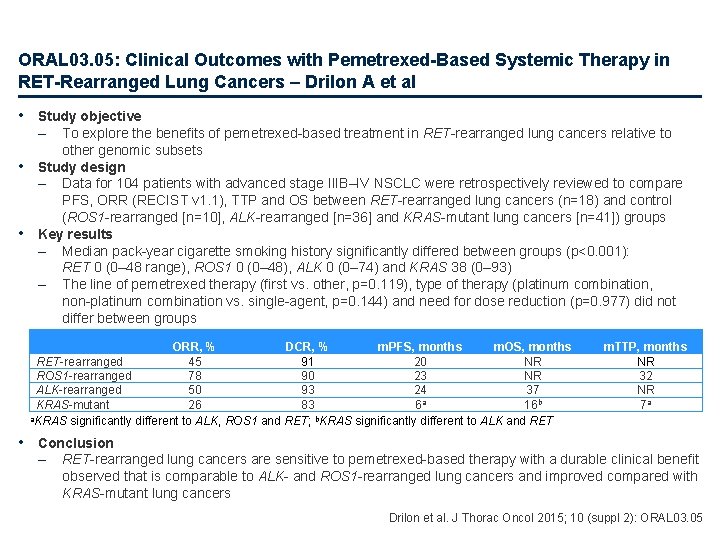
ORAL 03. 05: Clinical Outcomes with Pemetrexed-Based Systemic Therapy in RET-Rearranged Lung Cancers – Drilon A et al • Study objective – • • To explore the benefits of pemetrexed-based treatment in RET-rearranged lung cancers relative to other genomic subsets Study design – Data for 104 patients with advanced stage IIIB–IV NSCLC were retrospectively reviewed to compare PFS, ORR (RECIST v 1. 1), TTP and OS between RET-rearranged lung cancers (n=18) and control (ROS 1 -rearranged [n=10], ALK-rearranged [n=36] and KRAS-mutant lung cancers [n=41]) groups Key results – Median pack-year cigarette smoking history significantly differed between groups (p<0. 001): RET 0 (0– 48 range), ROS 1 0 (0– 48), ALK 0 (0– 74) and KRAS 38 (0– 93) – The line of pemetrexed therapy (first vs. other, p=0. 119), type of therapy (platinum combination, non-platinum combination vs. single-agent, p=0. 144) and need for dose reduction (p=0. 977) did not differ between groups ORR, % DCR, % m. PFS, months m. OS, months RET-rearranged 45 91 20 NR ROS 1 -rearranged 78 90 23 NR ALK-rearranged 50 93 24 37 a KRAS-mutant 26 83 6 16 b a. KRAS significantly different to ALK, ROS 1 and RET; b. KRAS significantly different to ALK and RET m. TTP, months NR 32 NR 7 a • Conclusion – RET-rearranged lung cancers are sensitive to pemetrexed-based therapy with a durable clinical benefit observed that is comparable to ALK- and ROS 1 -rearranged lung cancers and improved compared with KRAS-mutant lung cancers Drilon et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 03. 05

Advanced NSCLC Not radically treatable stage III and stage IV Later lines

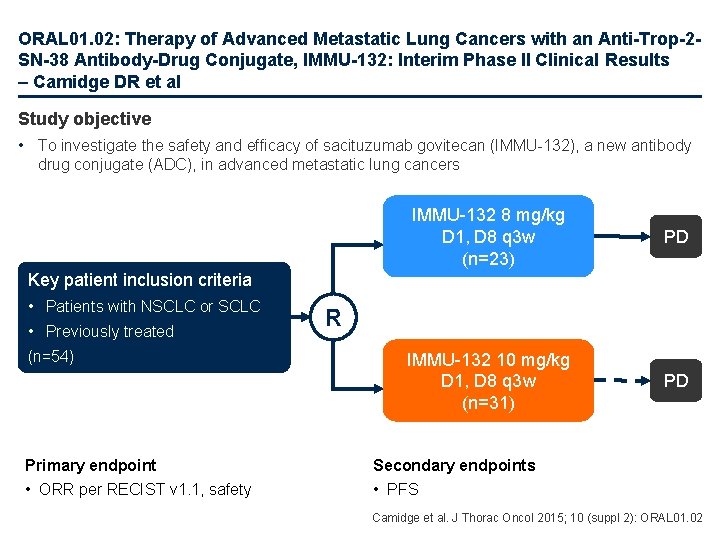
ORAL 01. 02: Therapy of Advanced Metastatic Lung Cancers with an Anti-Trop-2 SN-38 Antibody-Drug Conjugate, IMMU-132: Interim Phase II Clinical Results – Camidge DR et al Study objective • To investigate the safety and efficacy of sacituzumab govitecan (IMMU-132), a new antibody drug conjugate (ADC), in advanced metastatic lung cancers IMMU-132 8 mg/kg D 1, D 8 q 3 w (n=23) PD IMMU-132 10 mg/kg D 1, D 8 q 3 w (n=31) PD Key patient inclusion criteria • Patients with NSCLC or SCLC • Previously treated (n=54) Primary endpoint • ORR per RECIST v 1. 1, safety R Secondary endpoints • PFS Camidge et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 01. 02

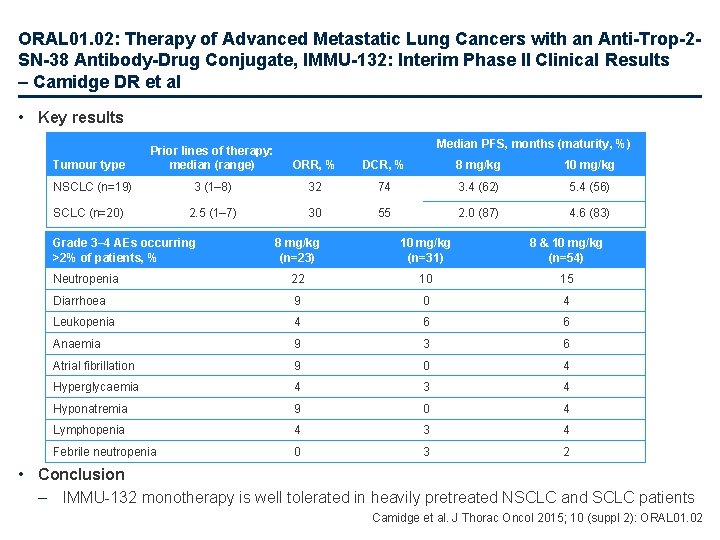
ORAL 01. 02: Therapy of Advanced Metastatic Lung Cancers with an Anti-Trop-2 SN-38 Antibody-Drug Conjugate, IMMU-132: Interim Phase II Clinical Results – Camidge DR et al • Key results Median PFS, months (maturity, %) Tumour type Prior lines of therapy: median (range) ORR, % DCR, % 8 mg/kg 10 mg/kg NSCLC (n=19) 3 (1– 8) 32 74 3. 4 (62) 5. 4 (56) 2. 5 (1– 7) 30 55 2. 0 (87) 4. 6 (83) SCLC (n=20) Grade 3– 4 AEs occurring >2% of patients, % 8 mg/kg (n=23) 10 mg/kg (n=31) 8 & 10 mg/kg (n=54) Neutropenia 22 10 15 Diarrhoea 9 0 4 Leukopenia 4 6 6 Anaemia 9 3 6 Atrial fibrillation 9 0 4 Hyperglycaemia 4 3 4 Hyponatremia 9 0 4 Lymphopenia 4 3 4 Febrile neutropenia 0 3 2 • Conclusion – IMMU-132 monotherapy is well tolerated in heavily pretreated NSCLC and SCLC patients Camidge et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 01. 02

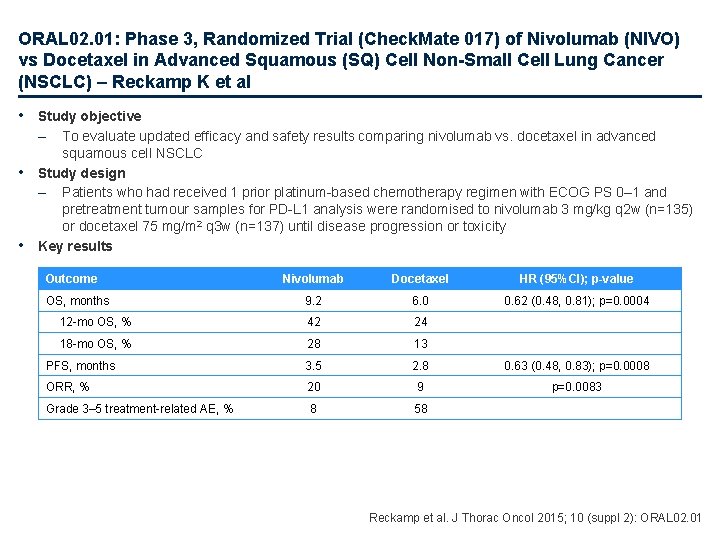
ORAL 02. 01: Phase 3, Randomized Trial (Check. Mate 017) of Nivolumab (NIVO) vs Docetaxel in Advanced Squamous (SQ) Cell Non-Small Cell Lung Cancer (NSCLC) – Reckamp K et al • Study objective – • • To evaluate updated efficacy and safety results comparing nivolumab vs. docetaxel in advanced squamous cell NSCLC Study design – Patients who had received 1 prior platinum-based chemotherapy regimen with ECOG PS 0– 1 and pretreatment tumour samples for PD-L 1 analysis were randomised to nivolumab 3 mg/kg q 2 w (n=135) or docetaxel 75 mg/m 2 q 3 w (n=137) until disease progression or toxicity Key results Outcome Nivolumab Docetaxel HR (95%CI); p-value 9. 2 6. 0 0. 62 (0. 48, 0. 81); p=0. 0004 12 -mo OS, % 42 24 18 -mo OS, % 28 13 PFS, months 3. 5 2. 8 0. 63 (0. 48, 0. 83); p=0. 0008 ORR, % 20 9 p=0. 0083 Grade 3– 5 treatment-related AE, % 8 58 OS, months Reckamp et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 02. 01

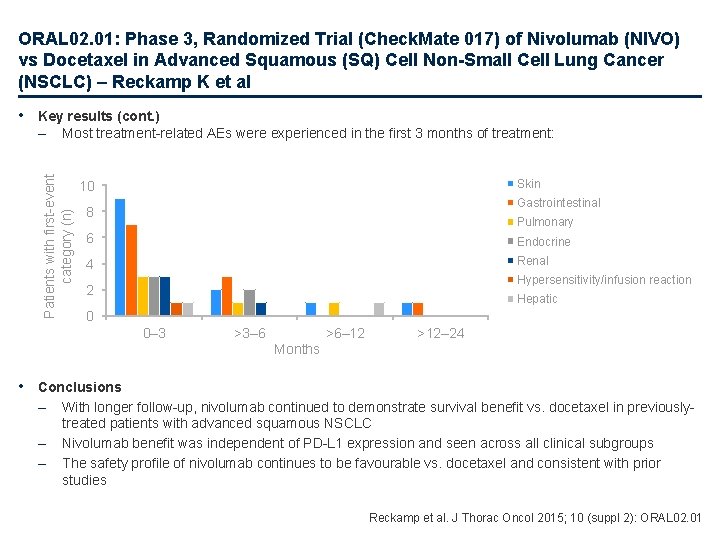
ORAL 02. 01: Phase 3, Randomized Trial (Check. Mate 017) of Nivolumab (NIVO) vs Docetaxel in Advanced Squamous (SQ) Cell Non-Small Cell Lung Cancer (NSCLC) – Reckamp K et al • Key results (cont. ) Most treatment-related AEs were experienced in the first 3 months of treatment: Patients with first-event category (n) – Skin 10 Gastrointestinal 8 Pulmonary 6 Endocrine 4 Renal Hypersensitivity/infusion reaction 2 Hepatic 0 0– 3 >3– 6 >6– 12 >12– 24 Months • Conclusions – – – With longer follow-up, nivolumab continued to demonstrate survival benefit vs. docetaxel in previouslytreated patients with advanced squamous NSCLC Nivolumab benefit was independent of PD-L 1 expression and seen across all clinical subgroups The safety profile of nivolumab continues to be favourable vs. docetaxel and consistent with prior studies Reckamp et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 02. 01

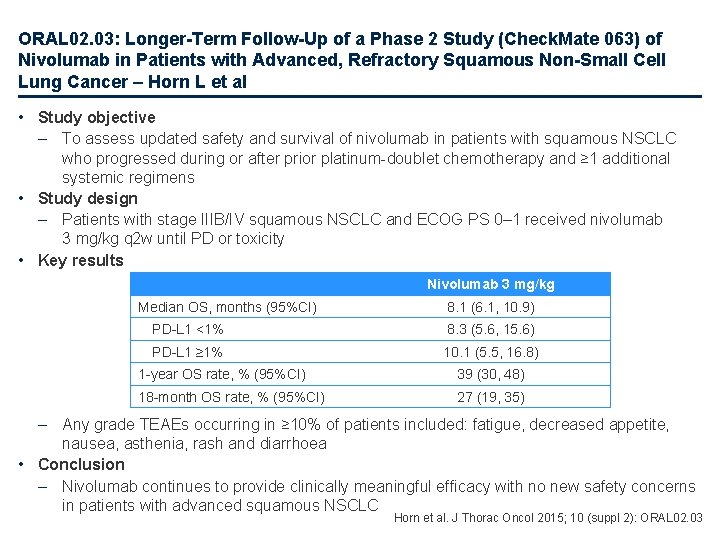
ORAL 02. 03: Longer-Term Follow-Up of a Phase 2 Study (Check. Mate 063) of Nivolumab in Patients with Advanced, Refractory Squamous Non-Small Cell Lung Cancer – Horn L et al • Study objective – To assess updated safety and survival of nivolumab in patients with squamous NSCLC who progressed during or after prior platinum-doublet chemotherapy and ≥ 1 additional systemic regimens • Study design – Patients with stage IIIB/IV squamous NSCLC and ECOG PS 0– 1 received nivolumab 3 mg/kg q 2 w until PD or toxicity • Key results Nivolumab 3 mg/kg Median OS, months (95%CI) 8. 1 (6. 1, 10. 9) PD-L 1 <1% 8. 3 (5. 6, 15. 6) PD-L 1 ≥ 1% 10. 1 (5. 5, 16. 8) 1 -year OS rate, % (95%CI) 39 (30, 48) 18 -month OS rate, % (95%CI) 27 (19, 35) – Any grade TEAEs occurring in ≥ 10% of patients included: fatigue, decreased appetite, nausea, asthenia, rash and diarrhoea • Conclusion – Nivolumab continues to provide clinically meaningful efficacy with no new safety concerns in patients with advanced squamous NSCLC Horn et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 02. 03
![ORAL 02 05 Safety and Efficacy of FirstLine Nivolumab NIVO Anti Programmed Death1 PD1 ORAL 02. 05: Safety and Efficacy of First-Line Nivolumab (NIVO; Anti. Programmed Death-1 [PD-1])](https://slidetodoc.com/presentation_image_h2/531de7db6f58a53e88c014b528ad16ad/image-59.jpg)
ORAL 02. 05: Safety and Efficacy of First-Line Nivolumab (NIVO; Anti. Programmed Death-1 [PD-1]) and Ipilimumab in Non-Small Cell Lung Cancer (NSCLC) – Rizvi NA et al • Study objective – • • To evaluate the safety and efficacy of first-line therapy with nivolumab plus ipilimumab in chemotherapy -naïve patients with advanced NSCLC Study design – Patients with stage IIIB/IV NSCLC (any histology), who had received no prior chemotherapy for advanced disease and ECOG PS 0 or 1 were randomised to nivolumab 1 mg/kg + ipilimumab 1 mg/kg both q 3 w, nivolumab 1 mg/kg q 2 w + ipilimumab 1 mg/kg q 6 w, nivolumab 3 mg/kg q 2 w + ipilimumab 1 mg/kg q 12 w or nivolumab 3 mg/kg q 2 w + ipilimumab 1 mg/kg q 6 w Key results NIVO 1 + IPI 1 q 3 w NIVO 1 q 2 w + IPI 1 q 6 w NIVO 3 q 2 w + IPI 1 q 12 w NIVO 3 q 2 w + IPI 1 q 6 w Treatment-related AEs leading to discontinuation, any grade, % 13 8 5 10 Treatment-related AEs leading to discontinuation, grade 3– 4, % 10 8 3 10 Confirmed overall response, % 13 25 39 31 10. 6 4. 9 8. 0 8. 3 55 NC 63 NC Outcome Median PFS, months 24 -week PFS, % • Conclusions – – First-line therapy with nivolumab plus ipilimumab demonstrated high level of clinical activity with durable responses Nivolumab plus ipilimumab is associated with a manageable safety profile Rizvi et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 02. 05

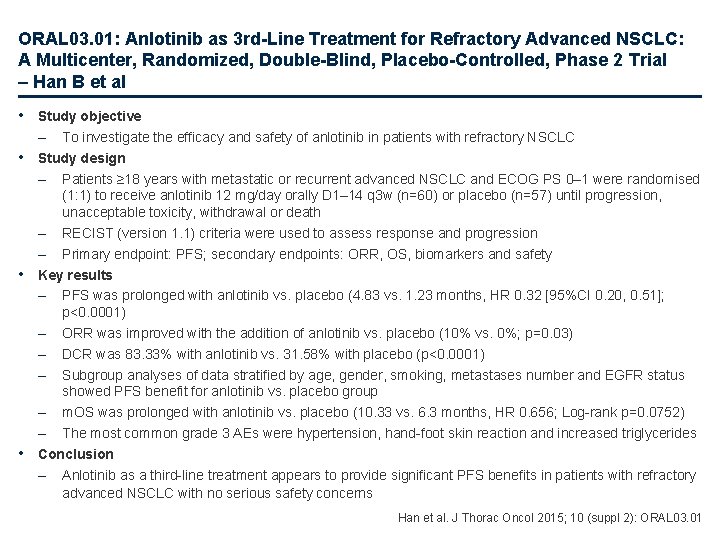
ORAL 03. 01: Anlotinib as 3 rd-Line Treatment for Refractory Advanced NSCLC: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial – Han B et al • Study objective – To investigate the efficacy and safety of anlotinib in patients with refractory NSCLC • Study design – Patients ≥ 18 years with metastatic or recurrent advanced NSCLC and ECOG PS 0– 1 were randomised (1: 1) to receive anlotinib 12 mg/day orally D 1– 14 q 3 w (n=60) or placebo (n=57) until progression, unacceptable toxicity, withdrawal or death – RECIST (version 1. 1) criteria were used to assess response and progression – Primary endpoint: PFS; secondary endpoints: ORR, OS, biomarkers and safety • Key results – PFS was prolonged with anlotinib vs. placebo (4. 83 vs. 1. 23 months, HR 0. 32 [95%CI 0. 20, 0. 51]; p<0. 0001) – ORR was improved with the addition of anlotinib vs. placebo (10% vs. 0%; p=0. 03) – DCR was 83. 33% with anlotinib vs. 31. 58% with placebo (p<0. 0001) – Subgroup analyses of data stratified by age, gender, smoking, metastases number and EGFR status showed PFS benefit for anlotinib vs. placebo group – m. OS was prolonged with anlotinib vs. placebo (10. 33 vs. 6. 3 months, HR 0. 656; Log-rank p=0. 0752) – The most common grade 3 AEs were hypertension, hand-foot skin reaction and increased triglycerides • Conclusion – Anlotinib as a third-line treatment appears to provide significant PFS benefits in patients with refractory advanced NSCLC with no serious safety concerns Han et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 03. 01

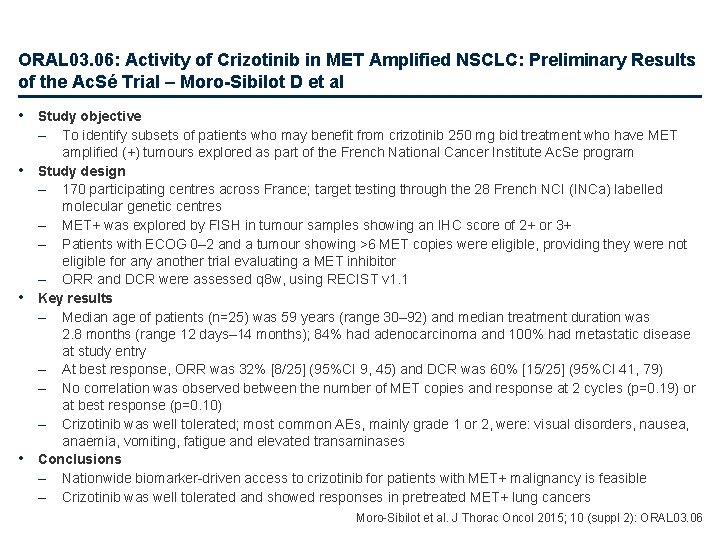
ORAL 03. 06: Activity of Crizotinib in MET Amplified NSCLC: Preliminary Results of the Ac. Sé Trial – Moro-Sibilot D et al • Study objective – • • • To identify subsets of patients who may benefit from crizotinib 250 mg bid treatment who have MET amplified (+) tumours explored as part of the French National Cancer Institute Ac. Se program Study design – 170 participating centres across France; target testing through the 28 French NCI (INCa) labelled molecular genetic centres – MET+ was explored by FISH in tumour samples showing an IHC score of 2+ or 3+ – Patients with ECOG 0– 2 and a tumour showing >6 MET copies were eligible, providing they were not eligible for any another trial evaluating a MET inhibitor – ORR and DCR were assessed q 8 w, using RECIST v 1. 1 Key results – Median age of patients (n=25) was 59 years (range 30– 92) and median treatment duration was 2. 8 months (range 12 days– 14 months); 84% had adenocarcinoma and 100% had metastatic disease at study entry – At best response, ORR was 32% [8/25] (95%CI 9, 45) and DCR was 60% [15/25] (95%CI 41, 79) – No correlation was observed between the number of MET copies and response at 2 cycles (p=0. 19) or at best response (p=0. 10) – Crizotinib was well tolerated; most common AEs, mainly grade 1 or 2, were: visual disorders, nausea, anaemia, vomiting, fatigue and elevated transaminases Conclusions – Nationwide biomarker-driven access to crizotinib for patients with MET+ malignancy is feasible – Crizotinib was well tolerated and showed responses in pretreated MET+ lung cancers Moro-Sibilot et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 03. 06

ORAL 03. 07: Response to crizotinib and cabozantinib in Stage IV Lung NSCLC Patients with Mutations That Cause MET Exon 14 Skipping – Paik P et al • Study objective – • • • To investigate responses to crizotinib and cabozantinib in patients with stage IV lung adenocarcinomas harbouring mutations leading to MET exon 14 skipping and report the clinical characteristics associated with this genotype Study design – Patients with stage IV NSCLC harbouring MET exon 14 splice site mutations (n=26) or a mutation deleting Y 1003 in exon 14 (n=1) were identified through a clinical assay based on hybrid capture/nextgeneration sequencing of 341 oncogenes and tumour suppressors (MSK-IMPACT) – Radiographic response to MET inhibition was assessed using RECIST 1. 1 and PERCIST criteria Key results – Overall, 56% patients had smoking history and 70% had adenocarcinoma – Patients were treated with off-label crizotinib (n=7), crizotinib on a clinical trial (n=8; NCT 00585195) or cabozantinib (n=1; NCT 01639508) – To date, 5 patients have been treated with off-label crizotinib and 1 with cabozantinib and crizotinib • Four of six patients (66. 7%) developed a PR to crizotinib treatment, including one patient who had previously received cabozantinib and had shown SD by RECIST and PET imaging demonstrating a complete PERCIST response to treatment initially • The remaining 2 patients on off-label crizotinib have their first scan pending Conclusions – MET exon 14 skipping is a novel oncogenic target that can predict response to MET inhibitors – Patients with these splice site mutations should be treated on a clinical trial of a MET inhibitor Paik et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 03. 07

MINI 03. 01: Prior TKI Therapy in NSCLC EGFR Mutant Patients Associates with Lack of Response to Anti-PD-1 Treatment – Garon EB et al Study objective • To determine the relationship between prior TKI therapy and response to anti-PD-1 therapy in patients with NSCLC EGFR mutations (EGFRm) Study design • In vitro – Western blot analysis of PD-L 1 expression in NSCLC cell lines in response to erlotinib and afatinib • Clinical – Retrospective analysis of patients from KEYNOTE-001 clinical trial in which patients received pembrolizumab 2 mg/kg q 3 w or 10 mg/kg q 2– 3 w – Patients • Early in the trial, patients with EGFRm were excluded; a subsequent amendment required patients to have a known EGFR status • Those with EGFR sensitising mutations must have had documented disease progression on erlotinib, gefitinib or afatinib and documented disease progression on subsequent platinum-double chemotherapy Garon et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 03. 01

MINI 03. 01: Prior TKI Therapy in NSCLC EGFR Mutant Patients Associates with Lack of Response to Anti-PD-1 Treatment – Garon EB et al • Key results – In vitro • PD-L 1 levels decrease in response to EGFR TKI in cell lines sensitive to TKI – Clinical • Among 29 EGFRm patients in KEYNOTE-001, 2 of 3 EGFR-naïve patients achieved a PR vs. 1 of 26 with a history of prior EGFR TKI (p<0. 001) • 18 of these patients had a 9 -week follow-up scan – Both of the EGFR TKI-naïve patients achieved a PR compared with only 1 of 16 with a history of prior EGFR TKI (p<0. 001) • The 1 patient with a history of prior EGFR TKI therapy who achieved a PR harboured an exon 20 insertion • In EGFR wild-type patients, prior TKI did not affect RR (p<0. 601) • A lower proportion of EGFRm patients continued on trial to the 9 -week scan (62% [18/29]), compared with EGFR wild-type patients (87% [60/69]) • Conclusions – There was a strong correlation between response and lack of prior EGFR TKI treatment, especially in those with a sensitising mutation – PD-L 1 levels decreased in response to an EGFR in cell lines sensitive to TKI – PD-1/PD-L 1 inhibition prior to an EGFR TKI may be more efficacious than a strategy in which the PD-1/PD-L 1 inhibition follows, or is given concurrently with an EGFR TKI Garon et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 03. 01

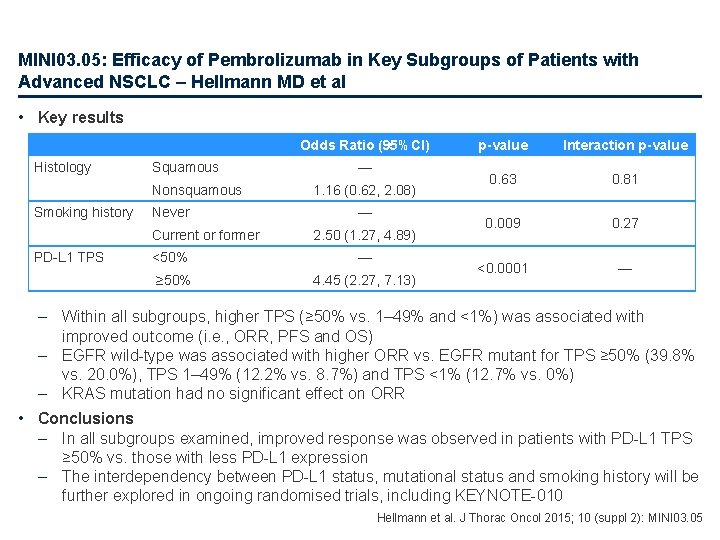
MINI 03. 05: Efficacy of Pembrolizumab in Key Subgroups of Patients with Advanced NSCLC – Hellmann MD et al Study objective – To assess the relationship between anti-tumour activity and the level of PD-L 1 expression in key patient subgroups Study design (KEYNOTE-001) – Treatment: pembrolizumab 2 mg/kg q 3 w, 10 mg/kg q 3 w or 10 mg/kg q 2 w until confirmed progression, intolerable toxicity or investigator decision – PD-L 1 expression: Assessed by IHC using a clinical assay and 22 C 3 antibody – Patients (n=550) • Evaluable patients for PD-L 1 had slides prepared within 6 months and for which a total proportion score (TPS) could be assigned • Key subgroups analysed: – Histology, smoking status, mutational status Hellmann et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 03. 05

MINI 03. 05: Efficacy of Pembrolizumab in Key Subgroups of Patients with Advanced NSCLC – Hellmann MD et al • Key results Odds Ratio (95%CI) Histology Squamous Nonsquamous Smoking history Never Current or former PD-L 1 TPS — 1. 16 (0. 62, 2. 08) — 2. 50 (1. 27, 4. 89) <50% — ≥ 50% 4. 45 (2. 27, 7. 13) p-value Interaction p-value 0. 63 0. 81 0. 009 0. 27 <0. 0001 — – Within all subgroups, higher TPS (≥ 50% vs. 1– 49% and <1%) was associated with improved outcome (i. e. , ORR, PFS and OS) – EGFR wild-type was associated with higher ORR vs. EGFR mutant for TPS ≥ 50% (39. 8% vs. 20. 0%), TPS 1– 49% (12. 2% vs. 8. 7%) and TPS <1% (12. 7% vs. 0%) – KRAS mutation had no significant effect on ORR • Conclusions – In all subgroups examined, improved response was observed in patients with PD-L 1 TPS ≥ 50% vs. those with less PD-L 1 expression – The interdependency between PD-L 1 status, mutational status and smoking history will be further explored in ongoing randomised trials, including KEYNOTE-010 Hellmann et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 03. 05

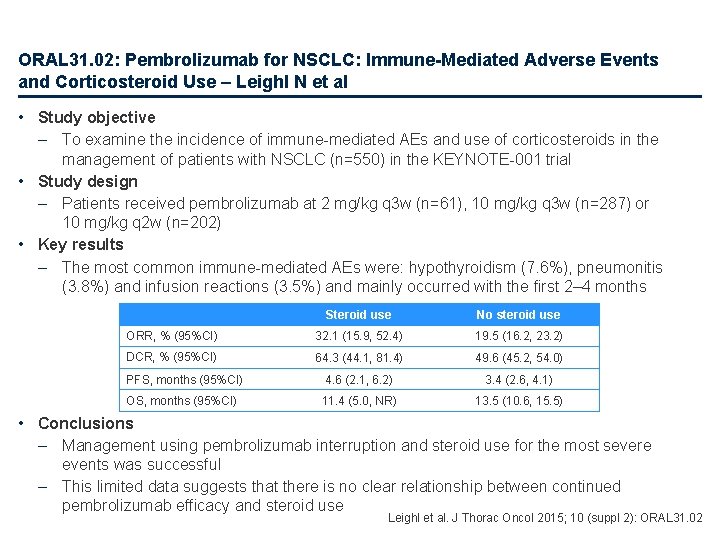
ORAL 31. 02: Pembrolizumab for NSCLC: Immune-Mediated Adverse Events and Corticosteroid Use – Leighl N et al • Study objective – To examine the incidence of immune-mediated AEs and use of corticosteroids in the management of patients with NSCLC (n=550) in the KEYNOTE-001 trial • Study design – Patients received pembrolizumab at 2 mg/kg q 3 w (n=61), 10 mg/kg q 3 w (n=287) or 10 mg/kg q 2 w (n=202) • Key results – The most common immune-mediated AEs were: hypothyroidism (7. 6%), pneumonitis (3. 8%) and infusion reactions (3. 5%) and mainly occurred with the first 2– 4 months Steroid use No steroid use ORR, % (95%CI) 32. 1 (15. 9, 52. 4) 19. 5 (16. 2, 23. 2) DCR, % (95%CI) 64. 3 (44. 1, 81. 4) 49. 6 (45. 2, 54. 0) PFS, months (95%CI) 4. 6 (2. 1, 6. 2) 3. 4 (2. 6, 4. 1) OS, months (95%CI) 11. 4 (5. 0, NR) 13. 5 (10. 6, 15. 5) • Conclusions – Management using pembrolizumab interruption and steroid use for the most severe events was successful – This limited data suggests that there is no clear relationship between continued pembrolizumab efficacy and steroid use Leighl et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 31. 02

MINI 03. 09: Role of T 790 M Mutation in EGFR-TKI Rechallenge for Patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer – Zhang Q-Y et al Study objective • To retrospectively evaluate the role of T 790 M mutation in EGFR-TKI rechallenge after failure of initial EGFR-TKIs in EGFR-mutant advanced NSCLC Study design • Data were analysed retrospectively from 515 patients with stage IIIB/IV EGFRmutant NSCLC who had previously received gefitinib or erlotinib – Of these, 51 patients had EGFR exon 19 or 21 L 858 R mutations and underwent rebiopsy after failure (at resistance) of initial EGFR-TKIs followed by EGFR-TKI rechallenge • EGFR detection was performed by Sanger sequencing or Amplification Refractory Mutation System methods • PFS and OS data were analysed using the Kaplan-Meier method and log-rank test • EGFR activating mutations still existed in all the 51 patients who received rebiopsy: – 18 patients had T 790 M mutations and 33 patients were without T 790 M mutations Zhang et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 03. 09

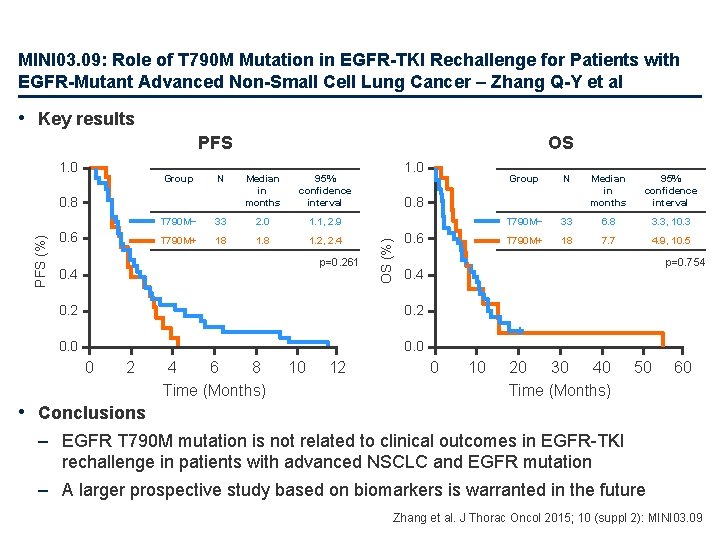
MINI 03. 09: Role of T 790 M Mutation in EGFR-TKI Rechallenge for Patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer – Zhang Q-Y et al • Key results PFS Group N PFS (%) 0. 8 0. 6 Median in months 95% confidence interval T 790 M− 33 2. 0 1. 1, 2. 9 T 790 M+ 18 1. 2, 2. 4 p=0. 261 0. 4 0. 2 1. 0 Group N Median in months 95% confidence interval T 790 M− 33 6. 8 3. 3, 10. 3 T 790 M+ 18 7. 7 4. 9, 10. 5 0. 8 OS (%) 1. 0 OS 0. 6 p=0. 754 + 0. 4 0. 2 + 0. 0 0 2 4 6 8 Time (Months) 10 12 0 10 20 30 40 Time (Months) 50 60 • Conclusions – EGFR T 790 M mutation is not related to clinical outcomes in EGFR-TKI rechallenge in patients with advanced NSCLC and EGFR mutation – A larger prospective study based on biomarkers is warranted in the future Zhang et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 03. 09

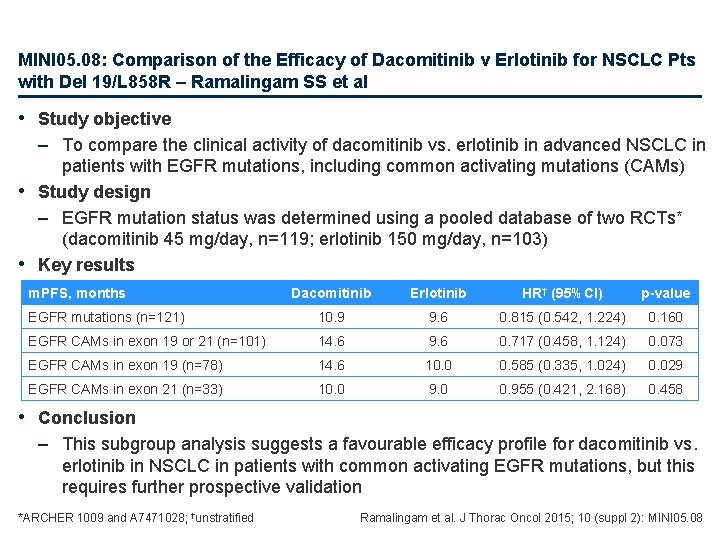
MINI 05. 08: Comparison of the Efficacy of Dacomitinib v Erlotinib for NSCLC Pts with Del 19/L 858 R – Ramalingam SS et al • Study objective – To compare the clinical activity of dacomitinib vs. erlotinib in advanced NSCLC in patients with EGFR mutations, including common activating mutations (CAMs) • Study design – EGFR mutation status was determined using a pooled database of two RCTs* (dacomitinib 45 mg/day, n=119; erlotinib 150 mg/day, n=103) • Key results m. PFS, months Dacomitinib Erlotinib HR† (95%CI) p-value EGFR mutations (n=121) 10. 9 9. 6 0. 815 (0. 542, 1. 224) 0. 160 EGFR CAMs in exon 19 or 21 (n=101) 14. 6 9. 6 0. 717 (0. 458, 1. 124) 0. 073 EGFR CAMs in exon 19 (n=78) 14. 6 10. 0 0. 585 (0. 335, 1. 024) 0. 029 EGFR CAMs in exon 21 (n=33) 10. 0 9. 0 0. 955 (0. 421, 2. 168) 0. 458 • Conclusion – This subgroup analysis suggests a favourable efficacy profile for dacomitinib vs. erlotinib in NSCLC in patients with common activating EGFR mutations, but this requires further prospective validation *ARCHER 1009 and A 7471028; †unstratified Ramalingam et al. J Thorac Oncol 2015; 10 (suppl 2): MINI 05. 08

ORAL 18. 02: MUC 1 -Targeted Dendritic Cell-Based Vaccine Immunotherapy in Patients with Standard Treatments-Refractory Non-Small-Cell Lung Cancer – Teramoto K et al • Study objective – To evaluate the efficacy of MUC 1 -targeted dendritic cell-based vaccine immunotherapy in patients with advanced NSCLC who are refractory to standard treatments • Study design – Patients (n=41) with histologically- or cytologically-confirmed NSCLC, ECOG PS 0– 2 were administered subcutaneous MUC 1 -targeted dendritic cell-based vaccine immunotherapy q 2 w – Tumour responses were assessed in 29 patients • Key results – Median number of vaccinations was 6 (range 1– 42) – Across all patients the median OS from first vaccination was 7. 4 months (95%CI 4. 5, 10. 3); 1 -year survival rate was 29. 3% (95%CI 15. 3, 41. 2) – In patients who received >6 vaccinations (n=29), median OS was 10. 1 months (95%CI 8. 3, 11. 9) from the first vaccination and 1 -year survival rate was 41. 4% (95%CI 23. 5, 59. 3) – Patients with immune-related AEs or peripheral white blood cells >20% had the best prognosis • Conclusion – MUC 1 -targeted dendritic cell-based vaccine immunotherapy demonstrated interesting early activity in patients with NSCLC refractory to standard treatments Teramoto et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 18. 02

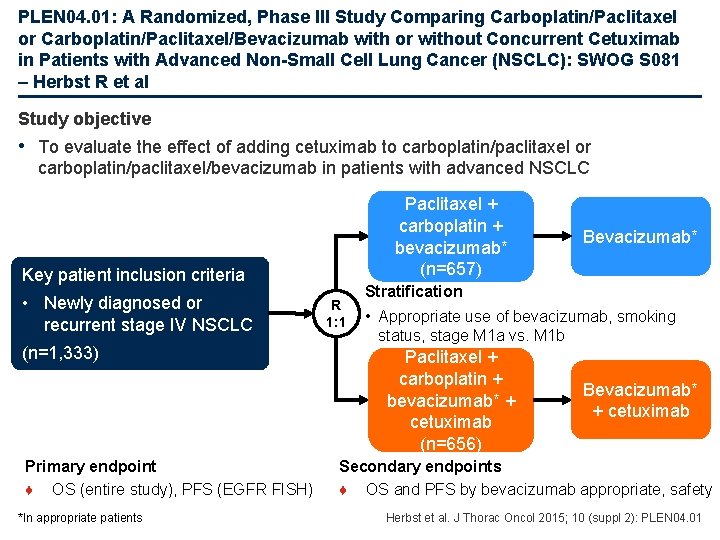
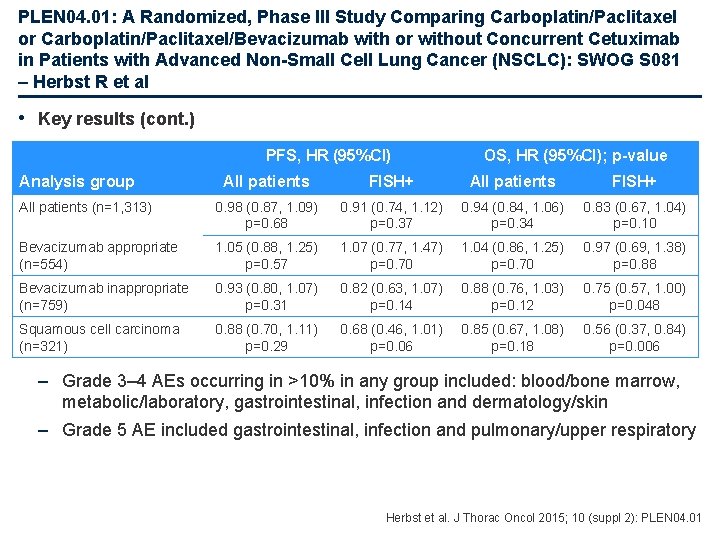
PLEN 04. 01: A Randomized, Phase III Study Comparing Carboplatin/Paclitaxel or Carboplatin/Paclitaxel/Bevacizumab with or without Concurrent Cetuximab in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): SWOG S 081 – Herbst R et al Study objective • To evaluate the effect of adding cetuximab to carboplatin/paclitaxel or carboplatin/paclitaxel/bevacizumab in patients with advanced NSCLC Paclitaxel + carboplatin + bevacizumab* (n=657) Key patient inclusion criteria • Newly diagnosed or recurrent stage IV NSCLC (n=1, 333) Primary endpoint ♦ OS (entire study), PFS (EGFR FISH) *In appropriate patients R 1: 1 Bevacizumab* Stratification • Appropriate use of bevacizumab, smoking status, stage M 1 a vs. M 1 b Paclitaxel + carboplatin + bevacizumab* + cetuximab (n=656) Bevacizumab* + cetuximab Secondary endpoints ♦ OS and PFS by bevacizumab appropriate, safety Herbst et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 01

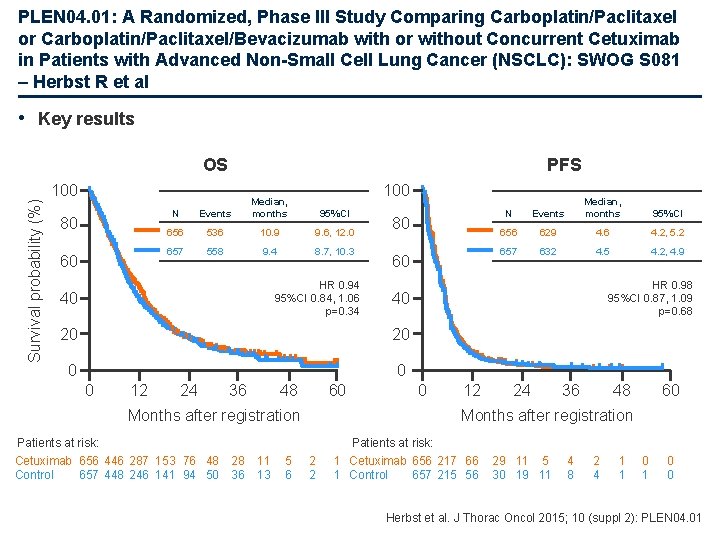
PLEN 04. 01: A Randomized, Phase III Study Comparing Carboplatin/Paclitaxel or Carboplatin/Paclitaxel/Bevacizumab with or without Concurrent Cetuximab in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): SWOG S 081 – Herbst R et al • Key results Survival probability (%) OS PFS 100 80 60 100 N Events Median, months 656 536 10. 9 9. 6, 12. 0 657 558 9. 4 8. 7, 10. 3 95%CI HR 0. 94 95%CI 0. 84, 1. 06 p=0. 34 40 80 60 20 0 0 12 24 36 48 60 Months after registration Patients at risk: Cetuximab 656 446 287 153 76 48 28 Control 657 448 246 141 94 50 36 11 13 5 6 Events Median, months 95%CI 656 629 4. 6 4. 2, 5. 2 657 632 4. 5 4. 2, 4. 9 HR 0. 98 95%CI 0. 87, 1. 09 p=0. 68 40 20 0 N 0 12 24 36 48 60 Months after registration 2 2 Patients at risk: 1 Cetuximab 656 217 66 1 Control 657 215 56 29 11 5 30 19 11 4 8 2 4 1 1 0 0 Herbst et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 01

PLEN 04. 01: A Randomized, Phase III Study Comparing Carboplatin/Paclitaxel or Carboplatin/Paclitaxel/Bevacizumab with or without Concurrent Cetuximab in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): SWOG S 081 – Herbst R et al • Key results (cont. ) PFS, HR (95%CI) Analysis group OS, HR (95%CI); p-value All patients FISH+ All patients (n=1, 313) 0. 98 (0. 87, 1. 09) p=0. 68 0. 91 (0. 74, 1. 12) p=0. 37 0. 94 (0. 84, 1. 06) p=0. 34 0. 83 (0. 67, 1. 04) p=0. 10 Bevacizumab appropriate (n=554) 1. 05 (0. 88, 1. 25) p=0. 57 1. 07 (0. 77, 1. 47) p=0. 70 1. 04 (0. 86, 1. 25) p=0. 70 0. 97 (0. 69, 1. 38) p=0. 88 Bevacizumab inappropriate (n=759) 0. 93 (0. 80, 1. 07) p=0. 31 0. 82 (0. 63, 1. 07) p=0. 14 0. 88 (0. 76, 1. 03) p=0. 12 0. 75 (0. 57, 1. 00) p=0. 048 Squamous cell carcinoma (n=321) 0. 88 (0. 70, 1. 11) p=0. 29 0. 68 (0. 46, 1. 01) p=0. 06 0. 85 (0. 67, 1. 08) p=0. 18 0. 56 (0. 37, 0. 84) p=0. 006 – Grade 3– 4 AEs occurring in >10% in any group included: blood/bone marrow, metabolic/laboratory, gastrointestinal, infection and dermatology/skin – Grade 5 AE included gastrointestinal, infection and pulmonary/upper respiratory Herbst et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 01

PLEN 04. 01: A Randomized, Phase III Study Comparing Carboplatin/Paclitaxel or Carboplatin/Paclitaxel/Bevacizumab with or without Concurrent Cetuximab in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): SWOG S 081 – Herbst R et al • Conclusions – In the overall study population there was no benefit in OS for adding cetuximab – In EGFR+ population, there was no benefit in PFS when adding cetuximab – There was an indication of benefit for OS in the EGFR FISH+ population, but this was not statistically significant – There was an indication of benefit on OS among bevacizumab-inappropriate EGFR FISH+ patients – In squamous cell FISH+ patients a significant improvement in OS was observed although this is an exploratory objective Herbst et al. J Thorac Oncol 2015; 10 (suppl 2): PLEN 04. 01

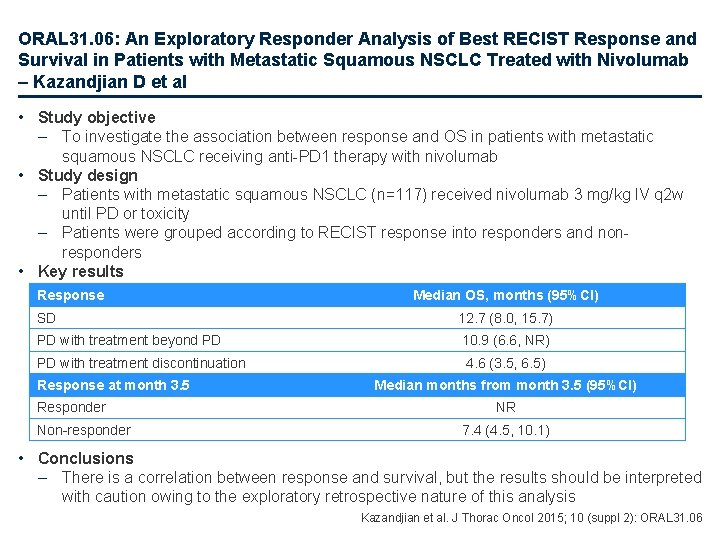
ORAL 31. 06: An Exploratory Responder Analysis of Best RECIST Response and Survival in Patients with Metastatic Squamous NSCLC Treated with Nivolumab – Kazandjian D et al • Study objective – To investigate the association between response and OS in patients with metastatic squamous NSCLC receiving anti-PD 1 therapy with nivolumab • Study design – Patients with metastatic squamous NSCLC (n=117) received nivolumab 3 mg/kg IV q 2 w until PD or toxicity – Patients were grouped according to RECIST response into responders and nonresponders • Key results Response Median OS, months (95%CI) SD 12. 7 (8. 0, 15. 7) PD with treatment beyond PD 10. 9 (6. 6, NR) PD with treatment discontinuation Response at month 3. 5 Responder Non-responder 4. 6 (3. 5, 6. 5) Median months from month 3. 5 (95%CI) NR 7. 4 (4. 5, 10. 1) • Conclusions – There is a correlation between response and survival, but the results should be interpreted with caution owing to the exploratory retrospective nature of this analysis Kazandjian et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 31. 06

Other malignancies SCLC and mesothelioma

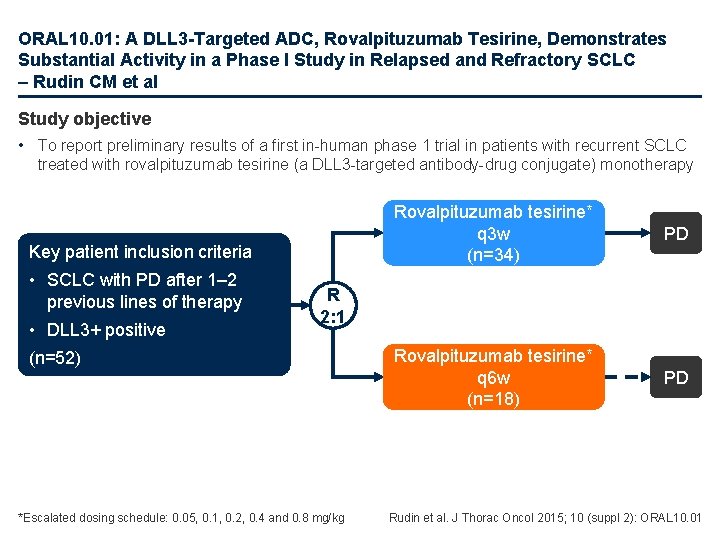
ORAL 10. 01: A DLL 3 -Targeted ADC, Rovalpituzumab Tesirine, Demonstrates Substantial Activity in a Phase I Study in Relapsed and Refractory SCLC – Rudin CM et al Study objective • To report preliminary results of a first in-human phase 1 trial in patients with recurrent SCLC treated with rovalpituzumab tesirine (a DLL 3 -targeted antibody-drug conjugate) monotherapy Key patient inclusion criteria • SCLC with PD after 1– 2 previous lines of therapy • DLL 3+ positive Rovalpituzumab tesirine* q 3 w (n=34) PD Rovalpituzumab tesirine* q 6 w (n=18) PD R 2: 1 (n=52) *Escalated dosing schedule: 0. 05, 0. 1, 0. 2, 0. 4 and 0. 8 mg/kg Rudin et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 01

ORAL 10. 01: A DLL 3 -Targeted ADC, Rovalpituzumab Tesirine, Demonstrates Substantial Activity in a Phase I Study in Relapsed and Refractory SCLC – Rudin CM et al • Key results – Maximum tolerated doses: 0. 2 mg/kg q 3 w x 3 cycles; 0. 3 mg/kg q 6 w x 2 cycles – Among the 16 confirmed DLL 3+ patients, 7 (44%) patients had PR and 8 (50%) patients had SD for a combined clinical benefit rate of 94% – In all evaluable patients (n=32), the ORR was 22% (n=7 PR) and SD 53% (n=17), for a combined clinical benefit rate of 75% – Similar response rates were observed among patients sensitive and refractory to first-line therapy and in the third-line setting – The most common TEAEs were: fatigue (40%), rash (39%), nausea (29%), dyspnoea (23%), decreased appetite (21%) and vomiting (21%) • Conclusions – Rovalpituzumab tesirine monotherapy had significant anti-tumour activity and a manageable toxicity in patients with recurrent DLL 3+ SCLC – A pivotal study is being planned Rudin et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 01

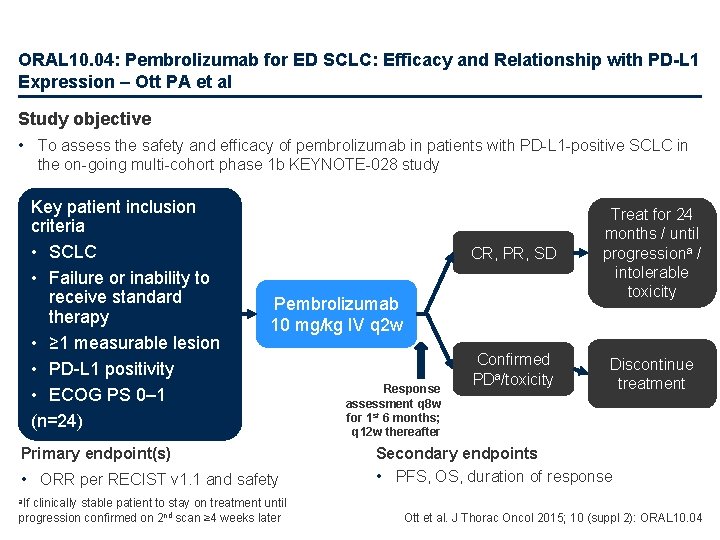
ORAL 10. 04: Pembrolizumab for ED SCLC: Efficacy and Relationship with PD-L 1 Expression – Ott PA et al Study objective • To assess the safety and efficacy of pembrolizumab in patients with PD-L 1 -positive SCLC in the on-going multi-cohort phase 1 b KEYNOTE-028 study Key patient inclusion criteria • SCLC • Failure or inability to receive standard therapy • ≥ 1 measurable lesion • PD-L 1 positivity • ECOG PS 0– 1 (n=24) CR, PR, SD Treat for 24 months / until progressiona / intolerable toxicity Confirmed PDa/toxicity Discontinue treatment Pembrolizumab 10 mg/kg IV q 2 w Primary endpoint(s) • ORR per RECIST v 1. 1 and safety clinically stable patient to stay on treatment until progression confirmed on 2 nd scan ≥ 4 weeks later Response assessment q 8 w for 1 st 6 months; q 12 w thereafter Secondary endpoints • PFS, OS, duration of response a. If Ott et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 04

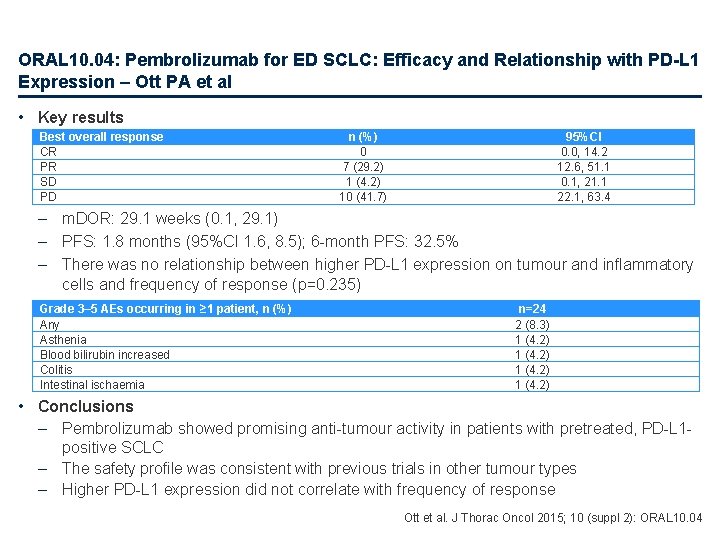
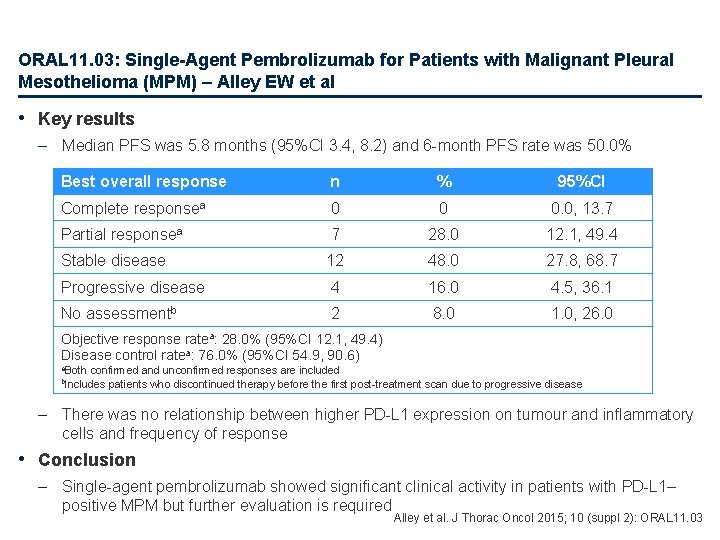
ORAL 10. 04: Pembrolizumab for ED SCLC: Efficacy and Relationship with PD-L 1 Expression – Ott PA et al • Key results Best overall response CR PR SD PD n (%) 0 7 (29. 2) 1 (4. 2) 10 (41. 7) 95%CI 0. 0, 14. 2 12. 6, 51. 1 0. 1, 21. 1 22. 1, 63. 4 – m. DOR: 29. 1 weeks (0. 1, 29. 1) – PFS: 1. 8 months (95%CI 1. 6, 8. 5); 6 -month PFS: 32. 5% – There was no relationship between higher PD-L 1 expression on tumour and inflammatory cells and frequency of response (p=0. 235) Grade 3– 5 AEs occurring in ≥ 1 patient, n (%) Any Asthenia Blood bilirubin increased Colitis Intestinal ischaemia n=24 2 (8. 3) 1 (4. 2) • Conclusions – Pembrolizumab showed promising anti-tumour activity in patients with pretreated, PD-L 1 positive SCLC – The safety profile was consistent with previous trials in other tumour types – Higher PD-L 1 expression did not correlate with frequency of response Ott et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 04

ORAL 10. 06: Long-Term Survival after Surgery for Pathologic N 1 and N 2 Small Cell Lung Cancer: A Comparison with Nonoperative Management – Yang C-F et al Study objective • To test whether or not surgery, in the setting of modern adjuvant therapies, offers a survival advantage among patients with node-positive SCLC compared with nonoperative management Study design • Patients were identified between 2003 and 2011 from the National Cancer Data Base: – Patients had to have p. T 1– 2 N 1– 2 M 0 SCLC – All patients underwent non-operative management (CT ± RT ≥ 45 Gy) or surgery (with adjuvant CT ± RT ≥ 45 Gy) – Patients with a history of unrelated malignancy and palliative-intent treatment were excluded • Data were assessed using Kaplan-Meier analyses and propensity score matching Yang et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 06

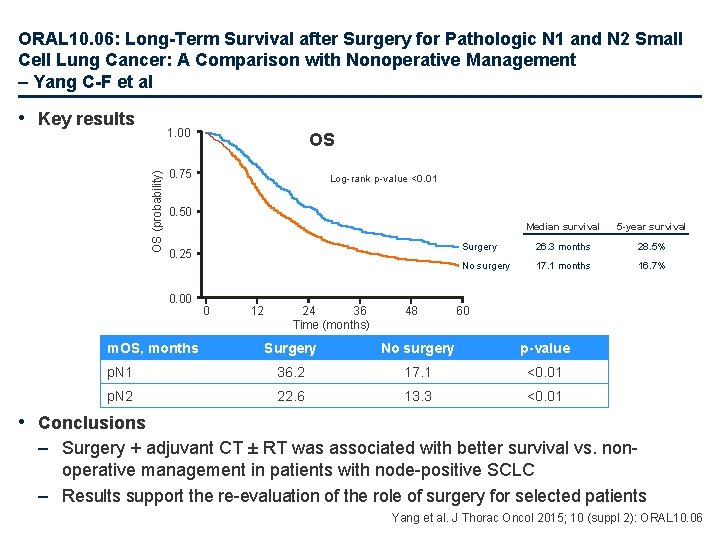
ORAL 10. 06: Long-Term Survival after Surgery for Pathologic N 1 and N 2 Small Cell Lung Cancer: A Comparison with Nonoperative Management – Yang C-F et al • Key results OS (probability) 1. 00 OS 0. 75 Log-rank p-value <0. 01 0. 50 0. 25 0. 00 m. OS, months 0 12 24 36 Time (months) 48 Median survival 5 -year survival Surgery 26. 3 months 28. 5% No surgery 17. 1 months 16. 7% 60 Surgery No surgery p-value p. N 1 36. 2 17. 1 <0. 01 p. N 2 22. 6 13. 3 <0. 01 • Conclusions – Surgery + adjuvant CT ± RT was associated with better survival vs. nonoperative management in patients with node-positive SCLC – Results support the re-evaluation of the role of surgery for selected patients Yang et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 10. 06

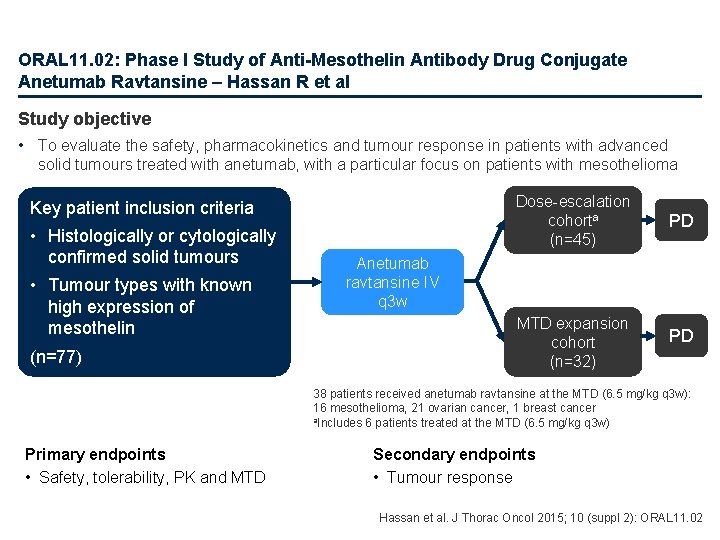
ORAL 11. 02: Phase I Study of Anti-Mesothelin Antibody Drug Conjugate Anetumab Ravtansine – Hassan R et al Study objective • To evaluate the safety, pharmacokinetics and tumour response in patients with advanced solid tumours treated with anetumab, with a particular focus on patients with mesothelioma Key patient inclusion criteria • Histologically or cytologically confirmed solid tumours • Tumour types with known high expression of mesothelin (n=77) Dose-escalation cohorta (n=45) PD MTD expansion cohort (n=32) PD Anetumab ravtansine IV q 3 w 38 patients received anetumab ravtansine at the MTD (6. 5 mg/kg q 3 w): 16 mesothelioma, 21 ovarian cancer, 1 breast cancer a. Includes 6 patients treated at the MTD (6. 5 mg/kg q 3 w) Primary endpoints • Safety, tolerability, PK and MTD Secondary endpoints • Tumour response Hassan et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 02

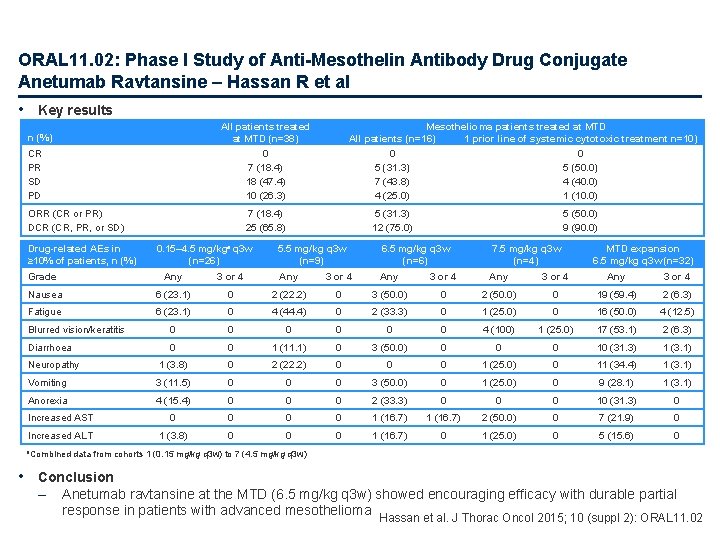
ORAL 11. 02: Phase I Study of Anti-Mesothelin Antibody Drug Conjugate Anetumab Ravtansine – Hassan R et al • Key results All patients treated at MTD (n=38) 0 7 (18. 4) 18 (47. 4) 10 (26. 3) n (%) CR PR SD PD ORR (CR or PR) DCR (CR, PR, or SD) Drug-related AEs in ≥ 10% of patients, n (%) Mesothelioma patients treated at MTD All patients (n=16) 1 prior line of systemic cytotoxic treatment n=10) 0 0 5 (31. 3) 5 (50. 0) 7 (43. 8) 4 (40. 0) 4 (25. 0) 1 (10. 0) 7 (18. 4) 25 (65. 8) 0. 15– 4. 5 mg/kga q 3 w (n=26) 5 (31. 3) 12 (75. 0) 5 (50. 0) 9 (90. 0) 5. 5 mg/kg q 3 w (n=9) 6. 5 mg/kg q 3 w (n=6) 7. 5 mg/kg q 3 w (n=4) MTD expansion 6. 5 mg/kg q 3 w (n=32) Grade Any 3 or 4 Any 3 or 4 Nausea 6 (23. 1) 0 2 (22. 2) 0 3 (50. 0) 0 2 (50. 0) 0 19 (59. 4) 2 (6. 3) Fatigue 6 (23. 1) 0 4 (44. 4) 0 2 (33. 3) 0 1 (25. 0) 0 16 (50. 0) 4 (12. 5) Blurred vision/keratitis 0 0 0 4 (100) 1 (25. 0) 17 (53. 1) 2 (6. 3) Diarrhoea 0 0 1 (11. 1) 0 3 (50. 0) 0 0 0 10 (31. 3) 1 (3. 1) Neuropathy 1 (3. 8) 0 2 (22. 2) 0 0 0 1 (25. 0) 0 11 (34. 4) 1 (3. 1) Vomiting 3 (11. 5) 0 0 0 3 (50. 0) 0 1 (25. 0) 0 9 (28. 1) 1 (3. 1) Anorexia 4 (15. 4) 0 0 0 2 (33. 3) 0 0 0 10 (31. 3) 0 Increased AST 0 0 1 (16. 7) 2 (50. 0) 0 7 (21. 9) 0 Increased ALT 1 (3. 8) 0 0 0 1 (16. 7) 0 1 (25. 0) 0 5 (15. 6) 0 a. Combined data from cohorts 1 (0. 15 mg/kg q 3 w) to 7 (4. 5 mg/kg q 3 w) • Conclusion – Anetumab ravtansine at the MTD (6. 5 mg/kg q 3 w) showed encouraging efficacy with durable partial response in patients with advanced mesothelioma Hassan et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 02

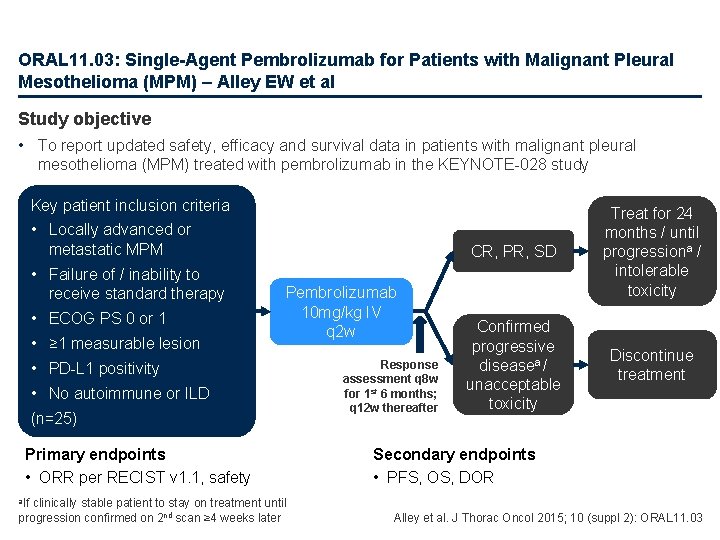
ORAL 11. 03: Single-Agent Pembrolizumab for Patients with Malignant Pleural Mesothelioma (MPM) – Alley EW et al Study objective • To report updated safety, efficacy and survival data in patients with malignant pleural mesothelioma (MPM) treated with pembrolizumab in the KEYNOTE-028 study Key patient inclusion criteria • Locally advanced or metastatic MPM • Failure of / inability to receive standard therapy • ECOG PS 0 or 1 • ≥ 1 measurable lesion Pembrolizumab 10 mg/kg IV q 2 w • PD-L 1 positivity • No autoimmune or ILD (n=25) Primary endpoints • ORR per RECIST v 1. 1, safety clinically stable patient to stay on treatment until progression confirmed on 2 nd scan ≥ 4 weeks later Response assessment q 8 w for 1 st 6 months; q 12 w thereafter CR, PR, SD Treat for 24 months / until progressiona / intolerable toxicity Confirmed progressive diseasea / unacceptable toxicity Discontinue treatment Secondary endpoints • PFS, OS, DOR a. If Alley et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 03

ORAL 11. 03: Single-Agent Pembrolizumab for Patients with Malignant Pleural Mesothelioma (MPM) – Alley EW et al • Key results – Median PFS was 5. 8 months (95%CI 3. 4, 8. 2) and 6 -month PFS rate was 50. 0% Best overall response n % 95%CI Complete responsea 0 0 0. 0, 13. 7 Partial responsea 7 28. 0 12. 1, 49. 4 Stable disease 12 48. 0 27. 8, 68. 7 Progressive disease 4 16. 0 4. 5, 36. 1 No assessmentb 2 8. 0 1. 0, 26. 0 Objective response ratea: 28. 0% (95%CI 12. 1, 49. 4) Disease control ratea: 76. 0% (95%CI 54. 9, 90. 6) a. Both confirmed and unconfirmed responses are included patients who discontinued therapy before the first post-treatment scan due to progressive disease b. Includes – There was no relationship between higher PD-L 1 expression on tumour and inflammatory cells and frequency of response • Conclusion – Single-agent pembrolizumab showed significant clinical activity in patients with PD-L 1– positive MPM but further evaluation is required Alley et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 03

ORAL 26. 01: Initial Analyses of the IASLC Malignant Pleural Mesothelioma (MPM) Database: Implications for the 8 th Edition AJCC and UICC Staging Manuals – Rusch V et al Study objective • To determine whether outcomes from the MPM database analysis warrant revisions to the upcoming 8 th edition of the AJCC/UICC staging manuals Study design • Electronic data capture to a new data dictionary used for case submissions • Minimum case submission requirements: complete clinical and/or post-surgical TNM stage with anatomical descriptors to support stage designation; accurate survival information; no conflict between descriptors and reported stage; and node positivity recorded by individual station • OS was analysed by Kaplan-Meier • Significance of individual T, N and M descriptors was evaluated by log-rank and Cox regression AJCC, American Joint Committee on Cancer; UICC, Union for International Cancer Control Rusch et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 26. 01

ORAL 26. 01: Initial Analyses of the IASLC Malignant Pleural Mesothelioma (MPM) Database: Implications for the 8 th Edition AJCC and UICC Staging Manuals – Rusch V et al • Key results – – 3, 519 cases were submitted; 2, 450 were included Stage available: clinical only 34%; post-surgical only 33%; both 34% A total of 1, 982 (81%) cases underwent surgery 5 -year OS for any N, MO showed no difference for T 1 a vs. T 1 b or for postsurgical T 2 vs. T 3 – 5 -year OS for any T, MO showed no difference for N 1 vs. N 2 • Conclusion – These initial results suggest some changes in the current MPM staging system are warranted, especially regarding T categories, although additional analyses are ongoing Rusch et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 26. 01

ORAL 26. 02: What Are the Risks and Benefits of Extended Pleurectomy Decortication for Mesothelioma? A Review of the Largest Institutional Series in the UK – Sharkey AJ et al Study objective • To assess the efficacy and safety of extended pleurectomy decortication (EPD) in patients with mesothelioma Study design • Retrospective analysis of case notes and pathological reports of 266 patients who underwent EPD within the previous 15 years • Duration of hospital stay, complication rates and survival were investigated Key results • Across all patients studied the median OS was 12. 2 months • Epithelioid p. NO disease was the most favourable subgroup with longer survival rate at 1, 3 and 5 years and longer overall median duration of survival OS rate (%) 1 year 3 years 5 years Median OS (months) All patients 48. 0 10. 3 2. 7 12. 2 Epithelioid p. NO 64. 9 17. 5 5. 2 23. 1 Sharkey et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 26. 02

ORAL 26. 02: What Are the Risks and Benefits of Extended Pleurectomy Decortication for Mesothelioma? A Review of the Largest Institutional Series in the UK – Sharkey AJ et al • Key results – The most common post-operative complication was persistent air leak and occurred in 31. 0% of patients, followed by atrial fibrillation in 16. 7% – Duration of intercostal drainage was significantly associated with the development of empyema (p<0. 001) and dehiscence of the neodiaphragm (p=0. 042) – Re-operation was required in 30 (11. 3%) patients – Post-operative complications significantly lengthened the median hospital stay by 26 days with empyema (p<0. 001), 7 days with removal of prosthetic patch (p=0. 003) and 6 days each with persistent air leak (p<0. 001) and atrial fibrillation (p=0. 037) – 90 -day mortality was significantly higher in patients who developed pleural empyema (71. 4% vs. 8. 6%, p=0. 001) – Complications prevented chemotherapy use in 28% of patients with empyema and 22. 7% of those with neodiaphragm dehiscence • Conclusions – In all but a selected subgroup of patients, EPD remains a palliative procedure – Reducing postoperative air leak, which increases pleural sepsis and perioperative risk and decreases adjuvant chemotherapy, is paramount Sharkey et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 26. 02

Other malignancies Rare tumours

ORAL 06. 01: Genomic Characterization of Large-Cell Neuroendocrine Lung Tumors – Fernandez-Cuesta L et al • Study objective – To perform genomic analyses of large-cell neuroendocrine carcinoma (LCNEC) using in house developed and published pipelines • Study design – 6. 0 SNP array analyses of 60 LCNECs, exome sequencing of 55 tumour-normal pairs, genome sequencing of 11 tumour-normal pairs, transcriptome sequencing of 69 tumours and expression arrays on 60 tumours were performed • Key results – Integrative analyses of copy number, mutations and expression patterns defined 2 major groups of LCNEC • SCLC-like group – with MYCL 1 and IRS 2 amplifications, concomitant mutations in RB 1 (27%) and TP 53 (75%) genes and inactivation of both TP 53 and RB 1, which is the hallmark of SCLC, in 20% of the cases • AD/SQ-like group – harbouring mutations in KEAP 1 (22%) and STK 11 (23%) • Conclusions – Although these results suggest that LCNEC might be a mix of different lung cancer subtypes, mutation clonality and expression analyses show that they are likely to be a separate entity, sharing molecular characteristics with the other lung cancer subtypes – The heterogeneity of the two groups suggests that LCNEC might represent an evolutionary trunk that can branch to SCLC or AD/SQ Fernandez-Cuesta et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 06. 01

ORAL 11. 05: Phase II Trial of Single Agent Amrubicin in Patients with Previously Treated Advanced Thymic Malignancies – Wakelee HA et al Study objective • To investigate the safety and efficacy of amrubicin, a third-generation anthracycline and topoisomerase II inhibitor, in patients with previously treated advanced thymic malignancies Key patient inclusion criteria • Inoperable invasive thymoma or thymic carcinoma • Adequate bone marrow, hepatic, renal + cardiac function Amrubicin* 40 mg/m 2 IV D 1, 2, 3 per 21 d cycle PD (n=33) • ORR Secondary endpoints • PFS, DCR, toxicity *Modified to 35 mg/m 2 due to high febrile neutropenia Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 05 Primary endpoint

ORAL 11. 05: Phase II Trial of Single Agent Amrubicin in Patients with Previously Treated Advanced Thymic Malignancies – Wakelee HA et al • Key results n (%) All (n=33) Thymoma (n=14) Thymic carcinoma (n=19) PR 6 (18) 4 (29) 2 (11) SD 23 (70) 10 (71) 13 (68) PD 4 (12) 0 (0) 4 (21) DCR 29 (88) 14 (100) 15 (79) – m. PFS: 8. 5 months (95%CI 6. 7, inf. ); OS: 30. 1 months (95%CI 21. 6, inf. ) Grade 3– 4 AEs occurring in ≥ 10% of patients, n (%) All (n=33) Fatigue 7 (21) Febrile neutropenia 7 (21) Anaemia 4 (12) • Conclusions – Amrubicin monotherapy shows promise in previously treated patients with thymic malignancies – Toxicity was as expected with a high frequency of febrile neutropenia Wakelee et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 05

ORAL 11. 06: A Prospective Phase II Study of Cisplatin and Cremophor EL-Free Paclitaxel (Genexol-PM) in Patients with Unresectable Thymic Epithelial Tumors: Can 18 F-FDG PET/CT play a role? – Kim HS et al Study objective • To investigate the efficacy and safety of cisplatin plus cremophor EL-free paclitaxel (Genexol. PM) in patients with unresectable thymic epithelial tumours (TETs) Key patient inclusion criteria • Unresectable TETs (thymoma / thymic carcinoma) • No prior palliative chemotherapy • Measurable disease (RECIST 1. 1) • Age ≥ 18 years Cisplatin 70 mg/m 2 + Genexol-PM 230 mg/m 2 q 3 w Treat until maximum 6 cycles PD intolerance • ECOG PS 0– 1 (n=42) Primary endpoint • ORR Secondary endpoints • PFS, toxicity, predictive value of the early response using PET-CT Kim et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 06

ORAL 11. 06: A Prospective Phase II Study of Cisplatin and Cremorphor EL-Free Paclitaxel (Genexol-PM) in Patients with Unresectable Thymic Epithelial Tumors: Can 18 F-FDG PET/CT play a role? – Kim HS et al • Key results All (n=40) Thymoma (n=13) Thymic carcinoma (n=27) OR* 25 (62. 5); 47. 6, 77. 4 6 (46); 23. 3, 76. 9 19 (70); 52. 0, 82. 1 SD 13 (32. 5); 17. 6, 52. 3 7 (54); 23. 1, 76. 7 6 (22); 3. 8, 40. 6 PD 2 (5. 0); 0, 12. 4 0 (0) 2 (8); 0, 14. 8 + Progression-free survival 1. 0 0. 8 PFS ++ ++ + 0. 6 0. 4 + + + 0. 2 m. PFS: 9. 8 months 0. 0 0 Median follow-up: 15. 7 months 6 + 12 18 Time (months) + 24 + OS + ++++++ ++ + 1. 0 Overall survival Patients, n (%); 95%CI 0. 8 0. 6 0. 4 m. OS: not reached 2 -year OS: 71% 0. 2 0. 0 30 +++ + + 0 6 12 18 24 Time (months) 30 36 – • There were no treatment-related deaths; the most common grade 3/4 treatment-related AE was neutropenia in 11 (26%) patients – Early metabolic response assessed by PET CT was not correlated to PFS in responders vs. nonresponders (9. 2 vs. 12. 7 months; p=0. 232) Conclusion – The combination of cisplatin and Genexol-PM is highly effective and tolerable for the treatment of unresectable TETs, especially in patients with thymic carcinoma *OR includes only PR; there was no CR Kim et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 11. 06

Other malignancies Brain metastases

ORAL 16. 01: Tyrosine Kinase Inhibitors (TKIs) for the Treatment of Brain Metastases (BMs) from Advanced Lung Cancer: A Large Retrospective Cohort – Bennati C et al • Study objective – To evaluate the efficacy of TKIs in treating brain metastases in patients with advanced NSCLC • Study design – Retrospective analysis of 89 patients with NSCLC and brain metastases and known mutation status: ALK+, 33 (37. 1%); EGFR+, 38 (42. 7%); ALK/EGFR- controls, 18 (20. 2%) – Standard radiotherapy (WBRT or radiosurgery) was used prior to TKIs in 40/71 (56. 3%) patients, while the remainder received a TKI upfront: EGFR inhibitor (gefitinib/erlotinib/afatinib) in 13/38 (34. 2%); ALK TKI (crizotinib/ceritinib/alectinib) in 18/33 (54. 5%) • Key results – OIRR was achieved in 81. 5% of the EGFR+ cohort and 84. 8% of the ALK+ subset with TKI given either before or after radiotherapy – Among those who were treated with TKIs upfront the OIRR was 100%, including complete response in 4/18 (22. 2%) of patients with ALK+ • Conclusions – TKIs are strongly active in patients with brain metastases from NSCLC which harbour an EGFR or ALK driver alteration – First-line use of TKIs may be reasonable in asymptomatic patients with long survival expectation, for whom WBRT may be postponed until a later disease stage OIRR, overall intracranial response rate Bennati et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 16. 01

ORAL 31. 07: A Phase II Trial of Pembrolizumab for Untreated Brain Metastases from Non-Small Cell Lung Cancer – Goldberg SB et al • Study objective – To determine the safety and efficacy of pembrolizumab in patients with advanced NSCLC and untreated or progressive brain metastases • Study design – Patients with advanced NSCLC and at least 1 untreated or progressive brain metastases (5– 20 mm), ECOG PS 0– 1 received pembrolizumab 10 mg/kg q 2 w • Key results – At interim analysis, the brain metastases response (CR + PR) rate was 33% – Of 6 patients with systemic response, 5 had response in CNS – Duration of brain metastases response was ~6 months – 4 of 5 confirmed responses are still ongoing – Median OS 7. 7 months (95%CI 3. 5, NR) – There were 4 treatment-related grade ≥ 3 AE: fatigue, diarrhoea, pneumonitis and hypokalaemia • Conclusions – Pembrolizumab appears to have activity in CNS in patients with NSCLC and untreated brain metastases – Systemic immunotherapy for select patients with small, asymptomatic brain metastases may be an alternative to radiation warranting further study Goldberg et al. J Thorac Oncol 2015; 10 (suppl 2): ORAL 31. 07
 European university association
European university association European magnetism association
European magnetism association European biomass industry association
European biomass industry association European diy retail association
European diy retail association European delirium association
European delirium association European diagnostic manufacturers association
European diagnostic manufacturers association Johannes theiner
Johannes theiner Cupola in diaphragm
Cupola in diaphragm Inferior articular process
Inferior articular process Thalasemia
Thalasemia Vertebral cavity
Vertebral cavity Surface anatomy of the breast
Surface anatomy of the breast Thoracic duct
Thoracic duct Sacrum of ox
Sacrum of ox Thoracic membranes
Thoracic membranes Neurogeen thoracic outlet syndroom
Neurogeen thoracic outlet syndroom Pig thoracic cavity diagram
Pig thoracic cavity diagram How to measure ap diameter of chest
How to measure ap diameter of chest Corpus callosum anatomy radiology
Corpus callosum anatomy radiology Thoracic cavity
Thoracic cavity Intercostal space
Intercostal space Chest wall anatomy
Chest wall anatomy Thorax anatomy
Thorax anatomy Thoracic kyphoscoliosis definition
Thoracic kyphoscoliosis definition Latissimus dorsi innervation
Latissimus dorsi innervation Hemiazygos vein
Hemiazygos vein Intercostal muscles
Intercostal muscles Tfl counterstrain
Tfl counterstrain Thoracic surgeon
Thoracic surgeon O2 flow rate chart
O2 flow rate chart Posturing medical
Posturing medical Thoracic duct
Thoracic duct External intercostal muscle
External intercostal muscle Rajesh shah thoracic surgeon
Rajesh shah thoracic surgeon Pouchlike structure at the start of the thoracic duct
Pouchlike structure at the start of the thoracic duct Brachial pulse
Brachial pulse Thoracic surgery
Thoracic surgery Thorasic extension
Thorasic extension Thoracic cavity
Thoracic cavity Thoracic cage anterior view
Thoracic cage anterior view Aortic knob
Aortic knob Muscles of thoracic spine
Muscles of thoracic spine Jugular notch and sternal notch
Jugular notch and sternal notch Thoracic skin
Thoracic skin Soft, spongy, cone-shaped organs in the thoracic cavity.
Soft, spongy, cone-shaped organs in the thoracic cavity. Spinal cavity
Spinal cavity Arteries of thoracic wall
Arteries of thoracic wall Thoracic cavity
Thoracic cavity Thoracic inlet x ray
Thoracic inlet x ray Xiphoid process
Xiphoid process Thorcic spine
Thorcic spine Thoracic nerves
Thoracic nerves Groin lymph nodes drainage
Groin lymph nodes drainage Anastomosis around scapula
Anastomosis around scapula Kati mori
Kati mori Goblet cells of respiratory epithelium
Goblet cells of respiratory epithelium Thoracic aorta supplies blood to
Thoracic aorta supplies blood to Thoracic outlet wedge pillow
Thoracic outlet wedge pillow Chronic pain management irwin
Chronic pain management irwin Lumbar vertebrae characteristics
Lumbar vertebrae characteristics Atheromatous thoracic aorta
Atheromatous thoracic aorta Abdominopelvic cavity location
Abdominopelvic cavity location Fascia of thoracic wall
Fascia of thoracic wall Thoracolumbar plexus
Thoracolumbar plexus Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Phản ứng thế ankan
Phản ứng thế ankan Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tia chieu sa te
Tia chieu sa te điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Frameset trong html5
Frameset trong html5 Hệ hô hấp
Hệ hô hấp Thế nào là số nguyên tố
Thế nào là số nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chụp tư thế worms-breton
Chụp tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Thẻ vin
Thẻ vin Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hát lên người ơi
Hát lên người ơi Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Cong thức tính động năng
Cong thức tính động năng