DISEASES OF THE HEART MYOCARDIUM PERICARDIUM Dr Eman




















- Slides: 100

DISEASES OF THE HEART MYOCARDIUM & PERICARDIUM Dr Eman MS Muhammad

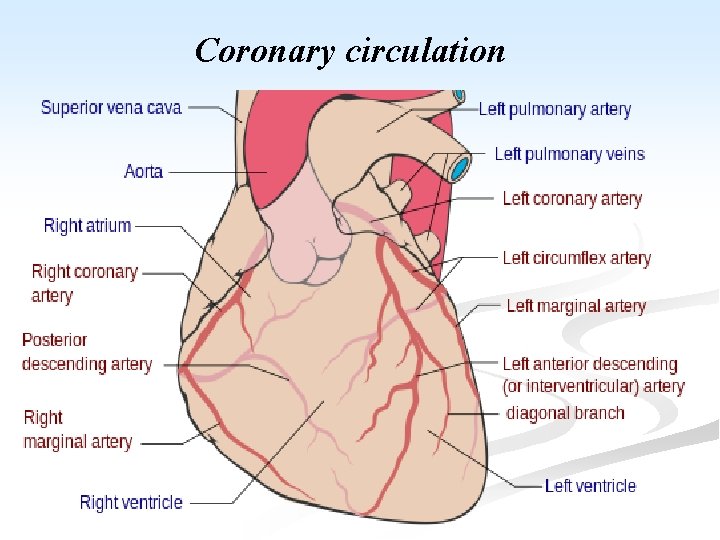
Coronary circulation

n Blood supply of the heart: n Generating energy almost exclusively by the oxidation of substrates, the heart relies heavily on an adequate flow of oxygenated blood through the coronary arteries. n With origin from the aorta immediately distal to the aortic valve, the coronary arteries consists of 5 -10 cm long, 2 -4 mm diameter, arise. n They run along the external surface of the heart and are called epicardial coronary arteries. n Whereas, the smaller vessels that penetrate the myocardium are the intramural arteries.

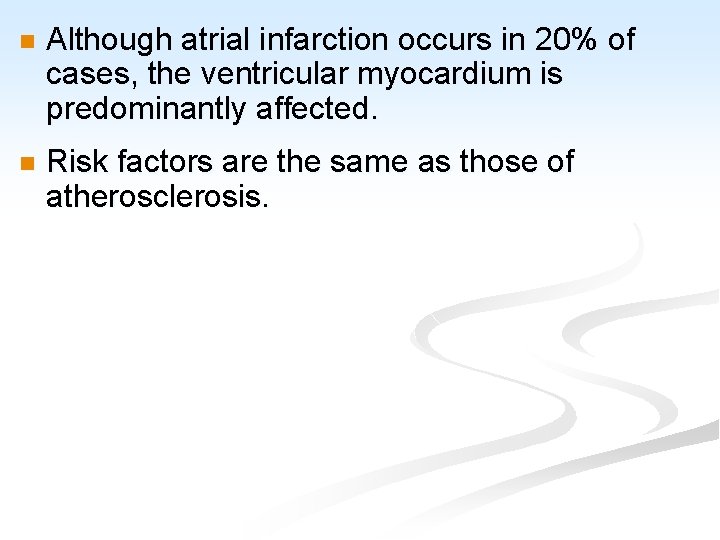
n The three major epicardial coronaries are: n The left anterior descending artery (LAD). n The left circumflex artery (LCX). n Both arising as branches from the bifurcation of the left (main) coronary artery. n The right coronary artery (RCA). n Branches of LAD are called diagonal and septal perforates. n Branches of LCX are obtuse marginals.

n Most coronary blood flow to the myocardium occurs during diastole, when the microcirculation is not compressed by myocardial contraction. n The LAD supplies most of the apex of the heart, the anterior wall of the left ventricle, and the anterior two thirds of the ventricular septum. n LCX peruses the lateral wall of the LV. n RCA supplies the right ventricular free wall, and the posterobasal wall of the LV and the posterior third of the ventricular septum.

ISCHAEMIC HEART DISEASE n General consideration and causal factors: n Ischemic heart diseases (IHD or CHD): are the leading cause of death in most industrialized countries. n Myocardial ischemia basically results from imbalance between myocardial oxygen supply and the demand of cardiac muscle.

This imbalance results from: n A. ↓ Coronary blood flow (90%): the most important factor, it is caused by: n Atherosclerosis + vasospasm + thrombosis n Other less common causes e. g. 1. Embolism from the aortic valve 2. Coronary artery spasm 3. Dissecting aneurysm 4. Polyartritis nodosa 5. Syphylitic aortic incompetence

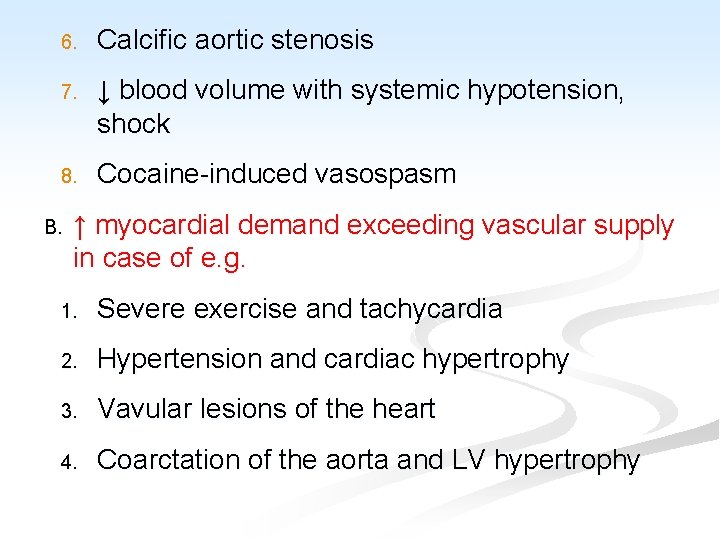
6. Calcific aortic stenosis 7. ↓ blood volume with systemic hypotension, shock 8. Cocaine-induced vasospasm B. ↑ myocardial demand exceeding vascular supply in case of e. g. 1. Severe exercise and tachycardia 2. Hypertension and cardiac hypertrophy 3. Vavular lesions of the heart 4. Coarctation of the aorta and LV hypertrophy

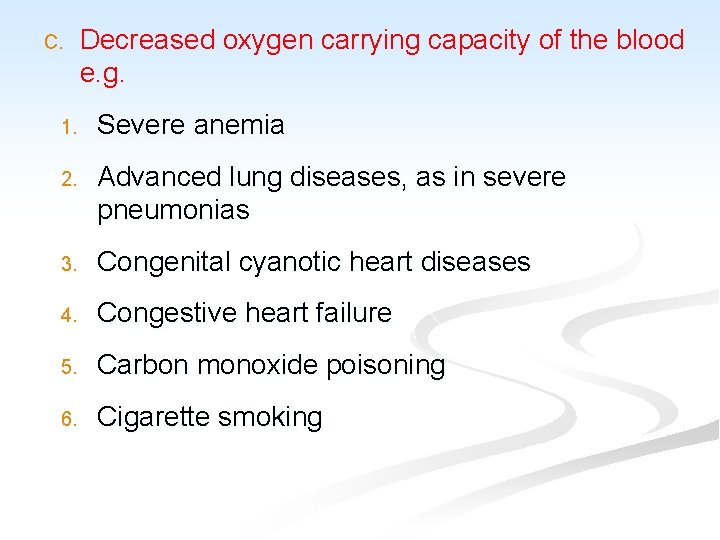
C. Decreased oxygen carrying capacity of the blood e. g. 1. Severe anemia 2. Advanced lung diseases, as in severe pneumonias 3. Congenital cyanotic heart diseases 4. Congestive heart failure 5. Carbon monoxide poisoning 6. Cigarette smoking

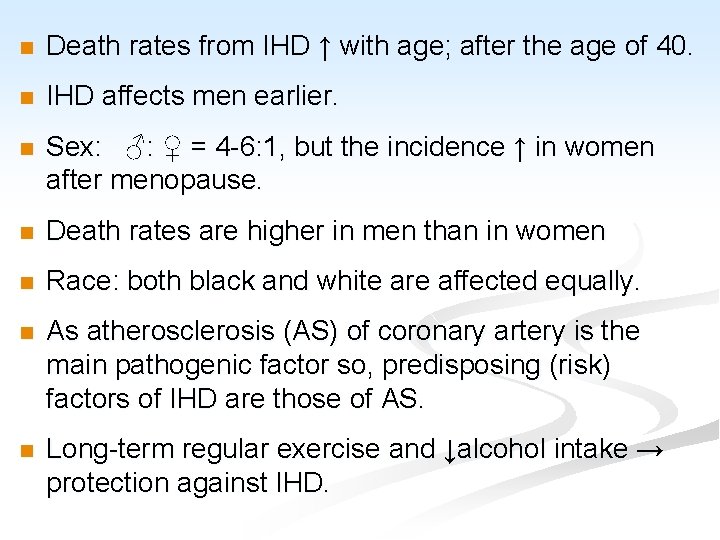
n Death rates from IHD ↑ with age; after the age of 40. n IHD affects men earlier. n Sex: ♂: ♀ = 4 -6: 1, but the incidence ↑ in women after menopause. n Death rates are higher in men than in women n Race: both black and white are affected equally. n As atherosclerosis (AS) of coronary artery is the main pathogenic factor so, predisposing (risk) factors of IHD are those of AS. n Long-term regular exercise and ↓alcohol intake → protection against IHD.

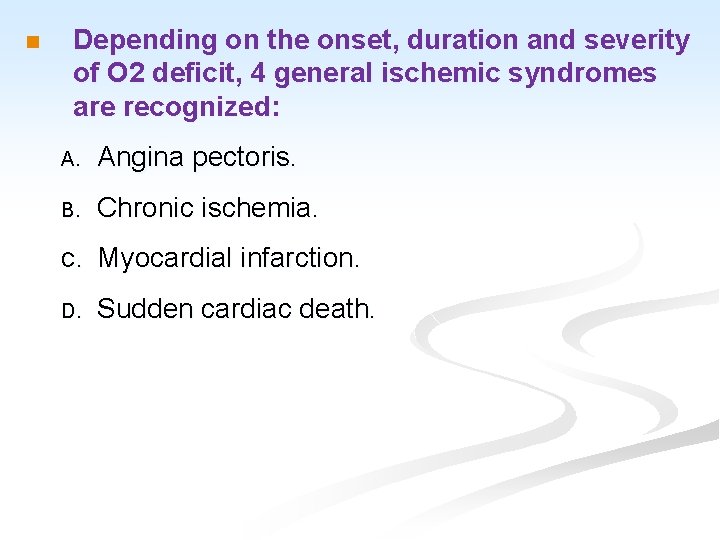
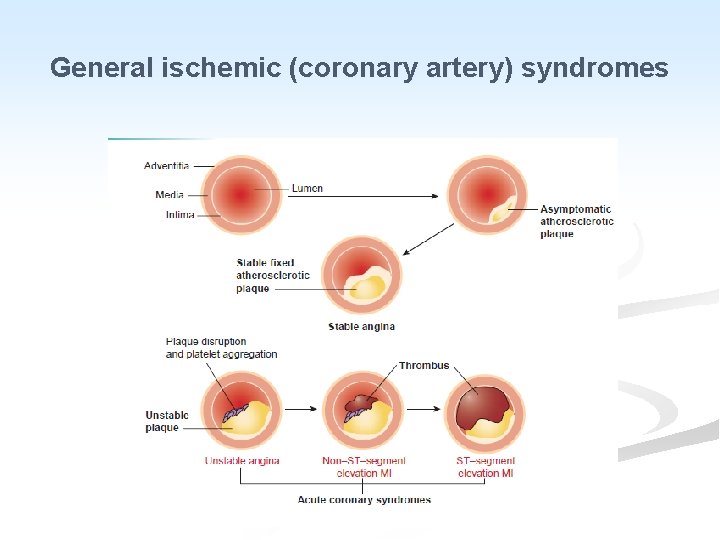
n Depending on the onset, duration and severity of O 2 deficit, 4 general ischemic syndromes are recognized: A. Angina pectoris. B. Chronic ischemia. C. Myocardial infarction. D. Sudden cardiac death.

Angina Pectoris n Angina is an episodic chest pain of variable severity often described as gripping, compressing or crushing. n It is due to transient reversible myocardial ischemia and is caused by an imbalance between myocardial oxygen supply and demand. n The pain is usually retro-sternal and may radiate to the neck and jaw or to the upper

n Attacks are brought on by factors which increase the work of the heart and includes; physical activity, exposure to cold, emotional stress, or heavy meal. n Ischemia induces release of adenosine, bradykinin that stimulate autonomic afferents → pain. n It is usually relieved by rest and/or vasodilators. n Ischemia, however, does not always give pain and up to 70% of ischemic episodes are silent. n Prognosis depends on the severity of

n Variants of angina are: I. Typical Stable Angina: n This is the commonest form of angina and many sufferers live for over 30 years. n It is due to progressive stenosing coronary artery by atheroma. n It is precipitated by exertion, emotional stress, and is relieved by rest or sublingual nitroglycerin. n Pain is classically described as a crushing or squeezing substernal sensation that radiate down the left arm or to the left jaw.

II. Variant Angina “Prinzmetal’s angina”: n In this form the attacks of pain are not related to exercise and can occur at rest. n It is caused by spasm of large or mediumsized coronary arteries, often at or near the site of atheromatous narrowing. n In 15% of cases, however, the coronary arteries appear normal. The mechanisms of vasospasm are not known. n Prinzmetal angina typically respond promptly to vasodilators as nitroglycerin and calcium channel blockers.

III. Unstable Angina (cresendo angina): n This clinical pattern may supervene in previously stable angina, or may commence de novo. n There is a sudden increase in the severity and duration of episodes of chest pain which begin to occur more frequently and often at rest. n The pathogenesis is similar to that of acute myocardial infarction. n It is associated with plaque disruption and superimposed thrombus, distal embolization of the thrombus and/or vasospasm. n In some patients it may progress to MI or sudden death.

General ischemic (coronary artery) syndromes

Chronic ischemic heart diseases It is progressive heart failure secondary to ischemic myocardial damage. n In most cases there is history of previous MI. n It occurs late in life. n

Gross: n LV hypertrophy and dilatation, discrete areas of grey white scarring from previous healed infarcts. n There is moderate to severe atherosclerosis of the coronary arteries, sometimes with total occlusion. n Microscopic: n This include myocardial hypertrophy, diffuse subendocardial vacuolization and fibrosis from previous infarction. n

Myocardial Infarction n It is commonly referred to as “heart attack”. n It is necrosis of heart muscle resulting from sudden severe myocardial ischemia. n The mortality of acute myocardial infarction (MI) is approximately 30 -35%, with 50% of these deaths occurring within an hour of onset from ventricular fibrillation (VF). n The survivors suffer from variable degrees of impaired cardiac function, including cardiac failure, arrhythmias and thrombo-emolism.

n Although atrial infarction occurs in 20% of cases, the ventricular myocardium is predominantly affected. n Risk factors are the same as those of atherosclerosis.

Myocardial infarction

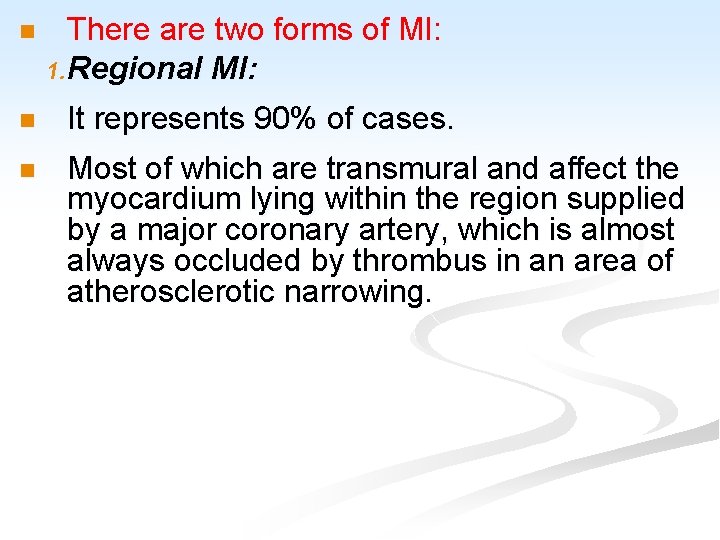
n There are two forms of MI: 1. Regional MI: n It represents 90% of cases. n Most of which are transmural and affect the myocardium lying within the region supplied by a major coronary artery, which is almost always occluded by thrombus in an area of atherosclerotic narrowing.

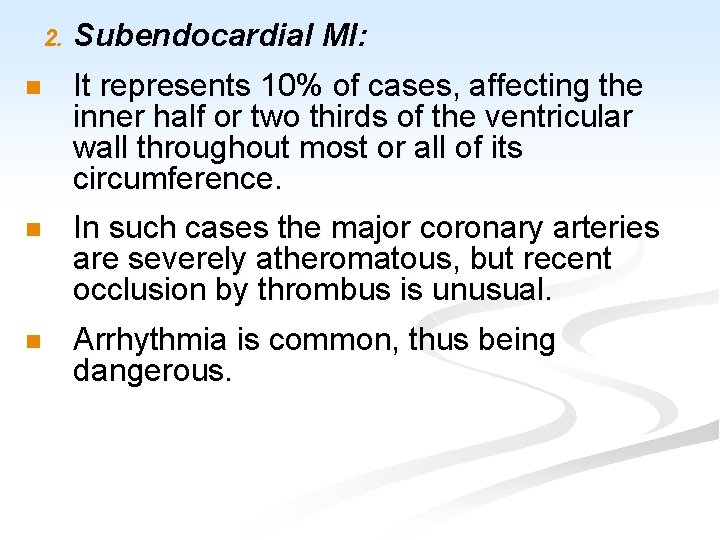
2. Subendocardial MI: n It represents 10% of cases, affecting the inner half or two thirds of the ventricular wall throughout most or all of its circumference. n In such cases the major coronary arteries are severely atheromatous, but recent occlusion by thrombus is unusual. n Arrhythmia is common, thus being dangerous.

Some describes a third variant of MI named: 3. Microscopic infarcts: occur in the setting of small vessel occlusions. n They can occur in cases of vasculitis, embolization of valve vegetations, or mural thrombi, or vessel spasm due to elevated catecholamines either endogenous (e. g. , pheochromocytoma or severe stress), or exogenous (e. g. , cocaine). n

I. Regional MI: n They vary greatly in size, but most are at least 2 cm across, and many are much larger. n The frequency of involvement of the coronary arteries and the distribution of infarction is: A. LAD (40 -50% of cases): the infarct is anterior extending from the apex, up to anterior wall of the LV, often involving the anterior part of the inter-ventricular septum, and adjacent anterior wall of the RV.

B. RCA (30 -40% of cases): The infarct is inferior (posterior) extending from the apex, up to inferior wall of the LV, often involving the adjacent parts of the inter-ventricular septum, and the inferior (posterior) wall of the RV. C. LCX (15% of cases): the infarct involves the lateral wall of the LV.

n MI is commonly caused by thrombosis of the left main coronary artery or much less commonly two main coronary arteries. n The extent of infarction depends on the site of occlusion (proximal or distal within the artery), and on the presence of collateral circulation. n Gradual atheromatous narrowing of a major branch may result in opening of the collateral vessels, so that when it is finally occluded by thrombus, only a small infarct is produced.

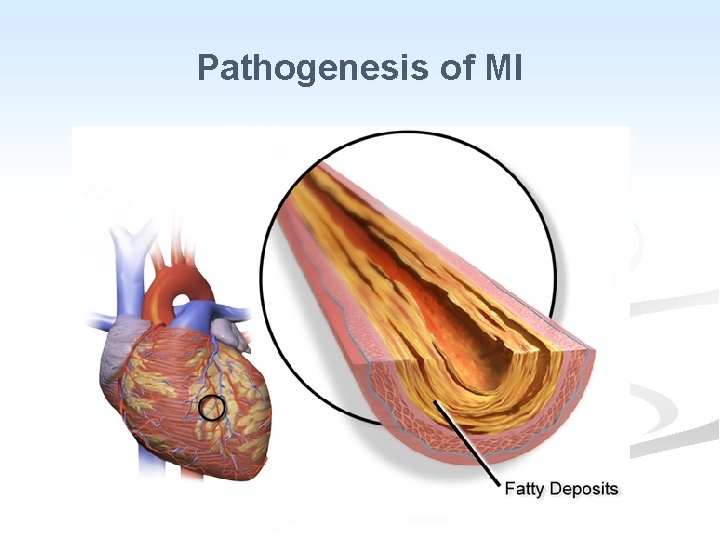
n Pathogenesis: n Three of the acute clinical events in IHD, unstable angina, MI, and sudden death, usually result from cracking or ulceration of an underlying atheromatous plaque. The sequence is: a. Fissuring, cracking or rupture of a plaque which has a lipid pool. b. Acute enlargement of the plaque with thrombosis of the lumen. c. Occlusion of the lumen. d. Penetration of vessels into the plaque.

n The role of thrombotic occlusion of coronary artery occurs in 90% 0 f MI. n Vasospasm may contribute to the occlusion, but the predominant cause is thrombus formation. n Spontaneous relieve of vasospasm and activation of the fibrinolytic system will relieve the occlusion. n Thrombi may occur at areas of endothelial damage over a severe atheromatous stenosis.

n Fissuring or ulceration of a plaque is thought to be the mechanisms which initiates the sequence of events in MI. n Healing of fissuring and thrombosis may be a factor in growth of the plaque. n The precipitating cause of severe fissuring leading to occlusion is unknown. n Shear stresses at the site of plague, increased blood pressure, and local vasospasm have all been implicated.


Pathogenesis of MI


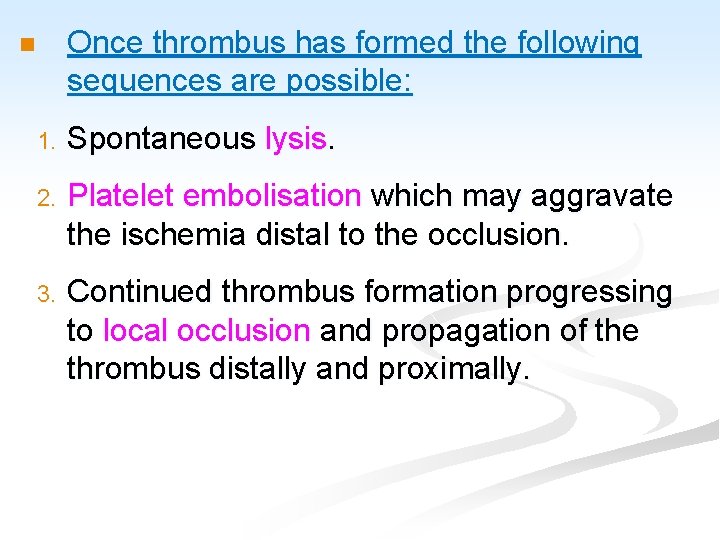
Once thrombus has formed the following sequences are possible: n 1. Spontaneous lysis. 2. Platelet embolisation which may aggravate the ischemia distal to the occlusion. 3. Continued thrombus formation progressing to local occlusion and propagation of the thrombus distally and proximally.

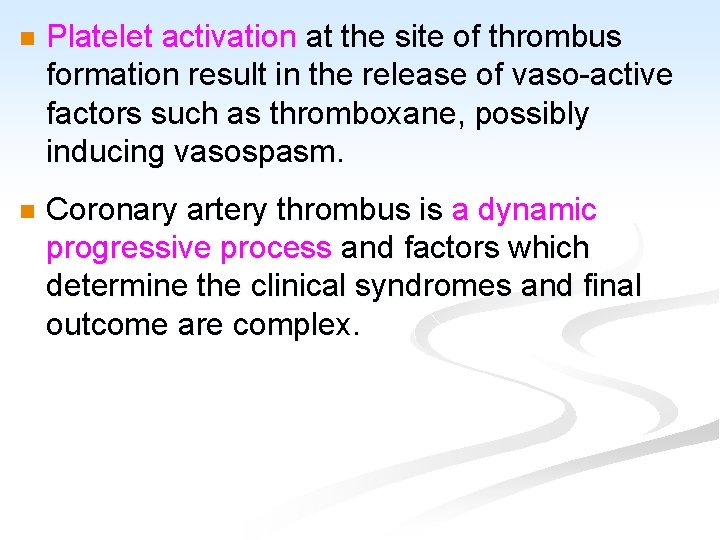
n Platelet activation at the site of thrombus formation result in the release of vaso-active factors such as thromboxane, possibly inducing vasospasm. n Coronary artery thrombus is a dynamic progressive process and factors which determine the clinical syndromes and final outcome are complex.

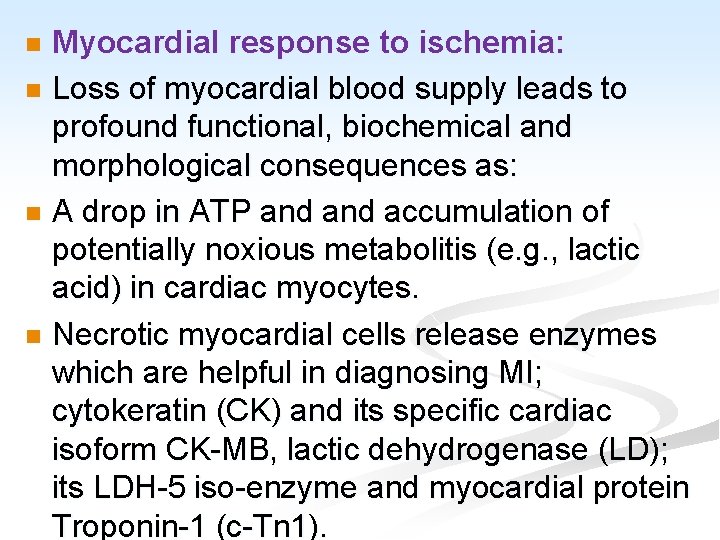
Myocardial response to ischemia: n Loss of myocardial blood supply leads to profound functional, biochemical and morphological consequences as: n A drop in ATP and accumulation of potentially noxious metabolitis (e. g. , lactic acid) in cardiac myocytes. n Necrotic myocardial cells release enzymes which are helpful in diagnosing MI; cytokeratin (CK) and its specific cardiac isoform CK-MB, lactic dehydrogenase (LD); its LDH-5 iso-enzyme and myocardial protein Troponin-1 (c-Tn 1). n

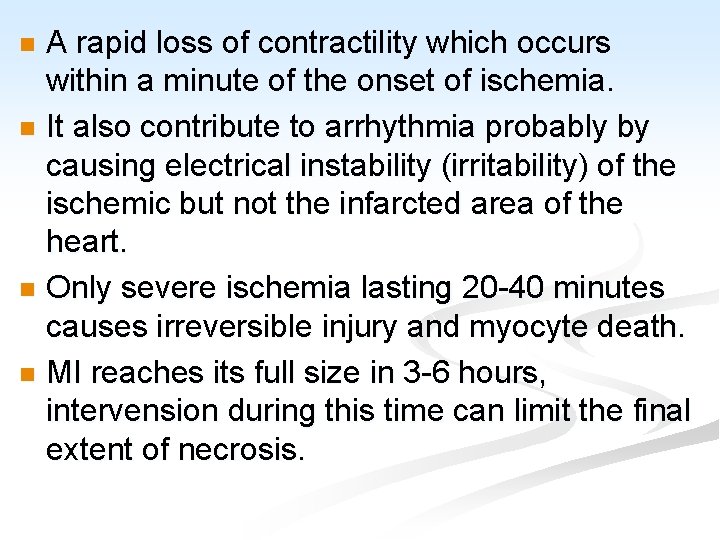
A rapid loss of contractility which occurs within a minute of the onset of ischemia. n It also contribute to arrhythmia probably by causing electrical instability (irritability) of the ischemic but not the infarcted area of the heart. n Only severe ischemia lasting 20 -40 minutes causes irreversible injury and myocyte death. n MI reaches its full size in 3 -6 hours, intervension during this time can limit the final extent of necrosis. n

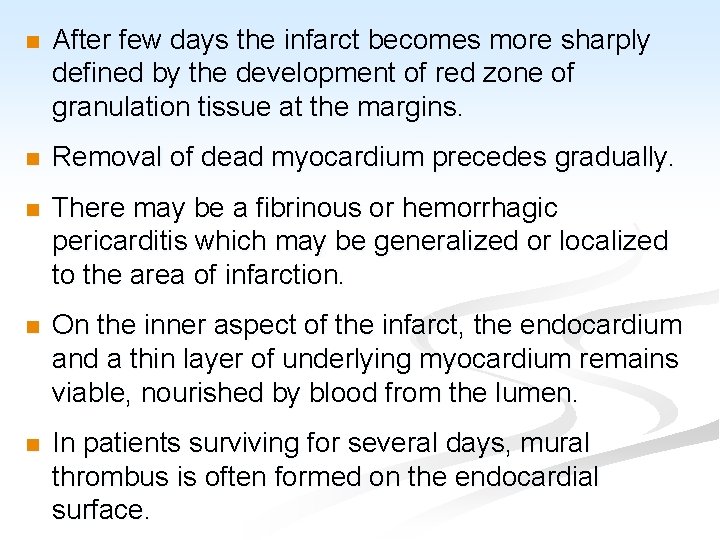
n Morbid anatomy: n Initially the necrotic muscle appears grossly and microscopically normal. n Myocardial necrosis can not be recognized in patients dying less than 6 -8 hours after the onset. n The first change visible at autopsy are blotchy pallor and congestion, followed in 24 -48 hours by palpable softening. n The color usually changes to grey-brown and then to yellow-grey. n Hemorrhages then appear at the margins.

n After few days the infarct becomes more sharply defined by the development of red zone of granulation tissue at the margins. n Removal of dead myocardium precedes gradually. n There may be a fibrinous or hemorrhagic pericarditis which may be generalized or localized to the area of infarction. n On the inner aspect of the infarct, the endocardium and a thin layer of underlying myocardium remains viable, nourished by blood from the lumen. n In patients surviving for several days, mural thrombus is often formed on the endocardial surface.

Acute Myocardial Infarction

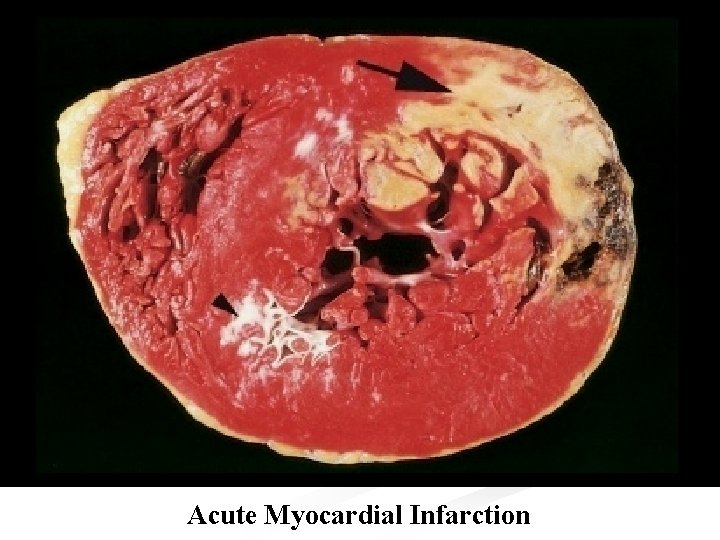
n Microscopically: n The infarcted muscle shows the changes of coagulative necrosis. n After about 8 hours it is infiltrated by PNL, and after a few days digestion by macrophages and organization can be seen at the margins. n The dead muscles is replaced by a fibrous scar over the next 6 -8 weeks. n Hypertrophy of the non infarcted myocardium occurs and the chamber enlarges to compensate for the decrease in contractility caused by the infarct, thus maintaining the stroke volume. n In 25 -30% of transmural regional infarct, stretching of the infarcted zone occurs, this may lead to aneurysmal formation.

MI: day 1, day 3, day 7

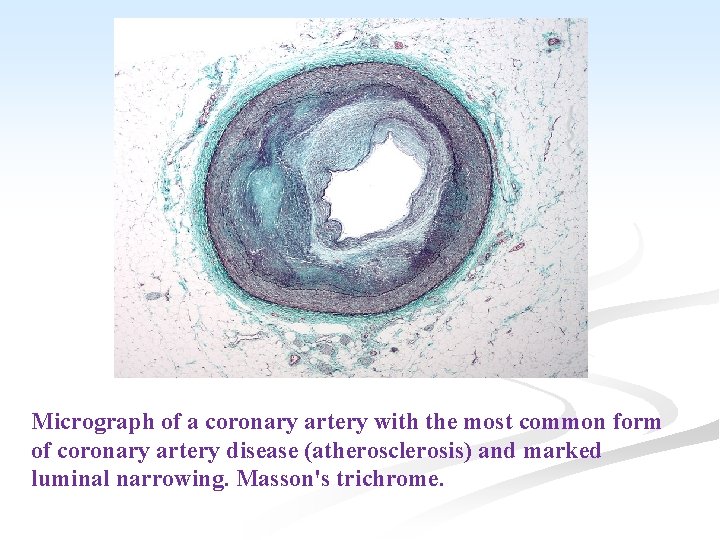
Micrograph of a coronary artery with the most common form of coronary artery disease (atherosclerosis) and marked luminal narrowing. Masson's trichrome.

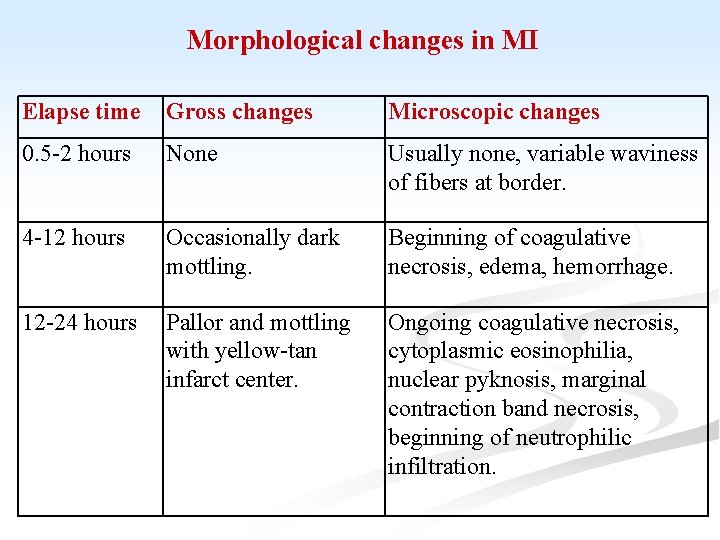
Morphological changes in MI Elapse time Gross changes Microscopic changes 0. 5 -2 hours None Usually none, variable waviness of fibers at border. 4 -12 hours Occasionally dark mottling. Beginning of coagulative necrosis, edema, hemorrhage. 12 -24 hours Pallor and mottling with yellow-tan infarct center. Ongoing coagulative necrosis, cytoplasmic eosinophilia, nuclear pyknosis, marginal contraction band necrosis, beginning of neutrophilic infiltration.

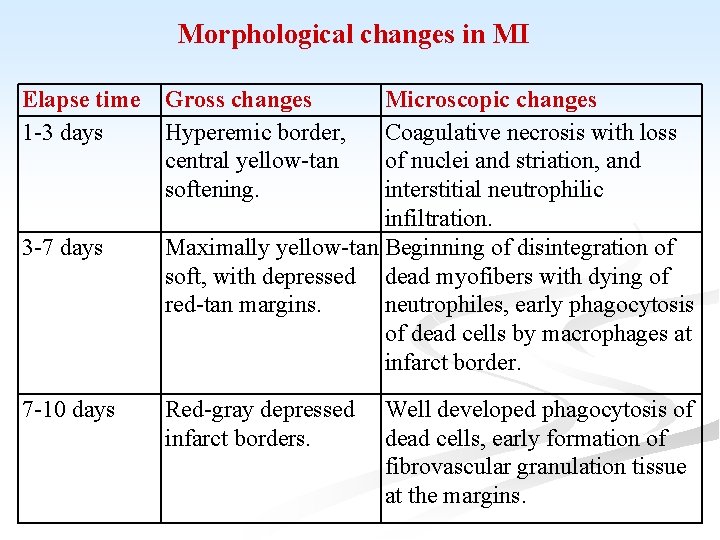
Morphological changes in MI Elapse time 1 -3 days 3 -7 days 7 -10 days Gross changes Hyperemic border, central yellow-tan softening. Microscopic changes Coagulative necrosis with loss of nuclei and striation, and interstitial neutrophilic infiltration. Maximally yellow-tan Beginning of disintegration of soft, with depressed dead myofibers with dying of red-tan margins. neutrophiles, early phagocytosis of dead cells by macrophages at infarct border. Red-gray depressed infarct borders. Well developed phagocytosis of dead cells, early formation of fibrovascular granulation tissue at the margins.

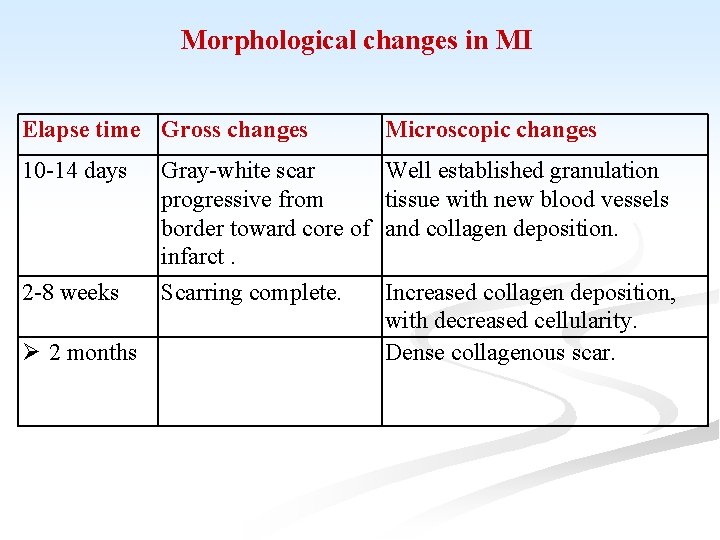
Morphological changes in MI Elapse time Gross changes Microscopic changes 10 -14 days Well established granulation tissue with new blood vessels and collagen deposition. 2 -8 weeks 2 months Gray-white scar progressive from border toward core of infarct. Scarring complete. Increased collagen deposition, with decreased cellularity. Dense collagenous scar.

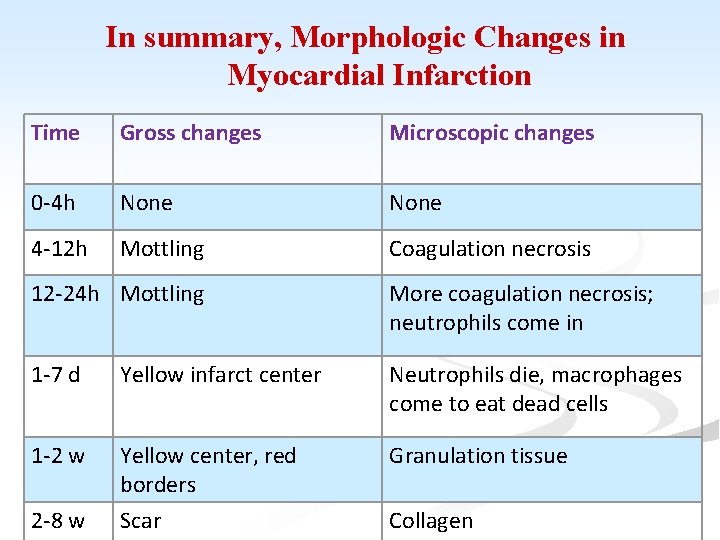
In summary, Morphologic Changes in Myocardial Infarction Time Gross changes Microscopic changes 0 -4 h None 4 -12 h Mottling Coagulation necrosis 12 -24 h Mottling More coagulation necrosis; neutrophils come in 1 -7 d Yellow infarct center Neutrophils die, macrophages come to eat dead cells 1 -2 w Yellow center, red borders Scar Granulation tissue 2 -8 w Collagen

n Size and rate of development of MI depend on: 1. The size and distribution of the affected vessel. 2. The rate of development and duration of occlusion. 3. Metabolic demands of the myocardium (affected by blood pressure and heart rate). 4. Extent of collateral circulation.

n Clinical feature and course: n The dominant symptom of MI is severe retrosternal pain, that does not relived by rest or vasodilators and persists for at least one or several hours. n It is usually accompanied by nausea, vomiting, sweating, weakness and prostration. n These early features are usually dramatic, but there is a spectrum of severity of symptoms. n In some cases MI is “silent” with little or no

n With the first few hours there is usually mild fever, moderate neutrophil leucocytosis, and characteristic ECG changes. n Necrosis of the myocardium is followed by release of cellular enzymes with consequent rise in their serum levels. n There is typical rise and gradual fall (troponin) or more rapid rise and fall (CKMB) of biochemical markers of myocardial necrosis. n Serum creatinine phosphokinase (CPK), and its isoenzyme (MB) rises in 2 -3 hours, peaks about 36 hours, and is diagnostic of

n Serum glutamic-oxaloacetic aminotransferase (SGOT) rises in 6 -8 hours, peaks at about 36 hours, and returns to normal usually within a week. n Lactic dehydrogenase (LDH-5 iso-enzyme) rises and peaks slightly later. n Acute MI is confirmed clinically by pathological Q waves on the ECG together with appropriate enzyme changes. n Electrocardiographic changes indicative of ischemia (ST segment elevation or depression).

n Outcome of myocardial infarction: n The mortality of heart attacks is 30 -50% with 50% of all deaths occurring within the first hour. n About 30 -50% of patients with MI die, mainly from VF, during the first week. n The “in hospital” mortality varies with the age of the patient, and the size of the infarct, but overall is about 15%. n The subsequent mortality in the first year and succeeding years is 10% and 5% respectively.

Complications of MI: n 1. Arrhythmias: VF is the most common cause of death in MI. n Primary VF occurs in the first 24 hours after infarction (usually the first hour). n It is thought to be responsible for most sudden deaths. n Secondary VF occurs some days later and is associated with extensive infarction. n The occurrence of frequent premature ventricular beats after the first month or so, indicates a particular liability to VF. n By involving the conducting system, MI may also

2. Cardiac failure: n Extensive infarction of LV can cause acute HF. n If MI progresses to cardiogenic shock mortality rate is 80%. n MI also predisposes to chronic HF which may develop at any time after infarction. n It indicates bad prognosis.

3. Mural thrombosis: n Release of tissue thromboplastin from the damaged muscle following acute MI, together with damage to the endocardium and localized stasis of blood, predispose to mural thrombosis in the ventricle. n This occurs in 30% of cases at autopsy. n Thrombus is eventually organized in patients who survive. n Systemic emboli may result but are less frequent than would be expected.

4. n Venous thrombosis: Systemic venous thrombosis affecting the leg veins in up to 30% of cases, but fatal pulmonary embolism is an uncommon cause of death in MI.

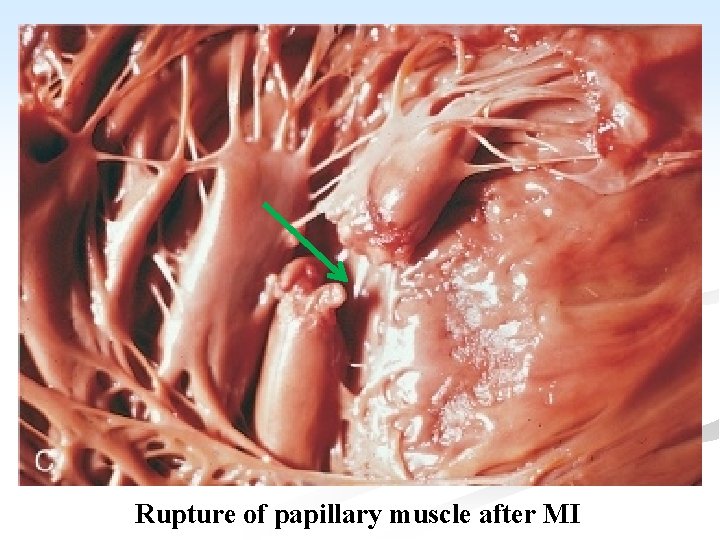
5. Rupture of infarcted myocardium: n It occurs in about 5% of cases. n The rupture occurs in the wall of the LV and causes hemopericardium and death from cardiac tamponade. n Rupture of either the intervenrliclar septum or papillary muscle may also occur precipitating or aggravating acute HF.

Rupture of papillary muscle after MI

6. Cardiac aneurysm: n The healing LV infarction may stretch to form cardiac aneurysm. n This occurs in 12 -15% of long term survivors. n Laminated thrombus tend to form in the cavity, and may cause embolism. n The aneurysm impairs ventricular function, causing cardiac failure.

7. n Angina pectoris: In some patients angina dates from the time of MI because occlusion of a major coronary artery render the surrounding areas of myocardium chronically ischemic.

8. Recurrence of infarction: n Individuals who have had MI are prone to infarction because of the underlying coronary artery diseases. n Cigarette smoking greatly ↑ the risk of this recurrence.

9. Post-infarction Dressler’s syndrome: n The patients of MI may develop pericardial and pleural effusions. n Raised ESR, fever and leucocytosis up to 10 weeks following infarction. n Raised titer of myocardial antibodies, and resolution of symptoms following corticosteroid therapy suggesting an autoimmune nature.

II. Subendocardial MI: n Less than 10% of cases of MI affecting the inner part of LV. n The infarct may be focal but when subendocardial MI affects most or all of the circumference of LV it is called “global infarction”. n The major coronary arteries are usually severely narrowed by atheroma. n At autopsy, in 75% of cases, there is no thrombotic occlusion.

n The immediate cause of infarction is failure of perfusion due to fall in blood pressure. n Hypotension may be precipitated by coronary artery occlusion. n Combined transmural and subendocardial infarction can occur. n Infarction may follow an episode of shock. n The combination of ventricular hypertrophy and coronary atheroma, predisposes to diffuse subendocardial infarction.

n In LV hypertrophy due to aortic valve disease, subendocardial infarction occurs even without serious coronary artery disease. n Subendocardial infarction is not complicated by pericarditis, nor by rupture of the ventricular wall or septum; otherwise its clinical features are those of regional MI. n In patients who recover, organization of the dead muscle leads to subendocardial scarring.

n Other effects of IHD: n Sudden death: n IHD is commonest cause of sudden cardiac death. n There may be a history of angina, previous infarction, or chest pain immediately before death, but sometimes there is no warning symptoms. n At autopsy there is usually severe atheroma, with or without old organized thrombotic occlusion. n IHD is the commonest cause of various

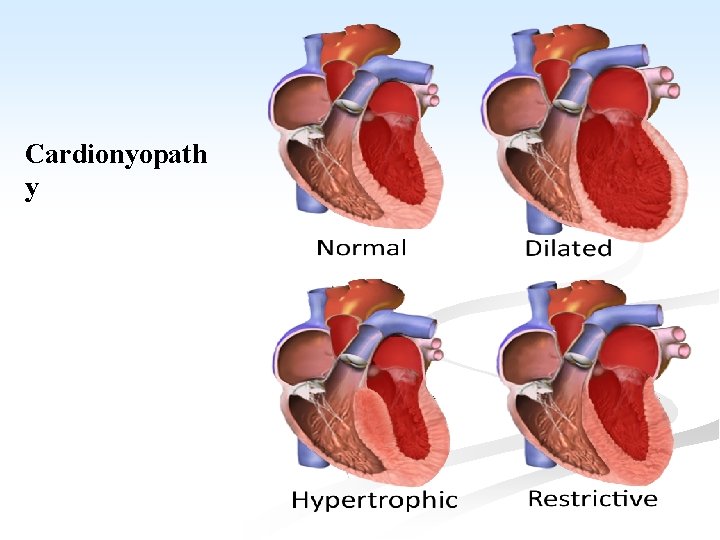
CARDIOMYOPATHY n A heterogenous group of disorders, in which there is chronic myocardial dysfunction of uncertain cause. n Cardiomyopathy is classified as hypertrophic, dilated and restrictive. n They may be further divided into, primary types of unknown etiology, and confined to the myocardium and secondary types which occur with some systemic disorders.

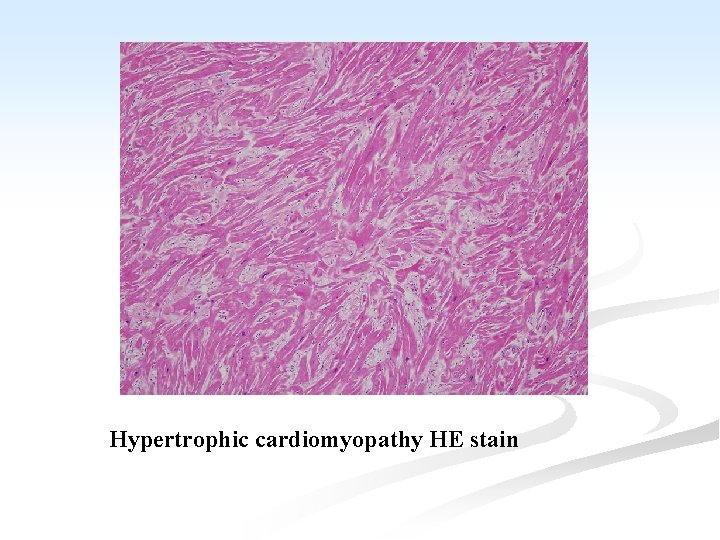
A. Primary Cardiomyopathy: the condition includes: I. Hypertrophic cardiomyopathy (HCM): n It is an autosomal dominant disorder characterized by variable but massive hypertrophy of the LV, especially of the interventicular septum, but with no dilatation. n Most cases of HCM are caused by point mutations in one of the genes encoding proteins that form the contractile apparatus. n Symptoms may occur at any age and sudden death is common.

n Function is affected by: A. ↓ compliance of the LV which interferes with diastolic filling. B. the asymmetrical hypertrophy of the septum, which may obstruct the outflow from the LV. C. mitral regurgitation due to distortion of the ventricle by the asymmetrical hypertrophy. n Microscopiclly: n Hypertrophy of the cardiac muscle, accompanied by interstitial fibrosis.

Hypertrophic cardiomyopathy HE stain

II. n n n Dilated (congestive) cardiomyopathy (DCM): It is characterized by progressive cardiac dilatation and contractile systolic dysfunction usually with concurrent hypertrophy. These patients present with unexplained congestive HF. In rare instances, DCM may shows familial aggregation, and hereditary basis in 20 -50% of cases.

n Coxsackie virus B and other enteroviruses can occasionally be detected in the myocardium from late stage DCM patients. n Some cases are associated with alcohol abuse. n Peripartum cardiomyopathy: occurs late in gestation or postpartum. n Iron overload: due to hereditary hemochromatosis may be blamed.

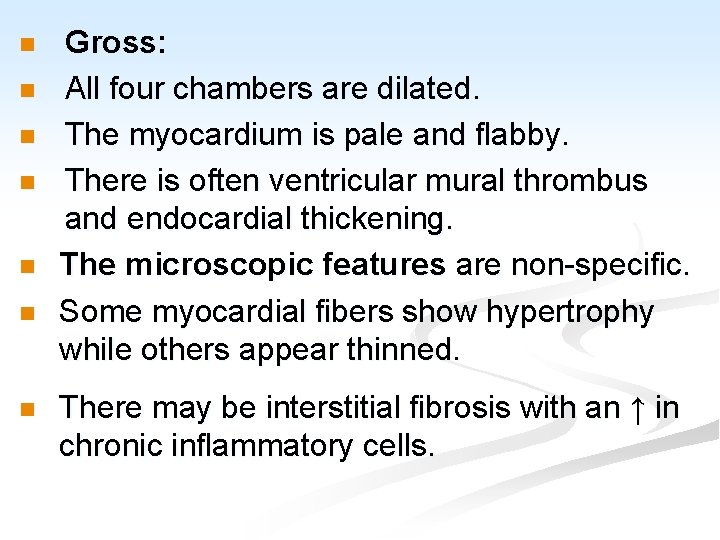
n n n n Gross: All four chambers are dilated. The myocardium is pale and flabby. There is often ventricular mural thrombus and endocardial thickening. The microscopic features are non-specific. Some myocardial fibers show hypertrophy while others appear thinned. There may be interstitial fibrosis with an ↑ in chronic inflammatory cells.

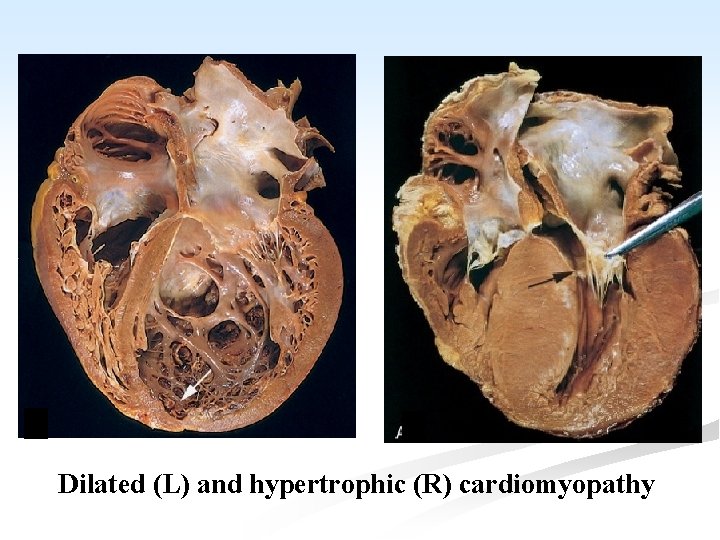
Dilated (L) and hypertrophic (R) cardiomyopathy

III. Restrictive (oblitrative) cardiomyopathy: n It is characterized by decrease in cardiac compliance resulting in impaired venricular filling during diastole. n The wall is stiff. n This is the least common form of cardiomyopathy. n It is of unknown etiology and includes two conditions. A. endomyocardial fibrosis.

n They are characterized by abnormal rigidity mainly due to gross endocardial thickening without myocardial disease. n The rigidity interferes with the filling and emptying of the affected chambers so that progressive diastolic HF results.

Endomyocardial fibrosis: A. n It is characterized by fibrosis of the endocardium, and the immediate underlying myocardium of the inflow tracts of either or both ventricles. n Mural thrombosis and embolic phenomena may occur.

B. Endomyocardial fibroelastosis: n A diffuse layer of dense, white, avascular tissue, composed largely of elastic fibers, developing in the endocardium, usually of the left atrium and ventricle. n It occurs sporadically world wide and is usually associated with eosinophilia. n The initial endocardial damage is thought to be related to eosinophilia. n It is endemic in tropical countries in children and young adults and is an unusual cause of heart failure in infants and young children.

Cardionyopath y

B. Secondary cardiomyopathy: n Hypertrophy in association with (a) Toxic conditions e. g. alcohol, co…etc. (b) Metabolic diseases e. g. hyperthyroidism (c) Storage diseases e. g. glycogen storage disease (d) Infiltrative diseases e. g. leukemia. (e) Vitamin deficiency e. g, beri.

MYOCARDITIS n Definition: n It is an inflammation of the myocardium, which occurs as a result of: a) Direct involvement by the causal agents. b) Toxin-mediated injury. A local hypersensitivity reaction. The commonest causes are viral. Toxoplasma affects the myocardium in neonates, and in immuno-compromised patients. c) n n

1. n n n Viral interstitial myocarditis: The commonest cause of myocarditis is viral infection. It may complicate viral diseases as coxsackie A and B virus, influenza virus, EBV, herpes virus, poliomyelitis virus, and viral hepatitis virus in infants and young adults, particularly males. The condition is usually mild and complete recovery is the role but it may be severe with fatal cardiac failure.

Asymptomatic cardiac involvement is believed to occur in many common viral illnesses; influenza, chickenpox, measles and mumps, and may be responsible for some of the sudden deaths during strenuous exercise in patients suffering from apparently trivial upper respiratory tract infection. n In some cases of congestive cardiomyopathy, a previous history suggestive of viral infection may be elicited. n Myocarditis is a common feature of intrauterine rubella infection. n

Histologically: n The interstitial tissue shows edema, and diffuse infiltration by lymphocytes, plasma cells and macrophages, and sometimes eosinophils. n Focal necrosis of muscle fibers. n

2. n n n Suppurative myocarditis: It is caused by pyogenic bacteria in cases of septicemia and pyemia. It also occurs as a serious complication of acute bacterial endocarditis. Staphylococcus aureus give rise to localized abscesses, while streptococcal pyogenes causes a spreading infection with extensive necrosis and hemorrhage. Focal suppurations occur in the interstitial tissue of the heart. The muscle fibers show degenerations and necrosis.

3. Toxic myocarditis: n It was a major manifestation of diphtheria. n A similar appearances, may be seen in pneumococcal pneumonia, typhoid fever. n It is also caused by chemical toxins as carbon monoxide, arsenic and phosphorus. n Microscopically: n There are numerous small foci of coagulative necrosis which are surrounded by macrophages and lymphocytes, although PML may be also present. n The necrotic muscle fibers undergo absorption and

4. n n Hypersensitivity reactions: Myocarditis is one of the most serious features of rheumatic fever. It may also complicate rheumatoid arthritis, and SLE. 5. Granulomatous myocarditis: n Granuloma are found in the myocardium in cases of TB myocarditis, syphilitic myocarditis and sarcoidosis.

DISEASES OF THE PERICARDIUM n The pericardium is a fibrous sac surrounds the heart. n It comprises visceral and parietal layers. n Accumulation of fluids and blood can occur and it may be the site of acute or chronic inflammation. n The pericardial sac can dilate to contain over a liter of fluid without rise in pressure, only if the fluid accumulates slowly. n If accumulation is rapid, even a small volume of fluid raises the pericardial pressure, interferes with the cardiac filling and leads to “cardiac tamponade”, a

There is severe hypotension with a low pulse pressure, during inspiration (pulsus paradoxus). n Cardiac tamponade is usually due to hemopericardium, but may be caused by tense effusion. n A pericardial effusion is a non inflammatory accumulation of fluid in the pericardial sac. n The commonest cause is metastatic carcinoma. n Effusions may also occur in cardiac failure, in other causes of edema; renal, hepatic, and nutritional. n It also occurs in some cases of myxedema. n

PERICARDITIS n The clinical features of acute pericarditis include fever, tachycardia, and usually chest pain, although the condition may be clinically silent. n If the volume of exudate in the sac is small as in early or mild pericarditis, pain results from rubbing together of the inflamed, roughened pericardium. n It is sharp and stabbing, and accompanied by an audible friction rub synchronous with heart beat. n If fluid is more abundant, it separates the pericardial layers and the heart sounds become muffled.

n Morphologically: n There are the usual features of acute inflammation of the serosal lining. n Active hyperemia, inflammatory edema, and emigration of leucocytes occur in the pericardial tissue. n Exudates accumulates in the pericardial sac and fibrin is deposited on its surface. n Depending on the cause, these changes may be mild and brief, with accumulation of clear or slightly turbid fluid and deposition of fine layer of fibrin on the inflamed surfaces.

n If the cause of a greater severity, there is turbid, blood stained or purulent exudates, and formation of thick layers of fibrin on the surfaces. n A fine deposit of fibrin on the pericardial surfaces undergoes lysis. n A thick layer is removed by organization, with consequent fibrous thickening and adhesion of the two layers of the pericardium, and if severe, constrictive pericarditis may result.

n Types of pericarditis: v Viral and idiopathic pericarditis: n A mild pericarditis occurs almost always in young adults. n It subsides within 2 weeks, but in 25 -30% of cases there may be recurrences. v Bacterial pericarditis: n It may complicate septicemia, pyemia, bacterial pneumonia, empyema, bronchogenic or esophageal carcinoma spreading to the outside. n Pyogenic bacteria are almost always responsible; staphylococcal, streptoccoci, and hemophilus

v Tuberculous pericarditis: n It is common in chronic pulmonary TB. n The route of infection is lymphatic, or direct spread from infected pleura. n There is an abundant turbid or blood stained exudate. n Tubercles may be seen on the pericardial surfaces. n It may progress to fibro-oblitrative calcification of the pericardial sac.

v Other causes of acute pericarditis: n Mild pericarditis may develop in the first week of acute MI. n A mild acute diffuse persistent pericarditis may occur as a part of post-infarction syndrome, poscardiotomy, post-traumatic syndrome, or non cardiac thoracic surgery (Dressler’s syndrome). n In all these cases autoimmune hypersensitivity reaction are triggered by injury to the heart. n Acute pericarditis occurs also in cases of SLE and rheumatoid arthritis. n Fibrinous pericarditis is also a feature of uremia, and is related to the level of blood urea and relieved spontaneously by dialysis.

v Constrictive pericarditis: n It is characterized by obliteration of the pericardial sac by a thick layer of dense fibrous tissue, which sometimes becomes calcified. n It results from prolonged pyogenic or from TB pericarditis. n It also occur as a complication of rheumatoid arthritis. n In many cases the etiology is unknown. n The fibrous tissue interferes with the filling of the heart. n This may be aggravated by constriction of the great veins, as they enter the atria. n The clinical picture is that of progressive congestive HF, associated with a small heart and a low stroke volume.

n Hydropericardium: n Hydropericardium is the accumulation of a clear serous fluid (transudate) in the pericardial sac. n It is a part of generalized edema (cardiac, renal and nutritional). n The lesion causes chronic HF. n Hemopericardium: n Blood accumulation in the pericardial sac. n Rapid blood accumulation in the pericardium causes acute HF.

n Causes: 1. Rupture of recent or healed cardiac infarct. 2. Rupture of cardiac aneurysm. 3. Rupture of aortic aneurysm. 4. Chest injuries and penetrating wounds of the heart. 5. Scurvy and hemorrhagic blood diseases. 6. Malignant tumors in the pericardium.

Thank you
 Eman nayyab
Eman nayyab Eman güvenlik
Eman güvenlik Richard kuebler
Richard kuebler Continuous capillaries
Continuous capillaries Sheep heart dissection labeled
Sheep heart dissection labeled Visceral pericardium covering heart
Visceral pericardium covering heart Pericardium fibrous
Pericardium fibrous Visceral pericardium
Visceral pericardium Nerve fibers
Nerve fibers Oblique and transverse sinus of heart
Oblique and transverse sinus of heart Pericardium
Pericardium Kussmaul sign
Kussmaul sign Reticulocyte characteristics
Reticulocyte characteristics What intercostal space is the pulmonic valve
What intercostal space is the pulmonic valve Applied anatomy of pericardium
Applied anatomy of pericardium Corpus adiposum retrosternale
Corpus adiposum retrosternale Pericardium serosum
Pericardium serosum Posterior mediastinum
Posterior mediastinum Tricuspid valve
Tricuspid valve Topogrphie
Topogrphie Pericardium-6
Pericardium-6 Borders of heart
Borders of heart Cural
Cural Pathology
Pathology Right border of the relative cardiac dullness is formed by
Right border of the relative cardiac dullness is formed by Sheep heart diagram
Sheep heart diagram Hrt 2 hrt
Hrt 2 hrt Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phản ứng thế ankan
Phản ứng thế ankan Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ điện thế nghỉ
điện thế nghỉ Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Gấu đi như thế nào
Gấu đi như thế nào Các số nguyên tố là gì
Các số nguyên tố là gì Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Tia chieu sa te
Tia chieu sa te Một số thể thơ truyền thống
Một số thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Tư thế worm breton là gì
Tư thế worm breton là gì Bổ thể
Bổ thể ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Giọng cùng tên là
Giọng cùng tên là Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Hát lên người ơi
Hát lên người ơi Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Chapter 17 reproductive system diseases and disorders
Chapter 17 reproductive system diseases and disorders 10 diseases of lymphatic system
10 diseases of lymphatic system Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Chapter 8 skin disorders and diseases review questions
Chapter 8 skin disorders and diseases review questions Non communicable diseases infographic
Non communicable diseases infographic Pflegmona
Pflegmona Capnia medical term
Capnia medical term Natural history of disease diagram
Natural history of disease diagram Seborrheic keratoses
Seborrheic keratoses Std
Std Periradicular diseases
Periradicular diseases Hyperglyceremia
Hyperglyceremia Referred pain chart
Referred pain chart Myth and fallacies about non-communicable diseases
Myth and fallacies about non-communicable diseases Zoonotic diseases
Zoonotic diseases Sarophyte
Sarophyte Non common communicable diseases
Non common communicable diseases Migrants
Migrants Chapter 24 lesson 1 sexually transmitted diseases
Chapter 24 lesson 1 sexually transmitted diseases Examples of communicable diseases
Examples of communicable diseases Different types of diseases
Different types of diseases Gingival diseases
Gingival diseases Flaccid gingiva
Flaccid gingiva Conclusion of plant diseases
Conclusion of plant diseases X linked diseases
X linked diseases Sperm fructose
Sperm fructose Chapter 6 musculoskeletal system
Chapter 6 musculoskeletal system Iceberg phenomenon definition
Iceberg phenomenon definition Grapevine trunk diseases
Grapevine trunk diseases Lifestyle modern
Lifestyle modern 8 common nail disorders
8 common nail disorders What causes genetic diseases
What causes genetic diseases