Endocrinology The pituitary gland the hypophysis It is







































































































- Slides: 154

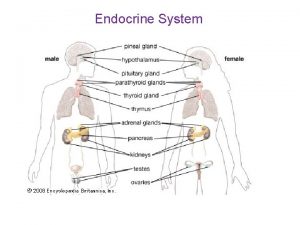
Endocrinology


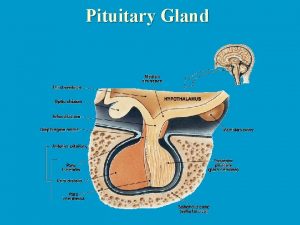
The pituitary gland (the hypophysis) It is a small gland situated at the base of the skull, in the sella turcica The pituitary gland consists of • Large ant lobe: Anterior pituitary gland (adenohypophysis) • Large post lobe: Posterior pituitary gland (neurohypophysis) • Small intermediate lobe


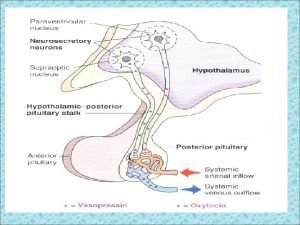
The pituitary gland (the hypophysis) Hypothalamic connections with the pituitary gland • A vascular connection (hypothalamo-hypophyseal portal circulation: between the hypothalamus and the ant lobe • A nervous connection (hypothalamo-hypophyseal tract): between the hypothalamus and the post lobe. Through these connections the hypothalamus act as a control centre to the endocrine system.



Adenohypophysis It’s called the master gland because it secretes tropic hormones that control most of the other endocrine glands This gland secretes protein hormones • Polypeptide hormones 1. Adrenocorticotropin (corticotropin) 2. Prolactin, promotes mammary gland development and milk production. 3. Growth hormone, systemic hormone that is not tropic to any specific endocrine gland • Glycoprotein hormones 1. Thyroid-stimulating hormone (thyrotropin) 2. Gonadotropic hormones, control the gonads in males and females A- follicle-stimulating hormone B- luteinizing hormone

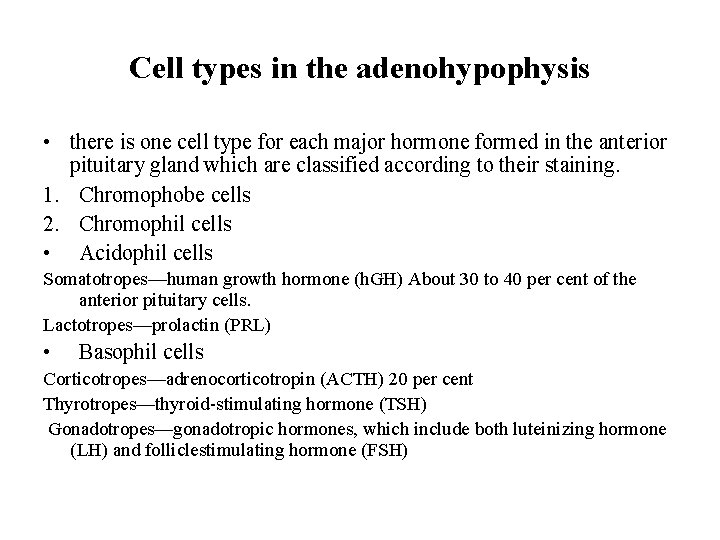
Cell types in the adenohypophysis • there is one cell type for each major hormone formed in the anterior pituitary gland which are classified according to their staining. 1. Chromophobe cells 2. Chromophil cells • Acidophil cells Somatotropes—human growth hormone (h. GH) About 30 to 40 per cent of the anterior pituitary cells. Lactotropes—prolactin (PRL) • Basophil cells Corticotropes—adrenocorticotropin (ACTH) 20 per cent Thyrotropes—thyroid-stimulating hormone (TSH) Gonadotropes—gonadotropic hormones, which include both luteinizing hormone (LH) and folliclestimulating hormone (FSH)


Regulation of anterior pituitary secretion • Hypothalamus • Feedback mechanism Hypothalamic control By releasing the hypophysiotropic hormones that reach the pituitary gland via the hypothalamo- hypophyseal portal circulation. 1. Releasing hormones 2. Release – inhibiting hormones

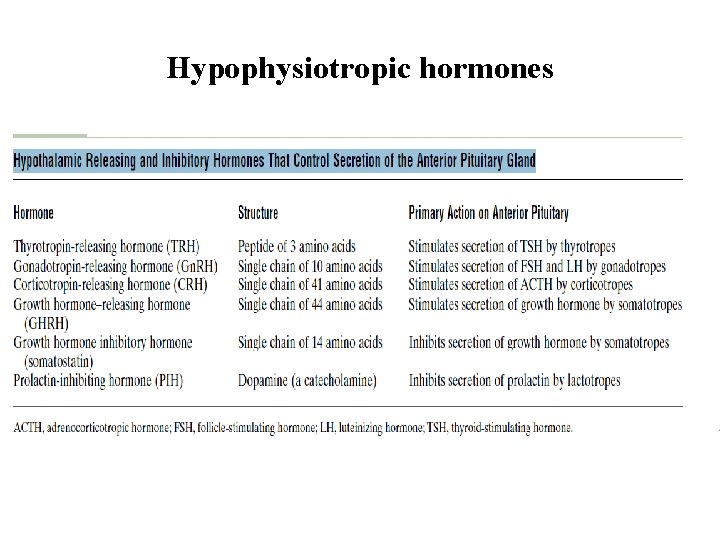
Hypophysiotropic hormones

Feedback mechanisms These constitute the relations between the blood levels of the hormones and the secretory rate of their pituitary tropic hormones as well as the hypothalmic hypophysiotropic hormones. There are 2 types • Positive feedback • Negative feedback


Growth hormone Action of GH • Induction of growth GH is the principle growth promoting factor in the body through 1. increasing the rate of both protein synthesis and cell division 2. Exerting many metabolic effect It leads to A- growth of almost all soft tissues in the body including skeletal muscles, skin and most viscera, it increases the metabolic rate NB. It doesn’t affect the nervous tissues and doesn’t produce tissue maturation B- Chondrogenesis, cartilage formation which is followed by ossification and growth of bones.

Growth hormone • Metabolic effect A- protein metabolism GH is a protein anabolic hormone B- Electrolyte metabolism GH increases the GIT absorption of ca+² and decreases the rate og urinary excretion of both Na and K C- fat metabolism GH mobilizes fat from adipose tissue and stimulates lipolysis, so it decrease the body fat content Increases the blood level of free fatty acids and keton bodies D- Carbohydrate metabolism GH is a diabetogenic by

Growth hormone 1. Increasing the hepatic glucose out put 2. Exerting anti-insulin effect NB. Prolonged adminstration or increased secretion of growth hormone→ exhausion of the beta cells → decreased insulin secretion → frank diabetes called pituitary or hypophyseal diabetes GH doesn’t produce most of its effects by it self, but through IGF-1 GH combines with IGF-1 in various proportions to produce its effect • GH exert an intrinsic lactogenic activity Due to its structural resembelance to prolactin


Control of growth hormone secretion GH secretion is controlled by • Hypophysiotropic hormones (GRH and GIH) • Feedback mechanism • The rate of growth hormone secretion undergoes marked fluctuation in response to a variety of stimuli




Diseases of the anterior pituitary gland • Excessive secretion of the GH Cause: acidophil somatotroph cell tumour Resulted in 1. gigantism in children 2. acromegaly in adult Gigantism Cause: excessive secretion of GH in children before closure of epiphysis Characterized by Overgrowth of the skeleton in normal proportion Overgrowth of soft tissues and viscera

Diseases of the anterior pituitary gland Infantile gonads may occur due to pressure of the tumour on the gonadotropes Diabetes mellitus may develop Signs due to local effect of the tumour may appear Acromegaly Cause: excessive secretion of GH in adults Characterized by Thick bones Enlargement of the lower jaw Overgrowth of vertebrae leading to kyphosis Enlargement of the viscera and other soft tissue

Diseases of the anterior pituitary gland Skin become coarse and thick, that of the forehead and scalp is thrown in to wrinkles (bulldog) scalp Enlargement of the heart may predispose to myocardial ischemia The body and limb hair is increased and the male sex organs may enlarge because the GH increases the action of androgens Diabetes may occur in 25 per cent of pts Gynecomastia, and lactation in 4 per cent of non pregnant female pts Signs due to the local effect of the pit tumour (increased ICT, bitemporal hemianopia and enlargement of the sella turcica)




Diseases of the anterior pituitary gland • Defficient secretion of GH In adults cause decrease of the tissue mass In young individual before puberty cause 1. Dwarfism or 2. Infantilism if associated by failure of sexual development due to simultaneous deficiency of GTHs (hypogonadism). 3. Panhypopituitarism (simmond’s diseases)


Diseases of the anterior pituitary gland Panhypopituitarism This is generalized failure of the ant pituitary gland Cause: - damage by disease - necrosis in some women in case of shock in sever postpartum hge (sheehan’s syndrom) It’s characterized by • Arrested growth in children and premature senility in adults • Atrophy of 1. Thyroid gland 2. Some zones of the adrenal cortex 3. gonads

Diseases of the anterior pituitary gland • Hypoglycemia • Sever pallor • Mild loss of weight due to lack of anabolic effect of both GH androgens


Causes of dwarfism (short stature or stunted growth) • Dwarfism • Infantilism • Cretinism • Laron dwarfism (growth hormone insensitivity) It occurs as a result of unresponsivenes of the GH receptors to the GH → deficiency of the circulating somatomedins • Precocious puberty • Certain bone and metabolic diseases • Some chromosomal diseases (turner).

Diagnosis of the disturbance of the ant pituitary • Measurement of the growth hormone level in the blood In hypofunction → midnight GH level ↓ In hypersecretion→ daytime level ↑ • Measurement of the somatomedins level in the blood • CT or MRI to discover any pituitary tumour MCQ • A neuroscientist is studying communication between the hypothalamus and pituitary in a rat model. She interrupts blood flow through the median eminence and then measures circulating levels of pituitary hormones following appropriate physiological stimulation. Secretion of which of the following hormones will be unaffected by the experimental manipulation? A. Growth hormone B. Prolactin C. Th yroid stimulating hormone D. Follicle-stimulating hormone E. Vasopressin

Diagnosis of the disturbance of the ant pituitary • Which of the following pituitary hormones is an opioid peptide? A. α-melanocyte-stimulating hormone (α-MSH) B. β-MSH C. ACTH D. Growth hormone E. β-endorphin • During childbirth, a woman suffers a serious hemorrhage and goes into shock. After she recovers, she displays symptoms of hypopituitarism. Which of the following will not be expected in this patient? A. Cachexia B. Infertility C. Pallor D. Low basal metabolic rate E. Intolerance to stress

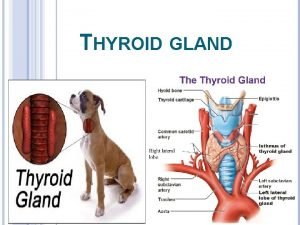
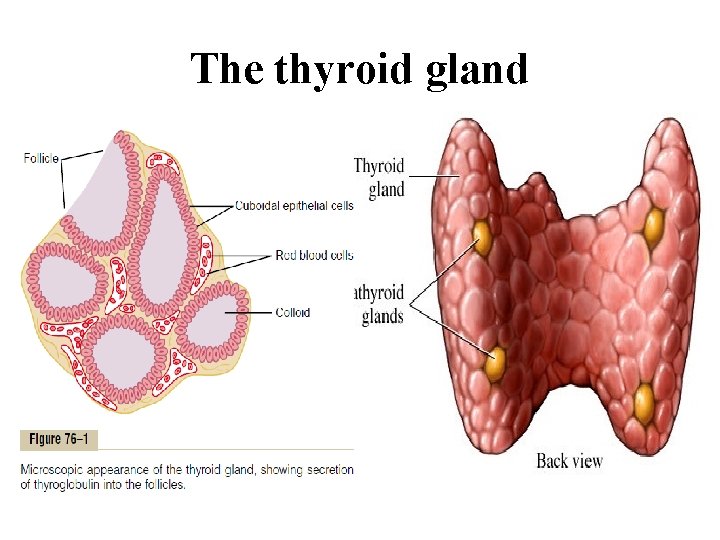
The thyroid gland It’s located in the front of the neck below the larynx on either side of the trachea. It’s formed of two lobes connected by a narrow isthmus, and some times there is a small pyramidal lobe arising from the isthmus. Histologically: The gland is made up of thyroid follicles Each follicle is lined by a single layer of epithelial cells The lumen of the follicles are filled with protein material (colloid) In between them there are special cells ( C or parafollicular cells)


The thyroid gland

The thyroid gland The thyroid hormones • • Thyroxin or T 4→ 80 mcg Triiodothyronin T 3→ 4 mcg Reverse triiodothyronin RT 3→ 2 mcg Calcitonin Daily requrements and supply of iodin Normally 2 mcg / Kg body weight Synthesis and secretion of thyroid hormones • Iodid trapping Active process, using 2 nd active transport (Na- I symport), and Na. KATPase activity

The thyroid gland • Oxidation of iodide to iodine By the activity of thyroid peroxidase enzyme • Binding of iodine to tyrosine Giving rise to MIT and DIT( biologically inactive) Catalyzed by thyroid peroxidase enzyme Occur in the colloid by combination of I and tyrosine residue (part of thyroglobulin). NB. Both the enzym and thyroglobulin are synthesyzed by the follicular cells then secreted in to the colloid by exocytosis. • Condensation of MIT and DIT Catalyzed by thyroid peroxidase enzyme

The thyroid gland • Secretion of the thyroid hormones The hormones remain bound to thyroglobulin till they are secreted The follicular cells ingest the colloid by endocytosis In the lysosomes, the peptide bonds between the iodinated compound and thyroglobulin are broken by protease enzyme Release of these compounds in the cytoplasm T 3 and T 4 are pass to the blood stream MIT, DIT are deiodinated by the iodotyrosin deiodinase enzyme Transport and concentration of the thyroid hormones Both T 3 and T 4 are present in 2 forms 1. Bound to plasma protein (albumin, prealbumin and thyroxin binding globulin) 2. Free (physiologically active).

The thyroid gland NB. T 3 is less bound, has shorter half life and its biological activity is 3 -5 times greater and its action is much more rapid than T 4. Mechanism of action of thyroid hormones Bind to specific nuclear receptors in the target cells → increasing the transcription of m. RNAs. Metabolism of thyroid hormones They are inactivated by • Deiodination By deiodinase enzymes. In the liver, kidney and many other tissues • Conjugation In the liver T 3 and T 4→ Sulphates and glucoronide

The thyroid gland Action of the thyroid hormones 1. Calorigenic effect ↑ the metabolic rate and O 2 consumption in all tissues except adult brain, testes, uterus, lymph nodes, spleen and ant pituitary. → ↑ heat production and body temprature. 2. Effect on the heart ↑ myocardial contractility, number of beta adrenergic receptors and enhance the response to catecholamines→ ↑ HR, strok volum, COP and systolic B. P LEAD TO ↑ in the pulse pressure and liability to develop arrhythmias. 3. Effect on the nervous system

The thyroid gland Essential for the normal development of the CNS 4. Effect on growth Promote bone growth and skeletal development early in life Exerting a permissive action on the GH Affect teeth growth, contour of the face and proportion of thebody Exert direct effect on tissue maturation 5. Effect on respiratory functions Help O 2 dissociation from HB by increasing 2, 3 DPG 6. Effect on sex functions Essential for normal menstrual cycle, sprrmatogenesis and milk secretion

The thyroid gland 7. Conversion of carotene to vitamin A in the liver 8. Effect on protein metabolism and skeletal muscles Protein catabolism and –ve nitrogen balance 9. Effect on carbohydrate metabolism ↑ CHO absorption from the GIT and glucose utilization in the tissues 10. Effect on lipid and cholesterol metabolism ↓ blood cholesterol level ↓ blood level of lipids


Regulation of thyroid gland activities • TSH Normal plasma level: 2 microunits Action: generalized stimulating effect on the thyroid gland Factors that control TSH secretion 1. 2. 3. Thyrotropin releasing hormone TRH Feedback control Other factors Dopamin, somatostatin and glucocorticoids inhibit TSH secretion shows a circadian rhythm Pregnancy • Dietary iodine intake


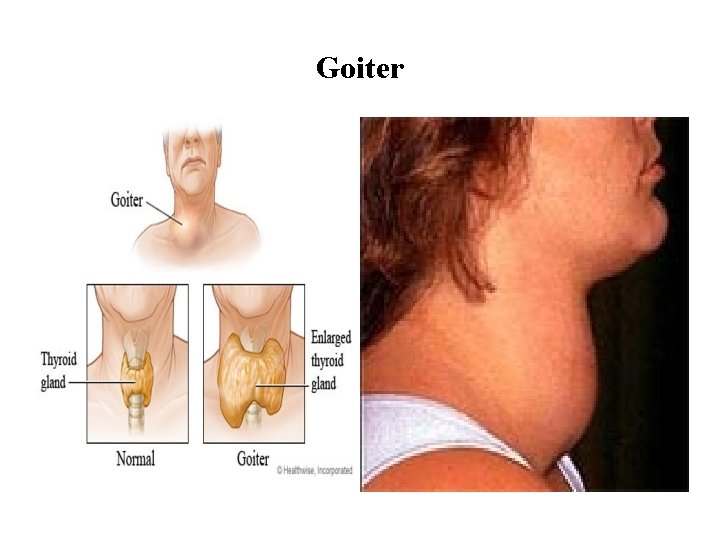
Goiter It’s a thyroid enlargement that is non-inflammatory and non malignant • Simple goiter Normal thyroid function Occurs due to - mild iodin defficiency - increased need to iodine • Colloid goiter Hypothyroidism Occurs due to – severe iodine defficiency • Toxic goiter Hyperthyroidism Occurs due to - excessive stimulation of the thyroid gland

Goiter

Diseases of the thyroid gland Hyperthyroidism Causes: 1. Grave’s disease: Autoimmune disease (TSH receptor- stimulating 2. 3. antibodies) Thyroid gland tumours TSH- secreting pituitary tumours Manifestations 1. 2. 3. 4. 5. 6. 7. 8. Exophthalmic goiter ↑ BMR Cardiovascular symptoms Nervous symptoms Metabolic changes Hyperphagia Increased intestinal movement Increased protein catabolism


Diseases of the thyroid gland Hypothyroidism 1. Hypothyroidism in adults (myxedema) Causes Primary hypothyroidism 2 nd hypothyroidism Manifestations 1. Skin changes 2. ↓ BMR 3. The muscles become weak and easily fatigued 4. Nervous symptoms 5. Husky and slow voice 6. Hypercholesterolemia 7. Depression of sex function 8. Microcytic hypochromic anemia



Diseases of the thyroid gland 2. Hypothyroidism in children (Cretinism) Causes Maternal causes Fetal causes Manifestations 1. Delayed physical growth 2. Mental retardation 3. Low BMR 4. Sexual retardation 5. The skin 6. General features



Thyroid functions tests A- Specific tests • • Measurement of serum T 4 by radioimunoassay Measurement of serum T 3 by radioimunoassay Measurement of pituitary production of TSH Measurement of TSH receptors antibodies Thyroid scan Functional stimulation test(TSH, TRH stimulation tests) Iodine suppression test Thyroid needle biopsy B- non specific tests • BMR • Serum cholesterol

Thyroid functions tests • A 40 -year-old woman comes to her primary care physician complaining of nervousness and an unexplained weight loss of 20 pounds over the past 3 month despite her impression that she is eating all the time. On physical examination, her eyes are found to be protruding, her skin is moist and warm, and her fingers have a slight tremor. Compared to a normal individual, a biopsy of her thyroid gland would most likely reveal which of the following: A. Decreased numbers of reabsorption lacunae B. Decreased evidence of endocytosis C. A decrease in the cross-sectional area occupied by colloid D. Increased levels of NIS in the basolateral membrane of thyrocytes E. Decreased evidence of lysosomal activity

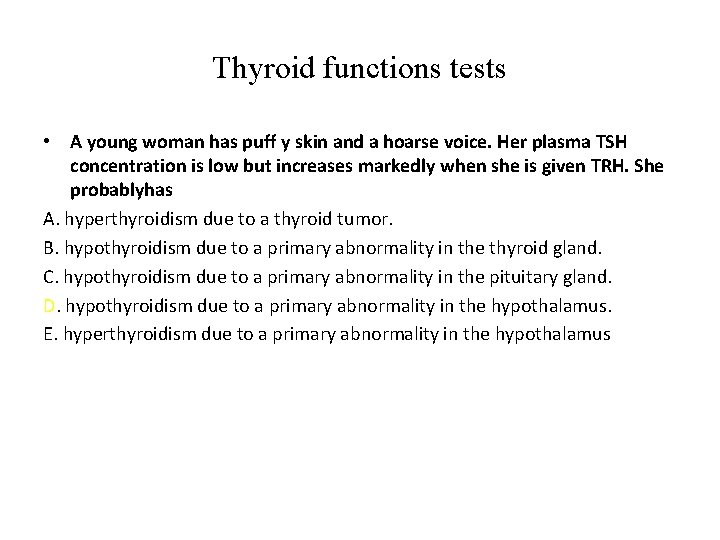
Thyroid functions tests • A young woman has puff y skin and a hoarse voice. Her plasma TSH concentration is low but increases markedly when she is given TRH. She probablyhas A. hyperthyroidism due to a thyroid tumor. B. hypothyroidism due to a primary abnormality in the thyroid gland. C. hypothyroidism due to a primary abnormality in the pituitary gland. D. hypothyroidism due to a primary abnormality in the hypothalamus. E. hyperthyroidism due to a primary abnormality in the hypothalamus

Parathyroid Glands Functional anatomy • there are four parathyroid glands in humans • located immediately behind the thyroid gland • Each parathyroid gland like a dark brown fat, is about 6 millimeters long, 3 millimeters wide, and 2 millimeters thick. • Removal of half the parathyroid glands → no major physiologic abnormalities • removal of three of the four normal glands → transient hypoparathyroidism

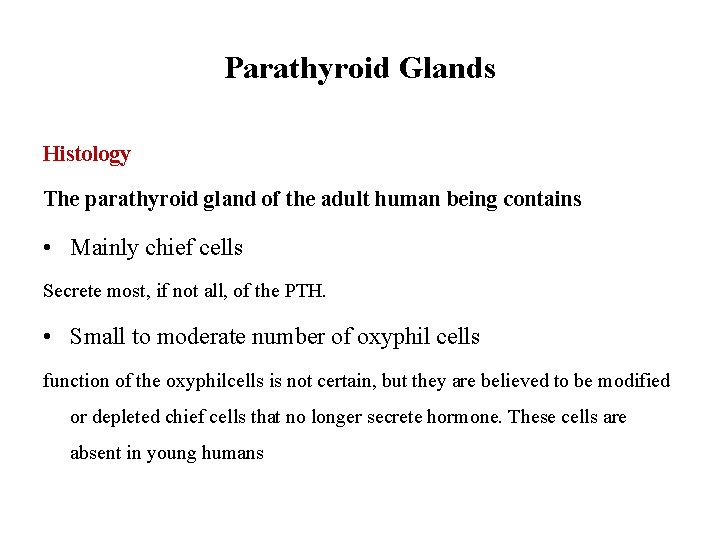
Parathyroid Glands Histology The parathyroid gland of the adult human being contains • Mainly chief cells Secrete most, if not all, of the PTH. • Small to moderate number of oxyphil cells function of the oxyphilcells is not certain, but they are believed to be modified or depleted chief cells that no longer secrete hormone. These cells are absent in young humans


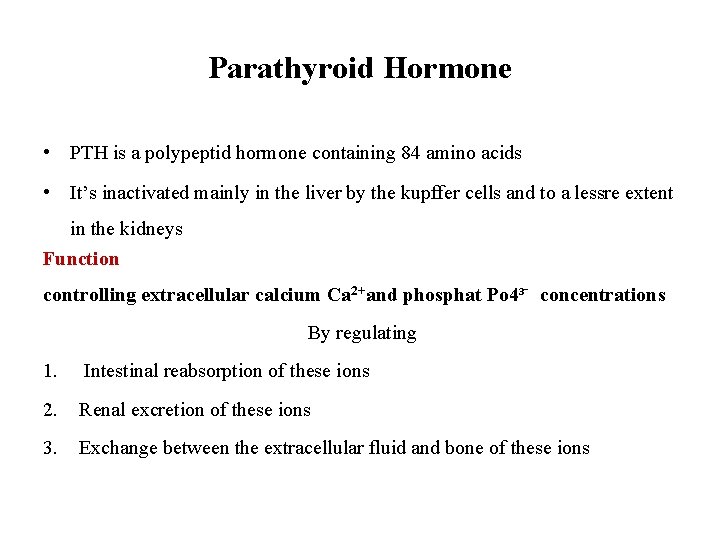
Parathyroid Hormone • PTH is a polypeptid hormone containing 84 amino acids • It’s inactivated mainly in the liver by the kupffer cells and to a lessre extent in the kidneys Function controlling extracellular calcium Ca 2+and phosphat Po 4³ concentrations By regulating 1. Intestinal reabsorption of these ions 2. Renal excretion of these ions 3. Exchange between the extracellular fluid and bone of these ions

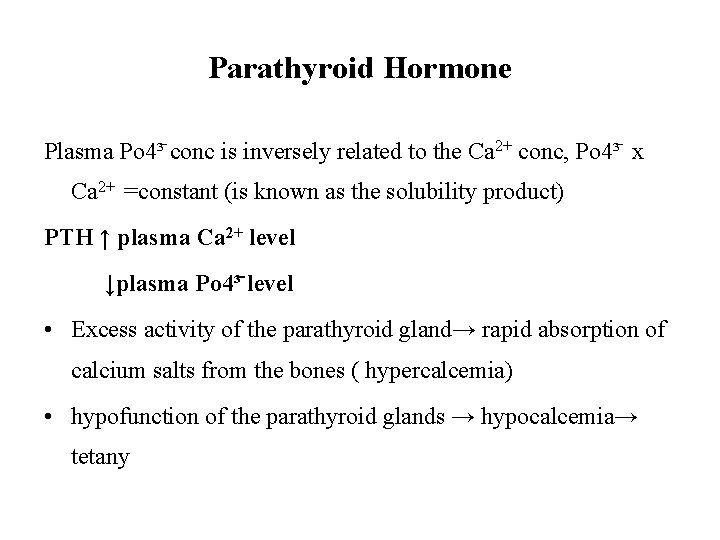
Parathyroid Hormone Plasma Po 4³ conc is inversely related to the Ca 2+ conc, Po 4³ x Ca 2+ =constant (is known as the solubility product) PTH ↑ plasma Ca 2+ level ↓plasma Po 4³ level • Excess activity of the parathyroid gland→ rapid absorption of calcium salts from the bones ( hypercalcemia) • hypofunction of the parathyroid glands → hypocalcemia→ tetany

Parathyroid Hormone Increases Calcium and Phosphate Absorption from the Bone Parathyroid Hormone Decreases Calcium Excretion and Increases Phosphate Excretion by the Kidneys • PTH diminish proximal tubular reabsorption of phosphate ions causes rapid loss of phosphate in the urine. N. B the strong effect of PTH to increase renal phosphate excretion, an effect that is usually great enough to override increased phosphate absorption from the bone.

Parathyroid Hormone • PTH also increases renal tubular reabsorption of calcium mainly in the late distal tubules, the collecting tubules, the early collecting ducts and to lesser extent the ascending loop of Henle. • It increases the rate of reabsorption of magnesium ions and hydrogen ions • It decreases the reabsorption of sodium, potassium, and amino acid ions in much the same way that it affects phosphate. Parathyroid Hormone Increases Intestinal Absorption of Calcium and Phosphate PTH greatly enhances both calcium and phosphate absorption from the intestines by increasing the formation of 1, 25 -dihydroxycholecalciferol from vitamin D in the kidneys

Parathyroid Hormone Mechanism of action of PTH acts through activating the adenylyl cyclase enzyme→ ↑the cyclic adenosine monophosphate (c. AMP) second messenger mechanism. Control of Parathyroid Secretion by Calcium Ion Concentration By –ve feedback mechanism • slightest decrease in plasma Ca 2+ → ↑ rate of PTH secretion within minutes. • In case of chronic decrease in plasma Ca 2+ sometimes fivefold or more → the glands will hypertrophy,

Parathyroid Hormone As in cases of ( rickets- pregnancy- lactation) • Increased plasma Ca 2+ → decreased activity and reduced size of the parathyroid glands As in cases of - excess quantities of calcium in the diet - increased vitamin D in the diet -bone absorption caused by factors other than PTH




Hypoparathyroidism • This occurs only when at least 3 parathyroid glands are damaged Causes • Autoimmunity • Accidental removal during thyroid surgery Effect • Hypocalcemia • Hyperphosphatemia N. B When the parathyroid glands are suddenly removed, the calcium level in the blood falls from the normal of 9. 4 mg/dl to 6 to 7 mg/dl within 2 to 3 days, and the blood phosphate concentration may double

Hypoparathyroidism Hypocalcemia • Extracellular fluid calcium concentration normally is 9. 4 mg/dl • calcium plays a key role in many physiologic processes - contraction of skeletal, cardiac, and smooth muscles - blood clotting - transmission of nerve impulse N. B neurons are very sensitive to changes in calcium ion concentrations 1. Hypercalcemia causes progressive depression of the nervous system 2. hypocalcemia causes the nervous system to become more excited

Hypoparathyroidism Effects of hypocalcemia • Numbness and tingling sensation • Muscle weakness, cardiac arrhythmias • Nervous System Excitement and Tetany. Due to increased neuronal membrane permeability to sodium ions, allowing easy initiation of action potentials. At plasma calcium ion concentrations about 50 per cent below normal, the peripheral nerve fibers become so excitable that they begin to discharge spontaneously, initiating trains of nerve impulses that pass to the peripheral skeletal muscles to elicit tetanic muscle contraction.

Hypoparathyroidism because of its action of increasing excitability in the brain hypocalcemia causes seizures. Tetany Definition It’s a state of spastic contraction of the skeletal muscles due to increased neuromuscular excitability that occurs as a result of a decrease in plasma Ca 2+. Causes Are those that cause hypocalcemia Hyperparathyroidism Renal failure Alkalemia Vit D defficiency Low calcium content in diet steatorrhea

Tetany Manifest tetany Plasma Ca 2+ level drops below 7 mg‰ Stiffness of skeletal muscles interrupted by attacks of spasmodic contraction Carpopedal spasm Generalized convulsions may occur Spasmodic contraction of the laryngeal muscles → respiratory distress and cyanosis If this sever or prolonged→ asphyxia


Tetany Latent tetany This occurs when the drop in the plasma Ca 2+ level doesn’t reach 7 mg ‰ tetany appears only in conditions that increase the neuromuscular excitability - Emotions - Pregnancy Diagnosis Measuring plasma Ca 2+ level Provocation tests - trousseau’s - Chvostek’s test - Erb’s test - hyperventilation - lactation

Tetany Treatment • the administration of extremely large quantities of vitamin D, to as high as 100, 000 units per day, along with intake of 1 to 2 grams of calcium, keeps the calcium ion concentration in normal range. • Dihydrotachysterol Has similar effect as PTH but it’s preferred as No antibody formation It acts for longer duration than PTH Less expensive

MCQS 1. A patient with parathyroid deficiency 10 days after inadvertent damage to the parathyroid glands during thyroid surgery would probably have A. low plasma phosphate and Ca 2 + levels and tetany. B. low plasma phosphate and Ca 2 + levels and tetanus. C. a low plasma Ca 2 + level, increased muscular excitability, and spasm of the muscles of the upper extremity (Trousseau sign). D. high plasma phosphate and Ca 2 + levels and bone demineralization. E. increased muscular excitability, a high plasma Ca 2 + level, and bone demineralization. 2. In an experiment, a rat is infused with a small volume of a calcium chloride solution, or sodium chloride as a control. Compared to the control condition, which of the following would result from the

MCQS calcium load? A. Bone demineralization. B. Increased formation of 1, 25 dihydroxycholecalciferol. C. Decreased secretion of calcitonin. D. Decreased blood coagulability. E. Increased formation of 24, 25 dihydroxycholecalciferol. 3. Which of the following is not involved in regulating plasma Ca 2+ levels? A. Kidneys B. Skin C. Liver D. Lungs E. Intestine

MCQS 5. Which of the following would you expect to find in a patient whose diet has been low in calcium for 2 months? A. Increased formation of 24, 25 dihydroxycholecalciferol. B. Decreased amounts of calcium-binding protein in intestinal epithelial cells. C. Increased parathyroid hormone secretion. D. A high plasma calcitonin concentration. E. Increased plasma phosphate.

The Adrenal Gland • There are two adrenal gland • Each of which weights about 4 grams • lie at the superior poles of the two kidneys. • each gland is composed of 1. The adrenal medulla The central 20 per cent of the gland, is functionally related to the sympathetic nervous system; it secretes the hormones epinephrine and norepinephrine in response to sympathetic stimulation. 2. The adrenal cortex secretes different group of hormones, called corticosteroids


Adrenal cortex The Adrenal Cortex Has Three Distinct Layers 1. The zona glomerulosa • a thin layer of cells that lies just underneath the capsule. • constitutes about 15 per cent of the adrenal cortex. • Its cells secret significant amounts of aldosterone because they contain the enzyme aldosterone synthase, • The secretion of these cells is controlled mainly by the extracellular fluid concentrations of angiotensin II and potassium.

Adrenal cortex 2. The zona fasciculata the middle and widest layer constitutes about 75 per cent of the adrenal cortex secretes the glucocorticoids cortisol and corticosterone, as well as small amounts of adrenal androgens and estrogens. The secretion of these cells is controlled in large part by the hypothalamicpituitary axis via (ACTH).

Adrenal cortex 3. The zona reticularis • the deep layer of the cortex • secretes the adrenal androgens dehydroepiandrosterone (DHEA) androstenedione, as well as small amounts of estrogens and some glucocorticoids. • The secretion of these cells is controlled by ACTH and cortical androgenstimulating hormone, released from the pituitary may also be involved.


Adrenocortical Hormones Are Steroids Derived from Cholesterol. • Mineralocorticoids (Aldosteron) they affect the electrolytes (the “minerals”) of the extracellular fluids-sodium and potassium • Glucocorticoids (cortisol) They exhibit important effects that increase blood glucose concentration. They affects both protein and fat metabolism • Small amounts of sex hormones androgenic hormones Has same effects in the body as the male sex hormone testosterone. Normally of only slight importance

Adrenocortical Hormones Mineralocorticoids • Aldosterone (very potent, accounts for about 90 per cent of all mineralocorticoid activity) • Desoxycorticosterone (1/30 as potent as aldosterone, but very small quantities secreted) • Corticosterone (slight mineralocorticoid activity) • 9ά-Fluorocortisol (synthetic, slightly more potent than aldosterone) • Cortisol (very slight mineralocorticoid activity, but large quantity secreted) • Cortisone (synthetic, slight mineralocorticoid activity)

Adrenocortical Hormones Glucocorticoids • Cortisol (very potent, accounts for about 95 per cent of all glucocorticoid activity) • Corticosterone (provides about 4 per cent of total glucocorticoid activity, but much less potent than cortisol) • Cortisone (synthetic, almost as potent as cortisol) • Prednisone (synthetic, four times as potent as cortisol) • Methylprednisone (synthetic, five times as potent as cortisol) • Dexamethasone (synthetic, 30 times as potent as cortisol)


Adrenocortical Hormones some of these hormones have both glucocorticoid and mineralocorticoid activities. e. g cortisol The intense glucocorticoid activity of the synthetic hormone dexamethasone, makes this an especially important drug for stimulating specific glucocorticoid activity Adrenocortical Hormones Are Bound to Plasma Proteins. • 90 to 95 per cent of the cortisol in the plasma binds to plasma proteins, especially a globulin called cortisol-binding globulin or transcortin and, to a lesser extent, to albumin.

Adrenocortical Hormones • this slows the elimination of cortisol from the plasma→ cortisol has a relatively long half life of 60 to 90 minutes→ a relatively uniform distribution of the adrenal hormones to the tissues. • Only about 60 per cent of circulating aldosterone combines with the plasma proteins→ it has a relatively short half-life of about 20 minutes • The adrenal steroids are degraded mainly in the liver, conjugated especially to glucuronic acid and, to a lesser extent, sulfates. • 25 per cent of these conjugates are excreted in the bile, The remaining conjugates filtered readily by the kidneys and excreted in the urine

Mineralocorticoid Functions of mineralocorticoid • Regulating Na and K metabolism and extracellular fluid volume - Aldosterone increases renal tubular reabsorption of sodium and secretion of Potassium by the renal tubular epithelial cells especially in the principal cells of the collecting tubules and, to a lesser extent, in the distal tubules and collecting ducts → sodium to be conserved in the extracellular fluid while increasing potassium excretion in the urine. • Aldosterone Stimulates Sodium and Potassium Transport in Sweat Glands, Salivary Glands, and Intestinal Epithelial Cells

Mineralocorticoid • Excess Aldosterone Increases Extracellular Fluid Volume and Arterial Pressure but Has Only a Small Effect on Plasma Sodium Concentration Aldosteron escape - when aldosterone secretion becomes zero, large amounts of salt are lost in the urine → ↓ the amount of sodium chloride in the extracellular fluid and decreasing the extracellular fluid volume→ severe extracellular fluid dehydration and low blood volume→ circulatory shock → death within a few days after the adrenal glands suddenly stop secreting aldosterone. • Excess Aldosterone Causes Hypokalemia and Muscle Weakness; Too Little Aldosterone Causes Hyperkalemia and Cardiac Toxicity.

Mineralocorticoid - Excess aldosteron causes loss of potassium ions from the extracellular fluid into the urine transport of potassium from the extracellular fluid into most cells of the body. → hypokalemia (4. 5 to 2 m. Eq/L) - deficient aldosterone → hyperkalemia When K rises to 60 to 100 per cent above normal→ serious cardiac toxicity • Excess Aldosterone Increases Tubular Hydrogen Ion Secretion, and Causes Mild Alkalosis. Aldosteron causes secretion of hydrogen ions in exchange for sodium in the intercalated cells of the- cortical collecting tubules

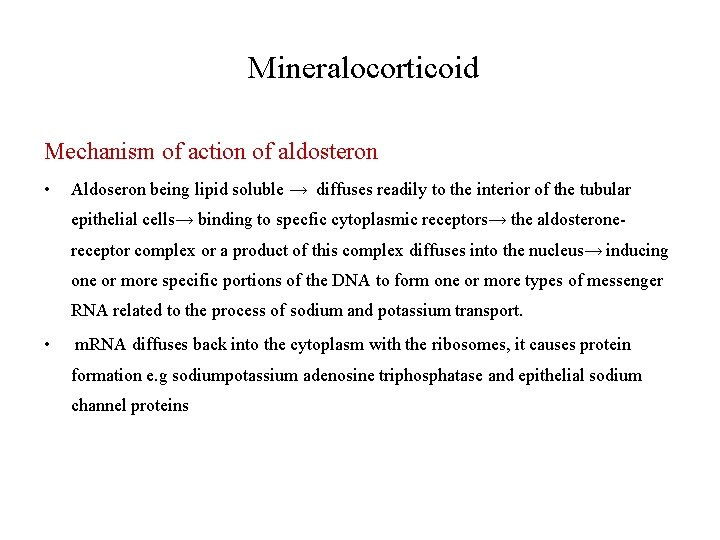
Mineralocorticoid Mechanism of action of aldosteron • Aldoseron being lipid soluble → diffuses readily to the interior of the tubular epithelial cells→ binding to specfic cytoplasmic receptors→ the aldosteronereceptor complex or a product of this complex diffuses into the nucleus→ inducing one or more specific portions of the DNA to form one or more types of messenger RNA related to the process of sodium and potassium transport. • m. RNA diffuses back into the cytoplasm with the ribosomes, it causes protein formation e. g sodiumpotassium adenosine triphosphatase and epithelial sodium channel proteins

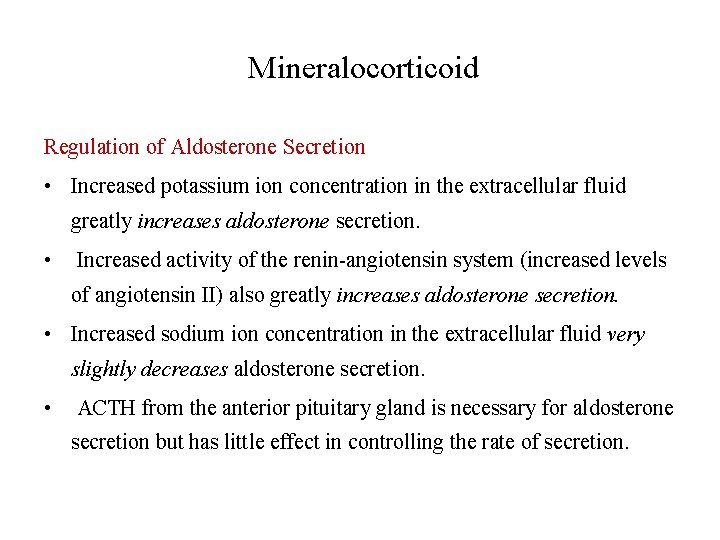
Mineralocorticoid Regulation of Aldosterone Secretion • Increased potassium ion concentration in the extracellular fluid greatly increases aldosterone secretion. • Increased activity of the renin-angiotensin system (increased levels of angiotensin II) also greatly increases aldosterone secretion. • Increased sodium ion concentration in the extracellular fluid very slightly decreases aldosterone secretion. • ACTH from the anterior pituitary gland is necessary for aldosterone secretion but has little effect in controlling the rate of secretion.

Glucocorticoids Functions of glucocorticoids Carbohydrate metabolism • Elevated Blood Glucose Concentration and “Adrenal Diabetes. ” The increase in blood glucose concentration is occasionally great enough (50 per cent or more above normal) Insulin is less effective in treating adrenal diabetes than pancreatic one due to tissue resistance • Stimulation of Gluconeogenesis Glucocortocoids increase the rate of gluconeogenesis as much as 6 - to 10 -fold. • Decreased Glucose Utilization by Cells

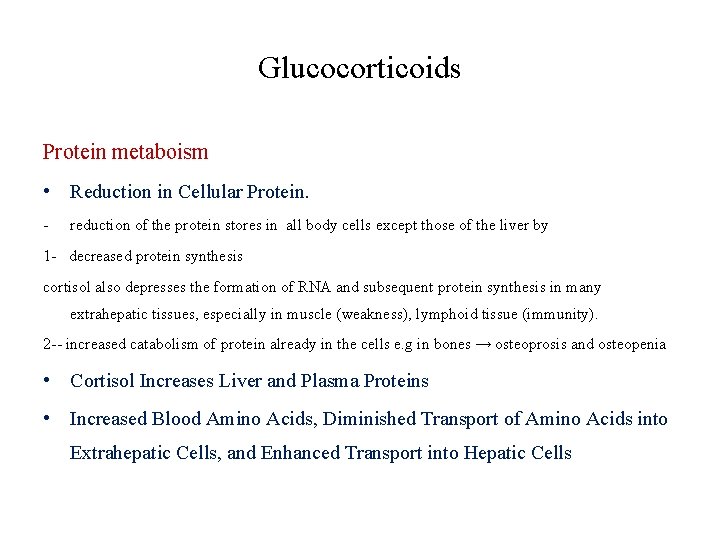
Glucocorticoids Protein metaboism • Reduction in Cellular Protein. - reduction of the protein stores in all body cells except those of the liver by 1 - decreased protein synthesis cortisol also depresses the formation of RNA and subsequent protein synthesis in many extrahepatic tissues, especially in muscle (weakness), lymphoid tissue (immunity). 2 -- increased catabolism of protein already in the cells e. g in bones → osteoprosis and osteopenia • Cortisol Increases Liver and Plasma Proteins • Increased Blood Amino Acids, Diminished Transport of Amino Acids into Extrahepatic Cells, and Enhanced Transport into Hepatic Cells

Glucocorticoids Lipid metabolism • cortisol promotes mobilization of fatty acids from adipose tissue→↑ conco free fatty acids in the plasma→ ↑ their utilization for energy • enhance the oxidation of fatty acids in the cells. These two effects lead to shift the metabolic systems of the cells from utilization of glucose for energy to utilization of fatty acids in times of starvation or other stresses. This is an important factor for long-term conservation of body glucose and glycogen. • Despit of this action excess cortisol secretion develop a peculiar type of obesity (buffalo-like torso and a rounded “moon face. ”)

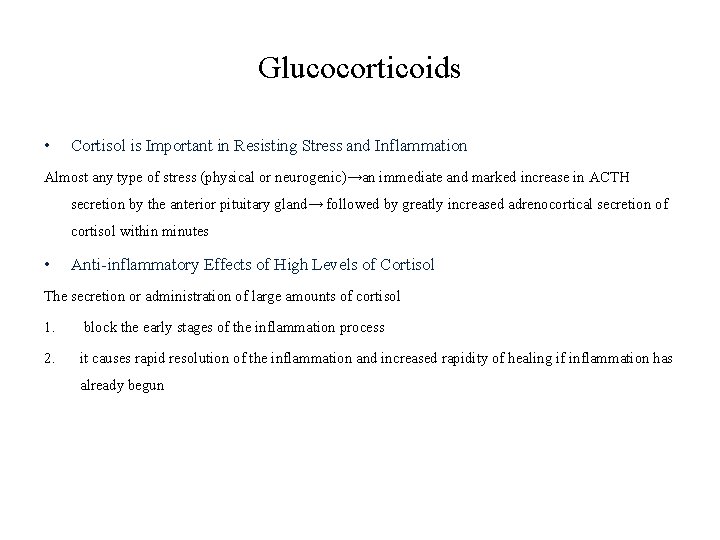
Glucocorticoids • Cortisol is Important in Resisting Stress and Inflammation Almost any type of stress (physical or neurogenic)→an immediate and marked increase in ACTH secretion by the anterior pituitary gland→ followed by greatly increased adrenocortical secretion of cortisol within minutes • Anti-inflammatory Effects of High Levels of Cortisol The secretion or administration of large amounts of cortisol 1. block the early stages of the inflammation process 2. it causes rapid resolution of the inflammation and increased rapidity of healing if inflammation has already begun

Glucocorticoids • Cortisol Blocks the Inflammatory Response to Allergic Reactions. cortisol effectively prevents shock or death in anaphylaxis • Effect on Blood Cells and on Immunity in Infectious Diseases. Cortisol decreases the number of eosinophils and lymphocytes in the blood the administration of large doses of cortisol causes significant atrophy of all the lymphoid tissue throughout the body→↓ output of T cells and antibodies→↓ immunity Cortisol increases the production of red blood cells Excess cortisol → polycythemia No cortisol→ anemia

Glucocorticoids • Permissive action Their presence is important for other hormones to exert their action eg adrenalin and glucagon • Effect on the respiratory system It accelerates the formation of surfactant in the lung in fetal life Regulation of Cortisol Secretion by ACTH from the Pituitary Gland Mechanism of action of ACTH and control of its secretion ACTH response to stress Diurnal rhythm of ACTH secretion



Adrenal sex hormones Adrenal androgen • Dehydroepiandrosterone DHEA • Androstendion Very small amounts of estrogen and progesteron Functions In males : early sexual maturation In females: growth of axillary and pubic hair at puberty

Disorders of the adrenal cortex Hypoadrenalism Addison’s Disease

Addison’s disease Causes • Failure of adrenal cortices to secrete adrenocortical hormones 1. Due to primary atrophy of the adrenal cortices is caused by autoimmunity in 80 per cent of the cases 2. 3. Tuberculosis cancer Mineralocorticoid Deficiency. • • Loss of excessive amount of Na, Cl and water in urin → greatly decreased extracellular fluid volume→↓ plasma volum and↑ conc of RBCs→↓COP→ shock and death may occur within 4 days to 2 weeks unless treated Hyponatremia hyperkalemia mild acidosis

Addison’s disease Glucocorticoid Deficiency • Failure to maintain normal blood glucoseconcentration between meals • Reduces the mobilization of both proteins and fats from the tissues→ depressing many metabolic functions of the body • Poor energy mobilization→ muscle weakness • Inability to tolerate stress Melanin Pigmentation • melanin pigmentation of the mucous membranes and skin.

Addison’s disease Treatment Small quntaties of mineralocorticoid and glucocorticoid daily Addisonian Crisis. • When pt with addison’s disease is subjected to stress he needs for excessive amounts of glucocorticoids and often must be given 10 or more times the normal quantities of glucocorticoids to prevent death. • This critical need for extra glucocorticoids and the associated severe debility in times of stress is called as Addisonian Crisis.




Hyperadrenalism-Cushing’s Syndrome Causes Excessive secretion of cortisol and to a little extent androgens as a result of • Adenomas of the anterior pituitary that secrete large amounts of ACTH→ adrenal hyperplasia and excess cortisol secretion (cushing’s disease) • Abnormal function of the hypothalamus that causes high levels of corticotropin-releasing hormone (CRH)→ excess ACTH release • Ectopic secretion of ACTH by a tumor elsewhere in the body, such as an abdominal carcinoma High ACTH and high cortisol level • Adenomas of the adrenal cortex. High cortisol→ -ve feedback ↓ACTH • Adminstration of large amounts of glucocorticoids

Hyperadrenalism-Cushing’s Syndrome Characteristics of Cushing’s syndrome • A buffalo torso • “moon face, ” due to edematous appearance of the face • acne and hirsutism due to androgenic potency of some of the hormones • Hypertension because of the slight mineralocorticoid effects of cortisol. • increased blood glucose concentration to values as high as 200 mg/dl after meals-as much as twice normal • Excessive protein catabolism - Sever muscle weakness - suppressed immune system - the subcutaneous tissues tear easily, resulting in developmentof large purplish striae - severe osteoporosis with consequent weakness of the bones.

Hyperadrenalism-Cushing’s Syndrome Treatment of Cushing’s Syndrome • Surgical removal of the tumour either pituitary or adrenal • In resistant cases or when surgery is not feasible, bilateral partial adrenalectomy followed by administration of adrenal steroids



Primary Aldosteronism (Conn’s Syndrome) Causes Secretion of large amounts of aldosterone caused by a small tumor of the zona glomerulosa Characteristics • Hypokalemia • slight increase in extracellular fluid volume and blood volume • Very slight increase in plasma sodium concentration (usually not more than a 4 to 6 m. Eq/L increase) • hypertension. • occasional periods of muscle paralysis caused by the hypokalemia • decreased plasma renin concentration


Adrenogenital Syndrome Causes • Excessive quantities of androgens that cause intense masculinizing effects throughout the body Characteristics • In a female, she develops virile characteristics • In the prepubertal male: as female plus rapid development of the male sexual organs • In the adult male: obscured by the normal virilizing characteristics of the testosterone secreted by the testes • Excretion of 17 -ketosteroids (which are derived from androgens)in the urine may be 10 to 15 times normal.



Adrenal function test Measurement of cortisol level in the plasma Measurement of aldosteron level in the plasma Plasma ACTH measurement Estimation of 17 - ketosteroid in urine Dexamethason suppression test ACTH adminstration test

The islets of langerhan’s Physiologic Anatomy of the Pancreas pancreas is composed of 1. 2. the acini: which secrete digestive juices into the duodenum the islets of Langerhans: which secrete insulin and glucagon into the blood The human pancreas has 1 to 2 million islets of Langerhans The islets of Langerhans containe • The beta cells: 60 per cent, secrete insulin and amylin • The alpha cells: 25 per cent, secrete glucagon. • The delta cells: 10 per cent , secrete somatostatin. • PP cell: secrete pancreatic polypeptide


Insulin Chemistry and Synthesis • Insulin is a glucose lowering polypeptide hormone • M. W: 5808 • 2 chains of amino acids linked by 2 disulphide bridges • Why c peptide plasma level is used as an index for the B cell function in pts receiving exogenous insulin? • insulin circulates in the blood stream in an unbound form • it has a plasma half-life that averages only about 6 minutes • Except for that portion of the insulin that combines with receptors in the target cells, the remainder is degraded by the enzyme insulinase mainly in the liver, to a lesser extent in the kidneys and muscles, andslightly in most other tissues.


Insulin Mechanism of action The insulin receptor is consisting of four subunits held together by disulfide linkages • two alpha subunits: extracellular and bind insulin • two beta subunits: that penetrate through the membrane, protruding into the cell cytoplasm. The intracellular portions have tyrosin kinase activity • The insulin binds with the alpha subunits → the intracellular portions of the beta subunits become autophosphorylated →activates a local tyrosine kinase→ phosphorylation of multiple other intracellular enzymes including a groupcalled insulin-receptor substrates (IRS)

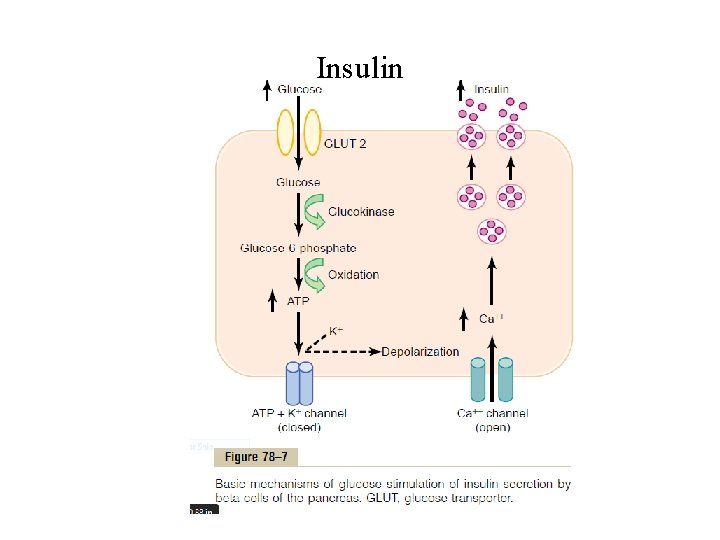
Insulin • Different types of IRS (e. g. IRS-1, IRS-2, IRS-3) are expressed in different tissues • The net effect is to activate some of these enzymes while inactivating others. • In this way, insulin directs the intracellular metabolic machinery toproduce the desired effects on carbohydrate, fat, and protein metabolism. This lead to • Within seconds after insulin binds with its membrane receptors, the membranes of about 80 per cent of the body’s cells markedly increase their uptake of glucose. (This is especially true of muscle cells and adipose cells but is not true of most neurons in the brain).


Insulin Glucose transport across cell membrane Action of insulin Effect of Insulin on Carbohydrate Metabolism • Insulin Promotes Muscle Glucose Uptake and Metabolism • Storage of Glycogen in Muscle • Insulin Promotes Liver Uptake, Storage, and Use of Glucose - insulin→ postprandial absorbed glucose to be stored almost immediately in the liver in the form of glycogen. - between meals→↓blood glucose, ↓insulin→liver glycogen is split back into glucose, released back into the blood to keep the glucose concentration from falling too low

Insulin - About 60 per cent of the glucose in the meal is stored in this way in the liver and then returned later. • Insulin Promotes Conversion of Excess Glucose into Fatty Acids and Inhibits Gluconeogenesis in the Liver • Insulin also inhibits gluconeogenesis by the liver • Lack of Effect of Insulin on Glucose Uptake and Usage by the Brain the brain cells are permeable to glucose and can use glucose without the intermediation of insulin.


Insulin Effect of Insulin on Fat Metabolism • Insulin stimulates lipogenesis in the liver and adipose tissue • Insulin prevent lipolysis in the fat cells By inhibiting the intracellular hormone sensitive lipase enzyme • Insulin promotes glucose transport through the cell membrane into the fat cells Some of this glucose is used to synthesize minute amounts of fatty acids Other glucose formslarge quantities of a-glycerol phosphate→ glycerol which combines with fatty acids to form triglycerides • Insulin decreases ketogenesis in the liver And increases the uptake of ketone bodies by the skeletal muscle

Insulin • Insulin Deficiency Increases Use of Fat for Energy • Insulin Deficiency Causes Lipolysis of Storage Fat and Release of Free Fatty Acids. • Insulin Deficiency Increases Plasma Cholesterol and Phospholipid Concentrations Effect of Insulin on Protein Metabolism • Insulin Promotes Protein Synthesis and Storage - Insulin stimulates transport of many of the amino acids into the cells. - Insulin inhibits the catabolism of proteins - • In the liver, insulin depresses the rate of gluconeogenesis. Insulin Lack Causes Protein Depletion and Increased Plasma Amino Acids. N. B The resulting protein wasting is one of the most serious of all the effects of severe diabetes mellitus. It can lead to extreme weakness as well as many deranged functionsof the organs.


Insulin • Effect of Insulin on Growth Insulin and growth hormone act synergistically to cause growth each promotes cellular uptake of a different selection of amino acids, all of which are required for growth. • Effect on plasma K level Insulin stimulate cellular uptake of K in the muscle and adipose tissue→ decreased plasma K level


Insulin


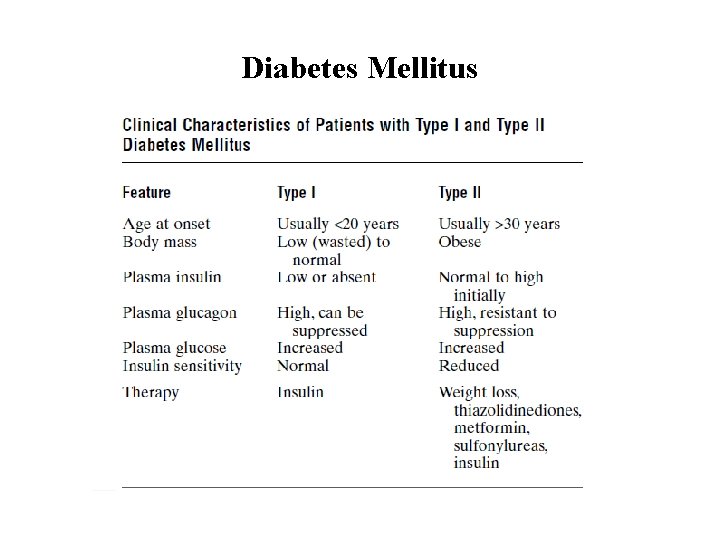
Diabetes Mellitus Diabetes mellitus: is a syndrome of impaired carbohydrate, fat, and protein metabolism caused by • lack of insulin secretion • decreased sensitivity of the tissues to insulin. Types of diabetes mellitus Type I diabetes (insulin-dependent diabetes mellitus (IDDM)) is caused by lack of insulin secretion. Type II diabetes (non–insulin-dependen diabetes mellitus (NIDDM)), is caused by decreased sensitivity of target tissues to the metabolic effect of insulin (insulin resistance)

Diabetes Mellitus

Diagnosis of diabetes Urinary Glucose To determine the quantity of glucose lost in the urine by • Simple office tests • quantitative laboratory tests Normally: undetectable amounts of glucose in urine Diabetes: urine contains glucose in small to large amounts, in proportion to the severity of disease and the intake of carbohydrates.

Diagnosis of diabetes Fasting Blood Glucose and Insulin Levels The fasting blood glucose level in the early morning is normally 80 to 90 mg/100 ml 110 mg/100 ml is considered to be the upper limit of normal A fasting blood glucose level above this value, it’s either • Diabetes mellitus • A least marked insulin resistance. • Type I diabetes→ plasma insulin levels are very low or undetectable during fasting and even after a meal. • Type II diabetes: plasma insulin concentration may be severalfold higher than normal and usually increases to a greater extent after ingestion of a standard glucose load during a glucose tolerance test

Diagnosis of diabetes Glucose Tolerance Test Glucose tolerance: is the ability of the body to utilize glucose without appearance of hyperglycemia and glucosuria. Indications Diagnosis of - Diabetes - Prediabetes - Gestational diabetes Precautions • No medication one week before the test especially steroid • Diet should contain 150 gm glucose / meal in the three days before the test

Diagnosis of diabetes • Moderate physical activity • The test shouldn’t be done in stressful conditions • Fasting 10 - 16 hrs before the test Procedure Pt is fasting and under complete physical and mental rest Fasting blood glucose is measured and urine for glucose Pt ingest 1 gram of glucose per kilogram of body weight(50 - 100 gms in 250 -300 ml water) Blood glucose is measured and urine is tested for glucose at 0. 5, 1, 2, 3, and 4 hrs after glucose ingestion

Diagnosis of diabetes Normal curve Fasting blood glucose : 70 -110 mg/100 ml The blood glucose rises and reaches maximum in one hour (120 to 140 mg/100 ml) The blood glucose falls back to below normal in about 2 hours of fasting. All urine samples contain no glucose Curve of diabetes mellitus the fasting blood glucose concentration is almost always above 110 mg/100 ml and often above 140 mg/100 ml. After one hour: much greater than normal rise in blood glucose level and may exceed the renal threshold the glucose level falls back to the control value only after 4 to 6 hours It also fails to fall below the control level In sever diabetes all urine samples contain glucose

 Thyoid gland
Thyoid gland Pituitary gland and pineal gland spiritual
Pituitary gland and pineal gland spiritual Htpothalamus
Htpothalamus Pituitary gland
Pituitary gland Pituitary gland division
Pituitary gland division Pancreas artery
Pancreas artery Dictalie
Dictalie Endocrine weight loss
Endocrine weight loss Embryonic development of pituitary gland
Embryonic development of pituitary gland Anterior pituitary
Anterior pituitary Blood supply of pituitary gland
Blood supply of pituitary gland Hypothalamus hormones
Hypothalamus hormones Nerve supply to pituitary gland
Nerve supply to pituitary gland Tuberculum impar
Tuberculum impar Hypothalamus
Hypothalamus Hypophyseal fossa and pituitary gland
Hypophyseal fossa and pituitary gland Origin of posterior pituitary gland
Origin of posterior pituitary gland Melatonin histology
Melatonin histology Evolution of pituitary gland
Evolution of pituitary gland Hypophyseal fossa and pituitary gland
Hypophyseal fossa and pituitary gland Human body organ systems
Human body organ systems Putery gland
Putery gland What is the name
What is the name Hypersecretion of prolactin
Hypersecretion of prolactin Pituitary gland disorders
Pituitary gland disorders Nerve supply of pituitary gland
Nerve supply of pituitary gland Pituitary gland
Pituitary gland Pituitary gland hormones
Pituitary gland hormones Hypophyseal fossa and pituitary gland
Hypophyseal fossa and pituitary gland N
N Hashitoxicosis
Hashitoxicosis Where is trh produced
Where is trh produced Hypophysis
Hypophysis Pars distalis
Pars distalis Hypophysis
Hypophysis Reproductive endocrinology near campbell
Reproductive endocrinology near campbell Dr betsy schwartz
Dr betsy schwartz Endocrinology
Endocrinology Disease artinya
Disease artinya 2232021
2232021 Reproductive biology and endocrinology
Reproductive biology and endocrinology Endocrinology of pregnancy
Endocrinology of pregnancy Anterior pituitary hormones
Anterior pituitary hormones Chapter 23 the endocrine system
Chapter 23 the endocrine system Pituitary dwarfism
Pituitary dwarfism Parathyroid gland chief cell
Parathyroid gland chief cell Pituitary adenoma
Pituitary adenoma Zona
Zona Difference between anterior and posterior pituitary
Difference between anterior and posterior pituitary Difference between anterior and posterior pituitary
Difference between anterior and posterior pituitary Hypoplexy
Hypoplexy Pituitary adenoma
Pituitary adenoma Hypothalamus
Hypothalamus Anterior pituitary
Anterior pituitary Hypothalamic pituitary portal system
Hypothalamic pituitary portal system Cl channel
Cl channel Anterior pituitary
Anterior pituitary Pituitary dwarfism
Pituitary dwarfism Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Phép trừ bù
Phép trừ bù Chúa sống lại
Chúa sống lại Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tiính động năng
Công thức tiính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates Dot
Dot Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Kể tên các môn thể thao
Kể tên các môn thể thao Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập điện thế nghỉ
điện thế nghỉ Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Ng-html
Ng-html Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tia chieu sa te
Tia chieu sa te Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Glasgow thang điểm
Glasgow thang điểm ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Tư thế ngồi viết
Tư thế ngồi viết Nodular goiter
Nodular goiter Warthin's tumor
Warthin's tumor Pancreas organ
Pancreas organ Serous acinus
Serous acinus Webers gland
Webers gland Seromucous gland
Seromucous gland Does urine and sperm come from the same tube
Does urine and sperm come from the same tube Sublingual gland
Sublingual gland Salivary gland disorders classification
Salivary gland disorders classification Parathyroid gland supplied by
Parathyroid gland supplied by Frontal sinus
Frontal sinus Pharynx food
Pharynx food Geniohyoid cat
Geniohyoid cat Sebaceous gland
Sebaceous gland Simple branched alveolar gland
Simple branched alveolar gland Submandibular gland excision
Submandibular gland excision Adrenal gland regions
Adrenal gland regions Seat of the soul pineal gland
Seat of the soul pineal gland Thumus
Thumus Oncocytoma salivary gland
Oncocytoma salivary gland Tubotympanic recess
Tubotympanic recess Xerotrachea
Xerotrachea Labia minora
Labia minora Bulbourethral gland
Bulbourethral gland Follicular cells of thyroid gland
Follicular cells of thyroid gland Coiled glands
Coiled glands Endokrin dan eksokrin
Endokrin dan eksokrin Pi-rads v
Pi-rads v Burning calories part/gland and the effect
Burning calories part/gland and the effect Lymph vessel
Lymph vessel Most common salivary gland tumor
Most common salivary gland tumor Simple branched alveolar gland
Simple branched alveolar gland Mammary gland
Mammary gland Pineal gland
Pineal gland Which is the largest gland of the body
Which is the largest gland of the body Endo crine gland
Endo crine gland Starfish aboral view
Starfish aboral view Gland shaped like a butterfly
Gland shaped like a butterfly Oncocytoma salivary gland
Oncocytoma salivary gland Pateys facio venous plane
Pateys facio venous plane Intercalated duct
Intercalated duct Alpha1-antitrypsin deficiency
Alpha1-antitrypsin deficiency Papillary thyroid carcinoma gross
Papillary thyroid carcinoma gross Basal cell adenoma
Basal cell adenoma Kow kant kick
Kow kant kick Git anatomy
Git anatomy Cheesy gland
Cheesy gland Thick
Thick Hormones definition psychology
Hormones definition psychology Adrenal gland sympathetic nervous system
Adrenal gland sympathetic nervous system A doughnut-shaped gland that is located below the bladder
A doughnut-shaped gland that is located below the bladder Function of the ductus deferens
Function of the ductus deferens Gastric gland
Gastric gland Underactive thyroid symptoms
Underactive thyroid symptoms A multicellular exocrine gland consists of ______.
A multicellular exocrine gland consists of ______.