Innovative Payment Approaches in Oncology Dr Ashley Woolmore









- Slides: 22

Innovative Payment Approaches in Oncology Dr Ashley Woolmore Senior Principal Global Real-world Evidence Solutions July 2015

Structure of this presentation Theme: Challenges of success in oncology • Demand • Supply • Payment 2 IMS Health Confidential

Structure of this presentation Theme: Challenges of success in oncology • Demand • Supply • Payment 3 IMS Health Confidential

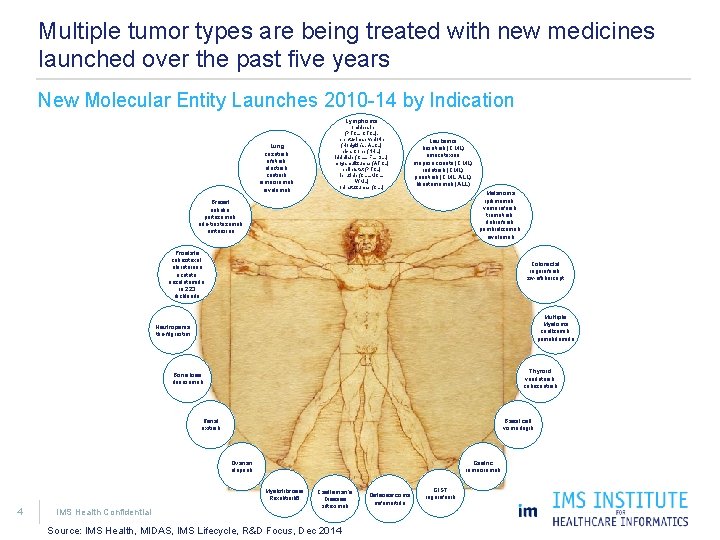
Multiple tumor types are being treated with new medicines launched over the past five years New Molecular Entity Launches 2010 -14 by Indication Lymphoma Lung crizotinib, afatinib, alectinib, ceritinib, ramucirumab, nivolumab romidepsin (PTCL, CTCL), brentuximab vedotin (Hodgkin’s, ALCL) pixantrone (NHL) idelalisib (CLL, FL, SLL) mogamulizumab (ATCL) belinostat (PTCL) ibrutinib (CLL, MCL, WML) obinutuzumab (CLL) Leukemia bosutinib (CML) omacetaxine mepesuccinate (CML) radotinib (CML) ponatinib (CML, ALL) blinatumomab (ALL) Melanoma ipilimumab, vemurafenib, trametinib, dabrafenib, pembrolizumab, nivolumab Breast eribulin, pertuzumab, ado-trastuzumab emtansine Prostate cabazitaxel, abiraterone acetate, enzalutamide, ra 223 dichloride Colorectal regorafenib, ziv-aflibercept Multiple Myeloma carfilzomib, pomalidomide Neutropenia tbo-filgrastim Thyroid vandetanib, cabozantinib Bone loss denosumab Renal axitinib Basal cell vismodegib Ovarian olaparib Gastric ramucirumab Myelofibrosis Ruxolitinib 6 4 IMS Health Confidential Castleman’s Disease siltuximab Source: IMS Health, MIDAS, IMS Lifecycle, R&D Focus, Dec 2014 Osteosarcoma mifamurtide GIST regorafenib

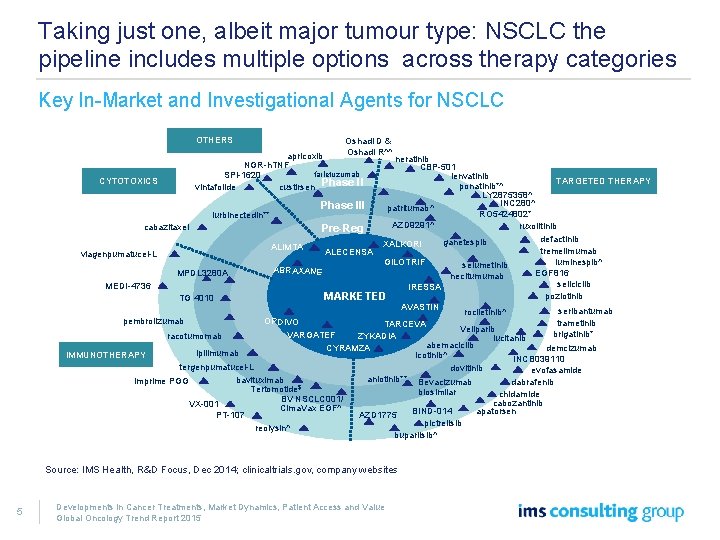
Taking just one, albeit major tumour type: NSCLC the pipeline includes multiple options across therapy categories Key In-Market and Investigational Agents for NSCLC OTHERS Oshadi D & Oshadi R^^ apricoxib NGR-h. TNF farletuzumab SPI-1620 custirsen Phase II vintafolide neratinib CBP-501 lenvatinib CYTOTOXICS TARGETED THERAPY ponatinib*^ LY 2875358^ INC 280^ Phase III patritumab^ RO 5424802* lurbinectedin** AZD 9291^ ruxolitinib cabazitaxel Pre-Reg defactinib ganetespib XALKORI ALIMTA tremelimumab ALECENSA viagenpumatucel-L luminespib^ GILOTRIF selumetinib ABRAXANE MPDL 3280 A EGF 816 necitumumab seliciclib MEDI-4736 IRESSA poziotinib MARKETED TG 4010 AVASTIN seribantumab rociletinib^ pembrolizumab OPDIVO trametinib TARCEVA Veliparib brigatinib* VARGATEF ZYKADIA racotumomab lucitanib abemaciclib demcizumab CYRAMZA ipilimumab IMMUNOTHERAPY icotinib^ INCB 039110 tergenpumatucel-L dovitinib evofasamide anlotinib** Bevacizumab bavituximab imprime PGG dabrafenib Tertomotide$ biosimilar chidamide BV NSCLC 001/ cabozantinib VX-001 Cima. Vax EGF^ apatorsen BIND-014 AZD 1775 PT-107 pictrelisib reolysin^ buparlisib^ Source: IMS Health, R&D Focus, Dec 2014; clinicaltrials. gov, company websites 5 Developments in Cancer Treatments, Market Dynamics, Patient Access and Value Global Oncology Trend Report 2015

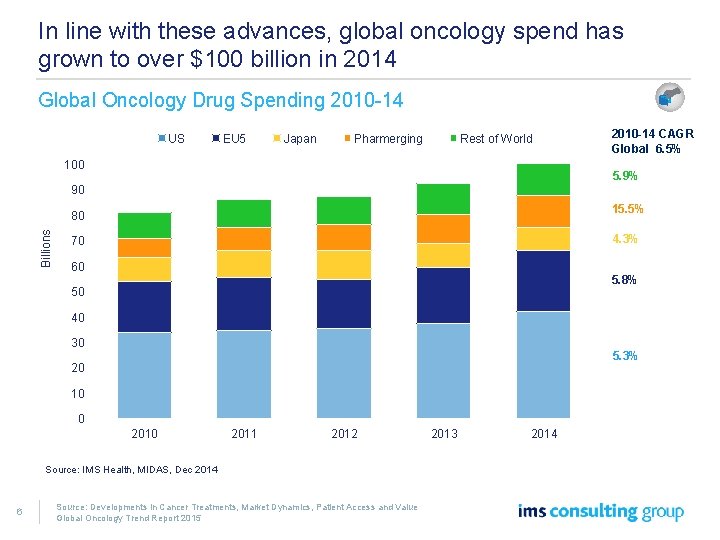
In line with these advances, global oncology spend has grown to over $100 billion in 2014 Global Oncology Drug Spending 2010 -14 US EU 5 Japan Pharmerging Rest of World 100 2010 -14 CAGR Global 6. 5% 5. 9% 90 15. 5% Billions 80 4. 3% 70 60 5. 8% 50 40 30 5. 3% 20 10 0 2011 2012 2013 Source: IMS Health, MIDAS, Dec 2014 6 Source: Developments in Cancer Treatments, Market Dynamics, Patient Access and Value Global Oncology Trend Report 2015 2014

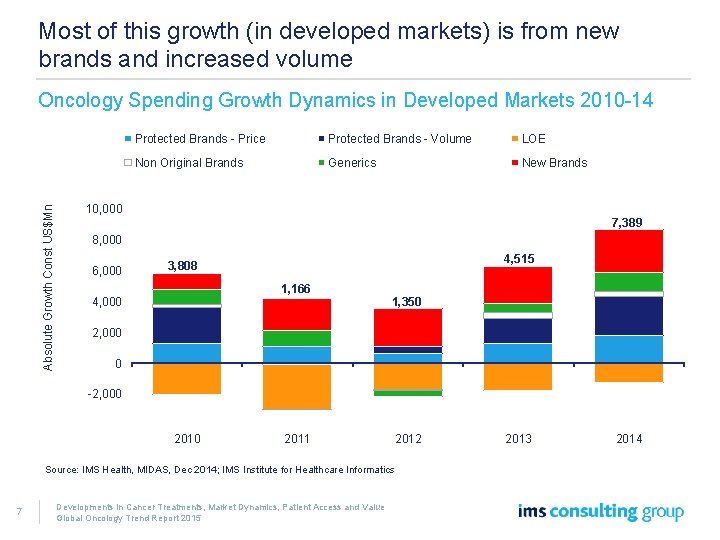
Most of this growth (in developed markets) is from new brands and increased volume Absolute Growth Const US$Mn Oncology Spending Growth Dynamics in Developed Markets 2010 -14 Protected Brands - Price Protected Brands - Volume LOE Non Original Brands Generics New Brands 10, 000 7, 389 8, 000 6, 000 4, 515 3, 808 1, 166 4, 000 1, 350 2, 000 0 -2, 000 -4, 000 2011 2012 Source: IMS Health, MIDAS, Dec 2014; IMS Institute for Healthcare Informatics 7 Developments in Cancer Treatments, Market Dynamics, Patient Access and Value Global Oncology Trend Report 2015 2013 2014

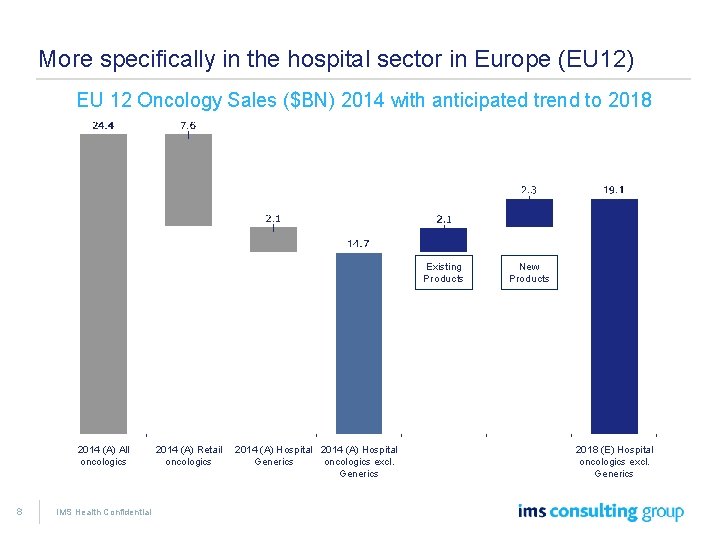
More specifically in the hospital sector in Europe (EU 12) EU 12 Oncology Sales ($BN) 2014 with anticipated trend to 2018 Existing Products 2014 (A) All oncologics 8 IMS Health Confidential 2014 (A) Retail oncologics 2014 (A) Hospital Generics oncologics excl. Generics New Products 2018 (E) Hospital oncologics excl. Generics

Above and beyond the number of new agents, three additional sources of complexity 9 A • Combination regimens B • Sequential use of high-cost products C • Multi-indication products IMS Health Confidential

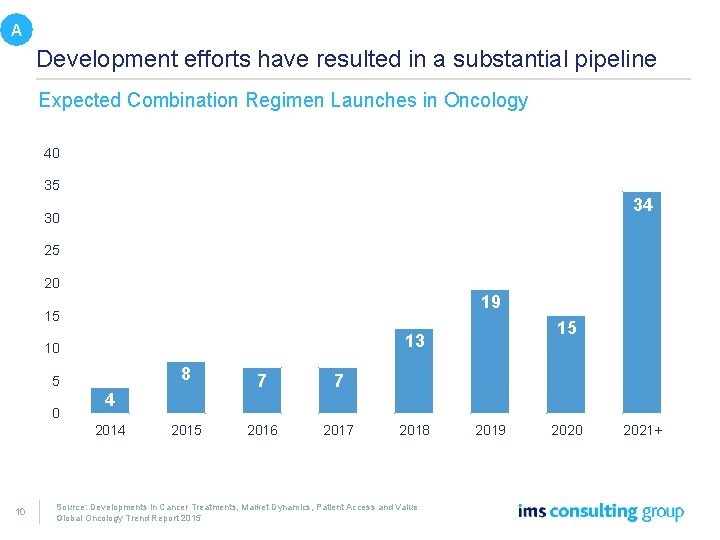
A Development efforts have resulted in a substantial pipeline Expected Combination Regimen Launches in Oncology 40 35 34 30 25 20 19 15 10 5 0 8 7 7 2015 2016 2017 4 2014 10 15 13 2018 2019 Source: Developments in Cancer Treatments, Market Dynamics, Patient Access and Value Global Oncology Trend Report 2015 2020 2021+

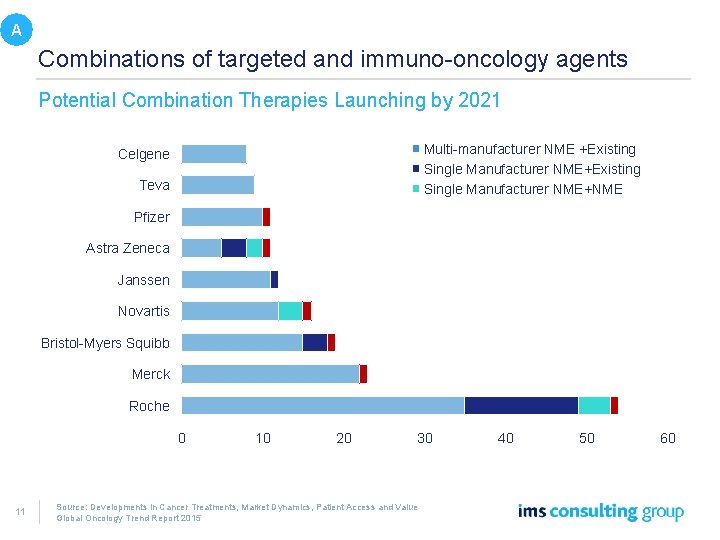
A Combinations of targeted and immuno-oncology agents Potential Combination Therapies Launching by 2021 Multi-manufacturer NME +Existing Single Manufacturer NME+NME Celgene Teva Pfizer Astra Zeneca Janssen Novartis Bristol-Myers Squibb Merck Roche 0 11 10 20 30 40 Source: Developments in Cancer Treatments, Market Dynamics, Patient Access and Value Global Oncology Trend Report 2015 50 60

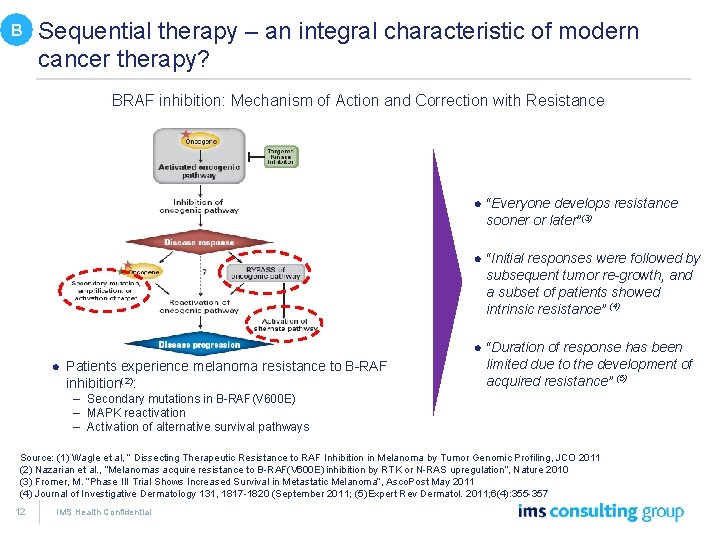
B Sequential therapy – an integral characteristic of modern cancer therapy? BRAF inhibition: Mechanism of Action and Correction with Resistance l “Everyone develops resistance sooner or later”(3) l Patients experience melanoma resistance to B-RAF inhibition(2): l “Initial responses were followed by subsequent tumor re-growth, and a subset of patients showed intrinsic resistance” (4) l “Duration of response has been limited due to the development of acquired resistance” (5) – Secondary mutations in B-RAF(V 600 E) – MAPK reactivation – Activation of alternative survival pathways Source: (1) Wagle et al, “ Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling, JCO 2011 (2) Nazarian et al. , “Melanomas acquire resistance to B-RAF(V 600 E) inhibition by RTK or N-RAS upregulation”, Nature 2010 (3) Fromer, M. “Phase III Trial Shows Increased Survival in Metastatic Melanoma”, Asco. Post May 2011 (4) Journal of Investigative Dermatology 131, 1817 -1820 (September 2011; (5) Expert Rev Dermatol. 2011; 6(4): 355 -357 12 IMS Health Confidential

B Excerpt from ‘The Emperor of all maladies’ 1 Atossa in the future “In 2050, [She] will arrive at her breast oncologist’s clinic with a thumb-size flash drive containing the entire sequence of her cancer’s genome, identifying every mutation in every gene The mutations will be organized into key pathways. An algorithm might identify the pathways that are contributing to the growth and survival of her cancer Therapies will be targeted against these pathways to prevent a relapse of the tumour after surgery She will begin with one combination of targeted drugs, expect to switch to a second cocktail when her cancer mutates and switch again when the cancer mutates again She will likely take some for of medicine, whether to prevent, cure of palliate her illness for the rest of her life” 13 IMS Health Confidential. 1: Siddharth Mukherjee, 2011, 4 th Estate

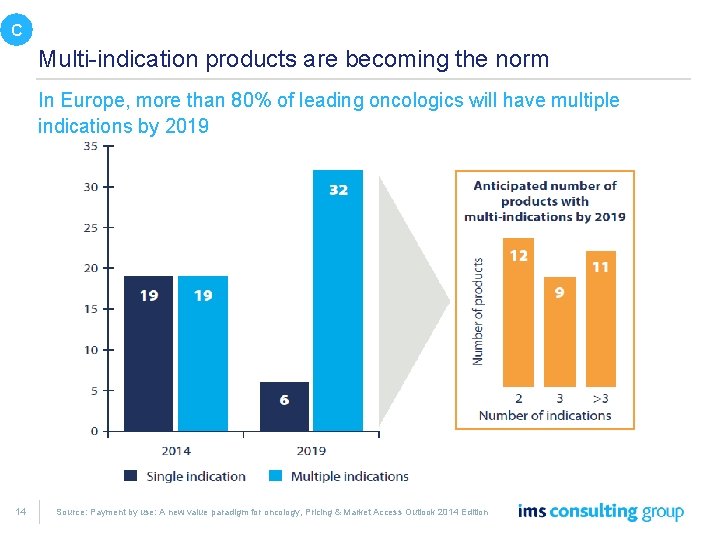
C Multi-indication products are becoming the norm In Europe, more than 80% of leading oncologics will have multiple indications by 2019 14 Source: Payment by use: A new value paradigm for oncology, Pricing & Market Access Outlook 2014 Edition

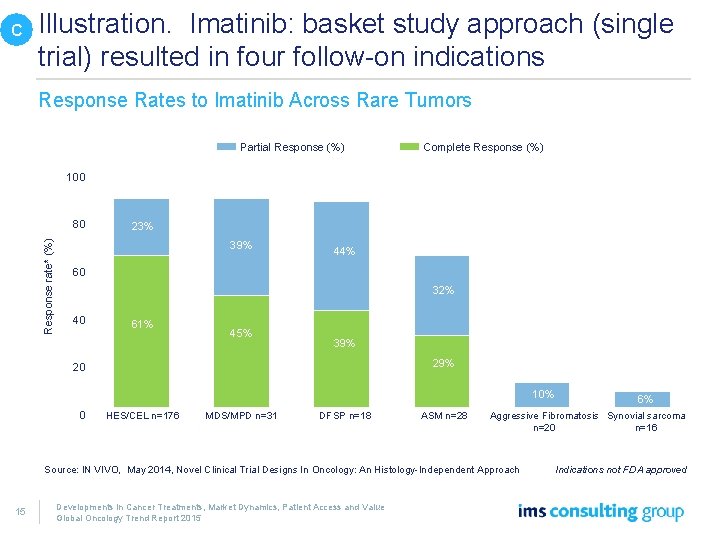
C Illustration. Imatinib: basket study approach (single trial) resulted in four follow-on indications Response Rates to Imatinib Across Rare Tumors Partial Response (%) Complete Response (%) 100 Response rate* (%) 80 23% 39% 44% 60 32% 40 61% 45% 39% 20 10% 0 HES/CEL n=176 MDS/MPD n=31 DFSP n=18 ASM n=28 Aggressive Fibromatosis Synovial sarcoma n=20 n=16 Source: IN VIVO, May 2014, Novel Clinical Trial Designs In Oncology: An Histology-Independent Approach 15 6% Developments in Cancer Treatments, Market Dynamics, Patient Access and Value Global Oncology Trend Report 2015 Indications not FDA approved

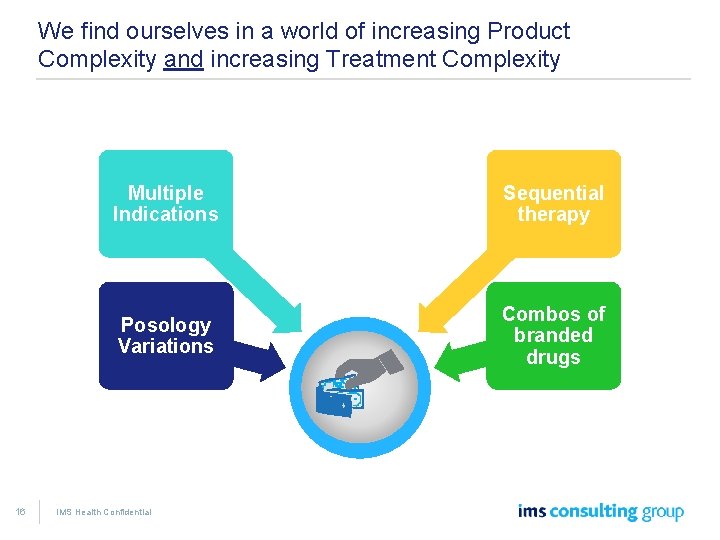
We find ourselves in a world of increasing Product Complexity and increasing Treatment Complexity 16 Multiple Indications Sequential therapy Posology Variations Combos of branded drugs IMS Health Confidential

Structure of this presentation Theme: Challenges of success in oncology • Demand • Supply • Payment 17 IMS Health Confidential

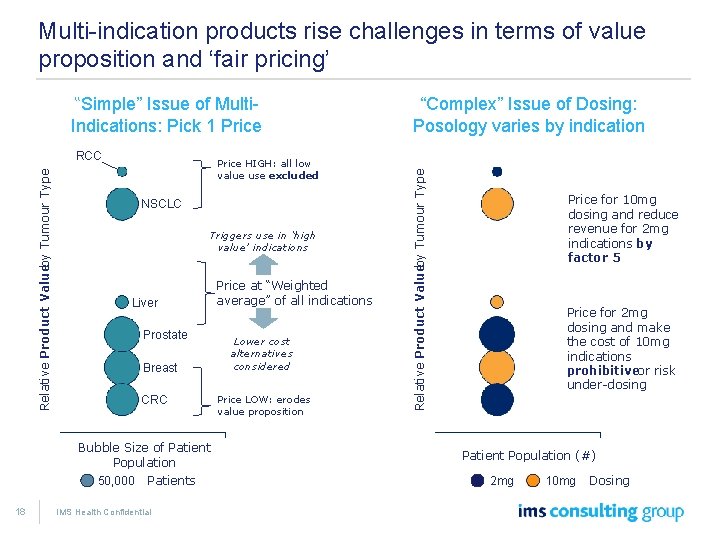
Multi-indication products rise challenges in terms of value proposition and ‘fair pricing’ Indications: Pick 1 Price Relative Product Valueby Tumour Type RCC Price HIGH: all low value use excluded NSCLC Triggers use in ‘high value’ indications Liver Prostate Breast CRC Bubble Size of Patient Population 50, 000 18 Patients IMS Health Confidential Price at “Weighted average” of all indications Lower cost alternatives considered Price LOW: erodes value proposition “Complex” Issue of Dosing: Posology varies by indication Relative Product Valueby Tumour Type “Simple” Issue of Multi- Price for 10 mg dosing and reduce revenue for 2 mg indications by factor 5 Price for 2 mg dosing and make the cost of 10 mg indications prohibitiveor risk under-dosing Patient Population (#) 2 mg 10 mg Dosing

Objectives of value-based schemes, including subscription What are these schemes trying to accomplish? • Address uncertainty of outcomes • Transparency of patient benefit • Balancing risk across stakeholders • Ensuring financial resources are used effectively • Clarity on revenue; disconnection to product 19 IMS Health Confidential

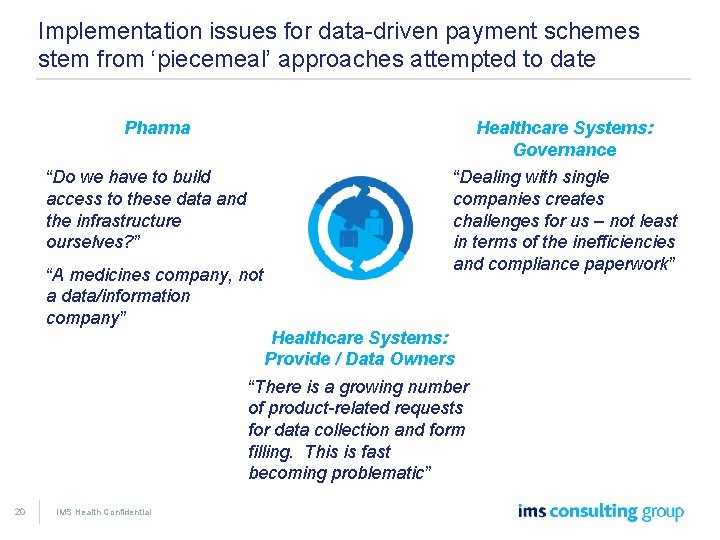
Implementation issues for data-driven payment schemes stem from ‘piecemeal’ approaches attempted to date Pharma Healthcare Systems: Governance “Do we have to build access to these data and the infrastructure ourselves? ” “A medicines company, not a data/information company” “Dealing with single companies creates challenges for us – not least in terms of the inefficiencies and compliance paperwork” Healthcare Systems: Provide / Data Owners “There is a growing number of product-related requests for data collection and form filling. This is fast becoming problematic” 20 IMS Health Confidential

A pan-pharma “Payment by Use” infrastructure is IMS’s proposed solution to these common challenges 21 IMS Health Confidential

Summary / Conclusion • Demand for progress and effective therapies has been successful: • War on Cancer: political will • Public demand for effective therapies • Focus of development into ‘smaller N’ specialty care • High launch ‘list price’ for single agents is the tip of the ice-berg • Sensitivity of value of survival • Signaling nature of oncology pricing • Complexity of ‘complete response’ pricing (pay now for future benefit) • Remainder of the ice-berg: • Chronicity of therapy • Sequential therapy • Combinations of high-cost products • ‘Arithmetic’ (1+1+1) pricing model is not tenable from an affordability perspective • Any shift toward more rational approaches, away from ‘product’ to therapy, or patient-level pricing must rely on an efficient information infrastructure 22 IMS Health Confidential
 Dr john woolmore
Dr john woolmore Innovative training techniques
Innovative training techniques Innovative train technology
Innovative train technology Innovative features of scripting languages
Innovative features of scripting languages Innovative features of scripting languages
Innovative features of scripting languages Innovative staff solution
Innovative staff solution Innovative business communication
Innovative business communication Aaarambbh revolution pvt ltd
Aaarambbh revolution pvt ltd Organizational innovation meaning
Organizational innovation meaning Innovative methods of teaching english language
Innovative methods of teaching english language Inventive and innovative
Inventive and innovative Innovative communication tools
Innovative communication tools Innovative management for a changing world
Innovative management for a changing world Innovative business models in agriculture
Innovative business models in agriculture Innovative features of scripting languages
Innovative features of scripting languages Splice
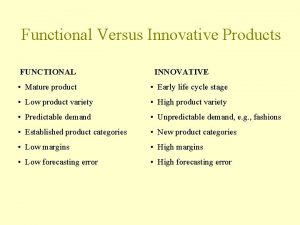
Splice Functional and innovative products
Functional and innovative products Milstat
Milstat Innovative university of eurasia
Innovative university of eurasia These are entrepreneurs who are to follow the path shown
These are entrepreneurs who are to follow the path shown Innovative management for turbulent times
Innovative management for turbulent times Innovative ideas to grow business
Innovative ideas to grow business Innovative library services
Innovative library services