DISEASES OF THE HEART MYOCARDIUM PERICARDIUM Dr Eman























- Slides: 86

DISEASES OF THE HEART MYOCARDIUM & PERICARDIUM Dr Eman MS Muhammad

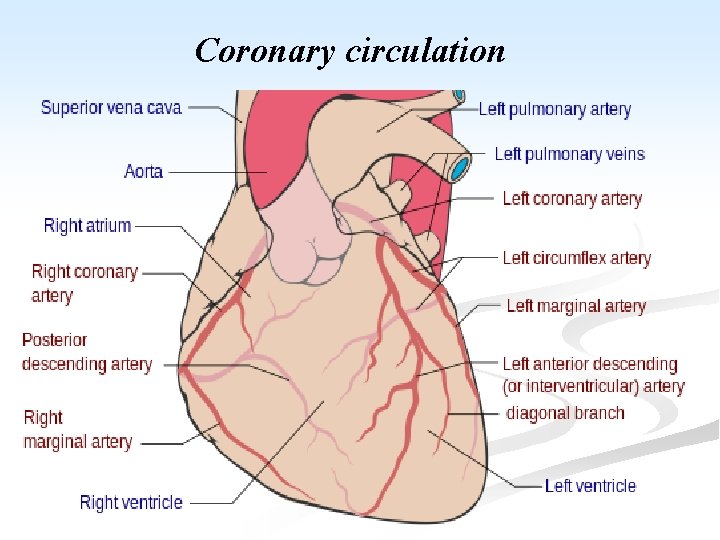
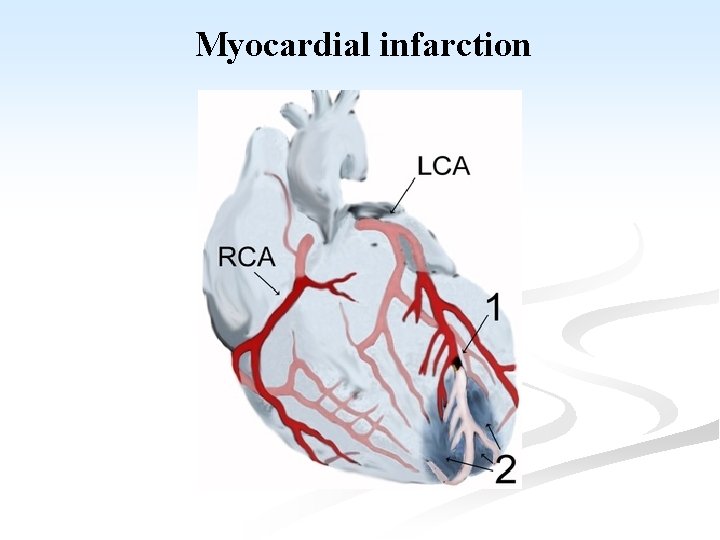
Coronary circulation

n Blood supply of the heart: n Generating energy almost exclusively by the oxidation of substrates, the heart relies heavily on an adequate flow of oxygenated blood through the coronary arteries. n With origin from the aorta immediately distal to the aortic valve, the coronary arteries consists of 5 -10 cm long, 2 -4 mm diameter, arise. n They run along the external surface of the heart and are called epicardial coronary arteries. n Whereas, the smaller vessels that penetrate the myocardium are the intramural arteries.

n The three major epicardial coronaries are: n The left anterior descending artery (LAD). n The left circumflex artery (LCX). n Both arising as branches from the bifurcation of the left (main) coronary artery. n The right coronary artery (RCA). n Branches of LAD are called diagonal and septal perforates. n Branches of LCX are obtuse marginals.

n Most coronary blood flow to the myocardium occurs during diastole, when the microcirculation is not compressed by myocardial contraction. n The LAD supplies most of the apex of the heart, the anterior wall of the left ventricle, and the anterior two thirds of the ventricular septum. n LCX peruses the lateral wall of the LV. n RCA supplies the right ventricular free wall, and the posterobasal wall of the LV and the posterior third of the ventricular septum.

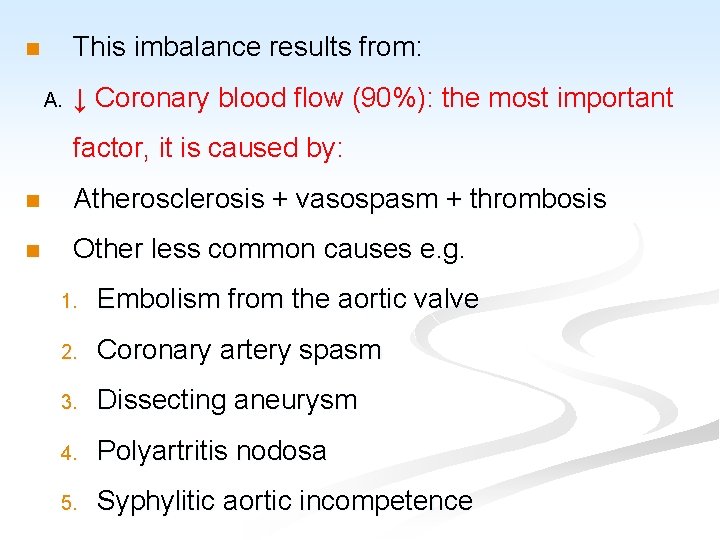
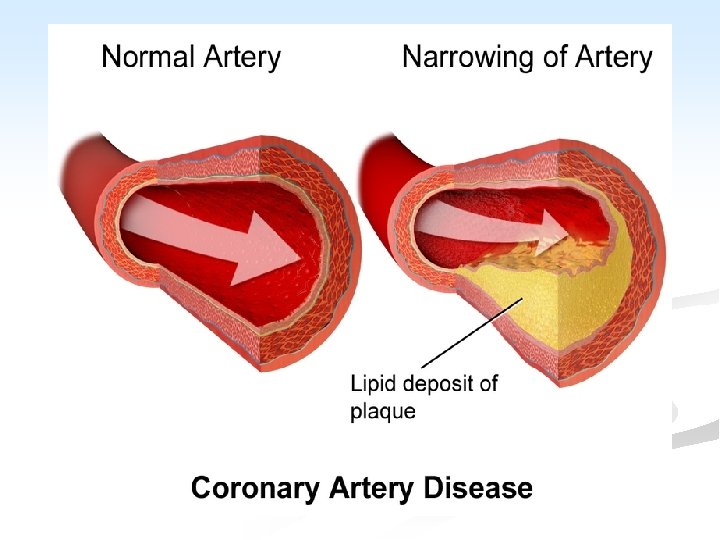
ISCHAEMIC HEART DISEASE n General consideration and causal factors: n Ischemic heart diseases (IHD or CHD): are the leading cause of death in most industrialized countries. n Myocardial ischemia basically results from imbalance between myocardial oxygen supply and the demand of cardiac muscle.

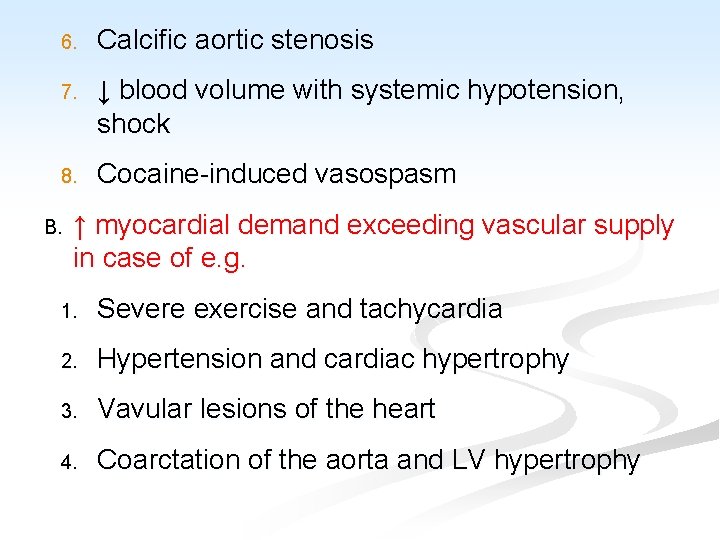
This imbalance results from: n A. ↓ Coronary blood flow (90%): the most important factor, it is caused by: n Atherosclerosis + vasospasm + thrombosis n Other less common causes e. g. 1. Embolism from the aortic valve 2. Coronary artery spasm 3. Dissecting aneurysm 4. Polyartritis nodosa 5. Syphylitic aortic incompetence

6. Calcific aortic stenosis 7. ↓ blood volume with systemic hypotension, shock 8. Cocaine-induced vasospasm B. ↑ myocardial demand exceeding vascular supply in case of e. g. 1. Severe exercise and tachycardia 2. Hypertension and cardiac hypertrophy 3. Vavular lesions of the heart 4. Coarctation of the aorta and LV hypertrophy

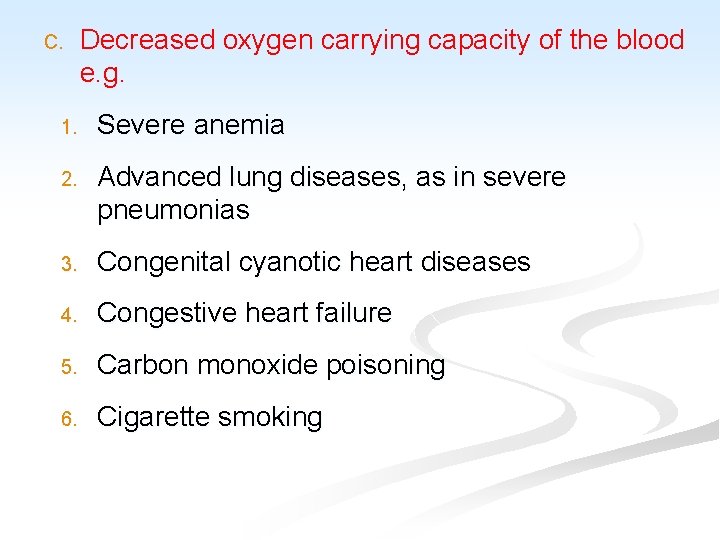
C. Decreased oxygen carrying capacity of the blood e. g. 1. Severe anemia 2. Advanced lung diseases, as in severe pneumonias 3. Congenital cyanotic heart diseases 4. Congestive heart failure 5. Carbon monoxide poisoning 6. Cigarette smoking

n Death rates from IHD ↑ with age; after the age of 40. n IHD affects men earlier. n Sex: ♂: ♀ = 4 -6: 1, but the incidence ↑ in women after menopause. n Death rates are higher in men than in women n Race: both black and white are affected equally. n As atherosclerosis (AS) of coronary artery is the main pathogenic factor so, predisposing (risk) factors of IHD are those of AS. n Long-term regular exercise and ↓alcohol intake → protection against IHD.

n Depending on the onset, duration and severity of O 2 deficit, 4 general ischemic syndromes are recognized: A. Angina pectoris. B. Chronic ischemia. C. Myocardial infarction. D. Sudden cardiac death.

Angina Pectoris n Angina is an episodic chest pain of variable severity often described as gripping, compressing or crushing. n The pain is usually retro-sternal and may radiate to the neck and jaw or to the upper aspect of either or both arms and hands. n It is due to transient reversible myocardial ischemia and is caused by an imbalance between myocardial oxygen supply and

n Attacks are brought on by factors which increase the work of the heart and includes; physical activity, exposure to cold, emotional stress, or heavy meal. n Ischemia induces release of adenosine, bradykinin that stimulate autonomic afferents → pain. n It is usually relieved by rest and/or vasodilators. n Ischemia, however, does not always give pain and up to 70% of ischemic episodes are silent. n Prognosis depends on the severity of

n Variants of angina are: I. Typical Stable Angina: n This is the commonest form of angina and many sufferers live for over 30 years. n It is due to progressive stenosing coronary artery by atheroma. n It is precipitated by exertion, emotional stress, and is relieved by rest or sublingual nitroglycerin. n Pain is classically described as a crushing or squeezing substernal sensation that radiate down the left arm or to the left jaw.

II. Variant Angina “Prinzmetal’s angina”: n In this form the attacks of pain are not related to exercise and can occur at rest. n It is caused by spasm of large or mediumsized coronary arteries, often at or near the site of atheromatous narrowing. n In 15% of cases, however, the coronary arteries appear normal. The mechanisms of vasospasm are not known. n Prinzmetal angina typically respond promptly to vasodilators as nitroglycerin and calcium channel blockers.

III. Unstable Angina (cresendo angina): n This clinical pattern may supervene in previously stable angina, or may commence de novo. n There is a sudden increase in the severity and duration of episodes of chest pain which begin to occur more frequently and often at rest. n The pathogenesis is similar to that of acute myocardial infarction. n It is associated with plaque disruption and superimposed thrombus, distal embolization of the thrombus and/or vasospasm. n In some patients it may progress to MI or sudden death.

Chronic ischemic heart diseases n It is progressive heart failure secondary to ischemic myocardial damage. It occurs late in life. In most cases there is history of previous MI.

Gross: n LV hypertrophy and dilatation, discrete areas of grey white scarring from previous healed infarcts. n There is moderate to severe atherosclerosis of the coronary arteries, sometimes with total occlusion. n Microscopic: n This include myocardial hypertrophy, diffuse subendocardial vacuolization and fibrosis from previous infarction. n

Myocardial Infarction n It is commonly referred to as “heart attack”. n It is necrosis of heart muscle resulting from sudden severe myocardial ischemia. n The mortality of acute myocardial infarction (MI) is approximately 30 -35%, with 50% of these deaths occurring within an hour of onset from ventricular fibrillation (VF). n The survivors suffer from variable degrees of impaired cardiac function, including cardiac failure, arrhythmias and thrombo-emolism.

n Although atrial infarction occurs in 20% of cases, the ventricular myocardium is predominantly affected. n Risk factors are the same as those of atherosclerosis.

Myocardial infarction

n There are two forms of MI: 1. Regional MI: It represents 90% of cases. Most of which are transmural and affect myocardial region supplied by a major coronary artery, which is almost always occluded by thrombus in an atherosclerotic area. n 2. Subendocardial MI: n It represents 10% of cases, affecting the inner ½ or 2/3 of the ventricular wall throughout most or all of its circumference. n Major coronary arteries are severely atheromatous, but recent occlusion by thrombus is unusual.

n Some describes a third variant of MI named: 3. Microscopic infarcts: occur in the setting of small vessel occlusions; vasculitis, embolization of valve vegetations, or mural thrombi, or vessel spasm due to elevated catecholamines either endogenous (e. g. , pheochromocytoma or severe stress), or exogenous (e. g. , cocaine).

I. Regional MI: n They vary greatly in size, but most are at least 2 cm across, and many are much larger. n The frequency of involvement of the coronary arteries and the distribution of infarction is: A. LAD (40 -50% of cases): the infarct is anterior extending from the apex, up to anterior wall of the LV, often involving the anterior part of the inter-ventricular septum, and adjacent anterior wall of the RV.

B. RCA (30 -40% of cases): The infarct is inferior (posterior) extending from the apex, up to inferior wall of the LV, often involving the adjacent parts of the inter-ventricular septum, and the inferior (posterior) wall of the RV. C. LCX (15% of cases): the infarct involves the lateral wall of the LV.

n MI is commonly caused by thrombosis of the left main coronary artery or much less commonly two main coronary arteries. n The extent of infarction depends on the site of occlusion (proximal or distal within the artery), and on the presence of collateral circulation. n Gradual atheromatous narrowing of a major branch → opening of the collaterals, so when it is finally occluded by thrombus, only a small infarct is produced.

n Pathogenesis: n Three of the acute clinical events in IHD, unstable angina, MI, and sudden death, usually result from cracking or ulceration of an underlying atheromatous plaque. The sequence is: a. Fissuring, cracking or rupture of a plaque which has a lipid pool. b. Acute enlargement of the plaque with thrombosis of the lumen. c. Occlusion of the lumen. d. Penetration of vessels into the plaque.

n Fissuring or ulceration of plaque is the mechanism which initiates the sequence of events in MI. The precipitating causes are unknown. Shear stresses at the site of plague, ↑ blood pressure, and local vasospasm have all been implicated. n Thrombi may occur at areas of endothelial damage over a severe atheromatous stenosis. Thrombotic occlusion occurs in 90% 0 f MI. Healing of fissuring and thrombosis → growth of the plaque. n Vasospasm may contribute to the occlusion.


Once thrombus has formed the following sequences are possible: n n 1. Spontaneous lysis. 2. Platelet embolisation → aggravate ischemia distal to the occlusion. 3. Continued thrombus formation progressing to local occlusion and propagation of the thrombus distally and proximally. Platelet activation at the site of thrombus → release of vaso-active factors such as thromboxane → vasospasm.

Myocardial response to ischemia: n Loss of myocardial blood supply leads to functional, biochemical and morphological consequences as: n A ↓ in ATP and accumulation of potentially noxious metabolites (e. g. , lactic acid) in cardiac myocytes. n Necrotic myocardial cells release enzymes helpful in diagnosing MI; cytokeratin (CK) and its specific cardiac isoform CK-MB, lactic dehydrogenase (LD); its LDH-5 iso-enzyme and myocardial protein Troponin-1 (c-Tn 1). n

Rapid loss of contractility within a minute of the onset of ischemia. n Electrical instability (irritability) of the ischemic but not the infarcted area of the heart → arrhythmia. n Only severe ischemia lasting 20 -40 minutes causes irreversible injury and myocyte death. n MI reaches its full size in 3 -6 hours; intervention during this time can limit the final extent of necrosis. n

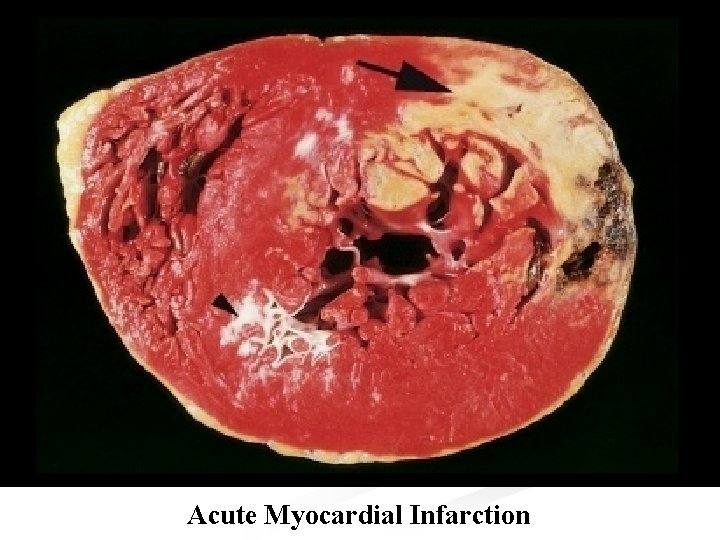
n Morbid anatomy: n Initially the necrotic muscle appears grossly and microscopically normal. Myocardial necrosis can not be recognized in patients dying less than 6 -8 hours after the onset. n The first change visible at autopsy are blotchy pallor and congestion, followed in 24 -48 hours by palpable softening. n The color changes to grey-brown. then to yellow-grey. Hemorrhages then appear at the margins.

n After few days the infarct becomes sharply defined by development of red zone of granulation tissue at the margins. Removal of dead myocardium precedes gradually. n There is fibrinous or hemorrhagic pericarditis which may be generalized or localized to the area of infarction. n On the inner aspect of the infarct, the endocardium and a thin layer of myocardium remains viable, nourished by blood from the lumen. n In patients surviving for several days, mural

Acute Myocardial Infarction

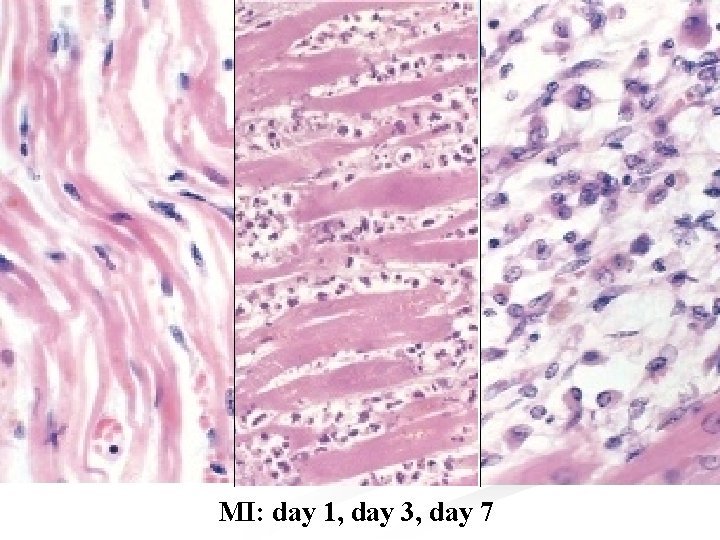
n Microscopically: n The infarcted muscle shows coagulative necrosis. n After 8 hours it is infiltrated by PNL, and after a few days digestion by macrophages and organization can be seen at the margins. n The dead muscles is replaced by a fibrous scar over the next 6 -8 weeks. n Hypertrophy of the non infarcted myocardium occurs and the chamber enlarges to compensate for ↓ in contractility thus maintaining the stroke volume. n In 25 -30% of transmural infarct → stretching

MI: day 1, day 3, day 7

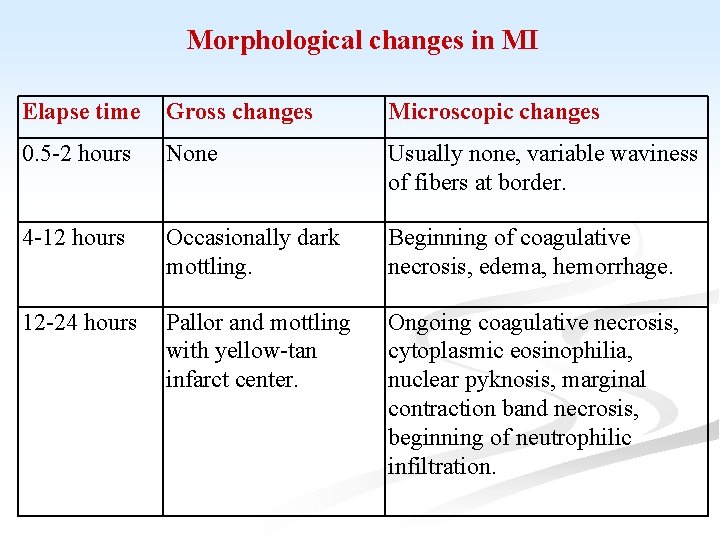
Morphological changes in MI Elapse time Gross changes Microscopic changes 0. 5 -2 hours None Usually none, variable waviness of fibers at border. 4 -12 hours Occasionally dark mottling. Beginning of coagulative necrosis, edema, hemorrhage. 12 -24 hours Pallor and mottling with yellow-tan infarct center. Ongoing coagulative necrosis, cytoplasmic eosinophilia, nuclear pyknosis, marginal contraction band necrosis, beginning of neutrophilic infiltration.

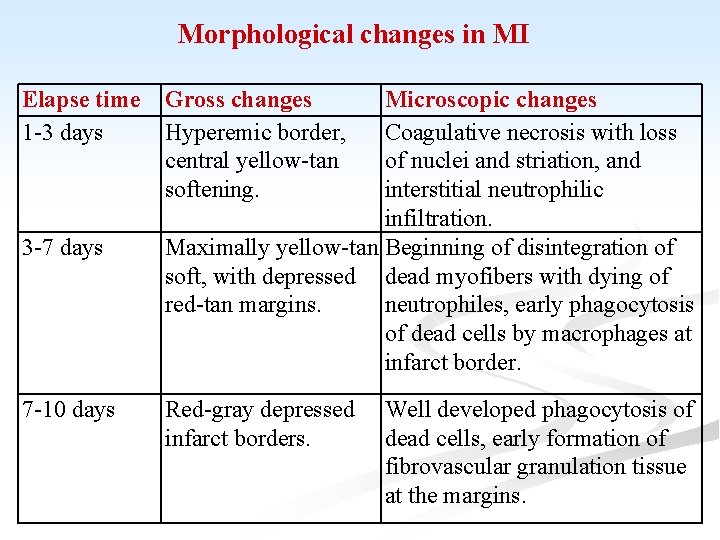
Morphological changes in MI Elapse time 1 -3 days 3 -7 days 7 -10 days Gross changes Hyperemic border, central yellow-tan softening. Microscopic changes Coagulative necrosis with loss of nuclei and striation, and interstitial neutrophilic infiltration. Maximally yellow-tan Beginning of disintegration of soft, with depressed dead myofibers with dying of red-tan margins. neutrophiles, early phagocytosis of dead cells by macrophages at infarct border. Red-gray depressed infarct borders. Well developed phagocytosis of dead cells, early formation of fibrovascular granulation tissue at the margins.

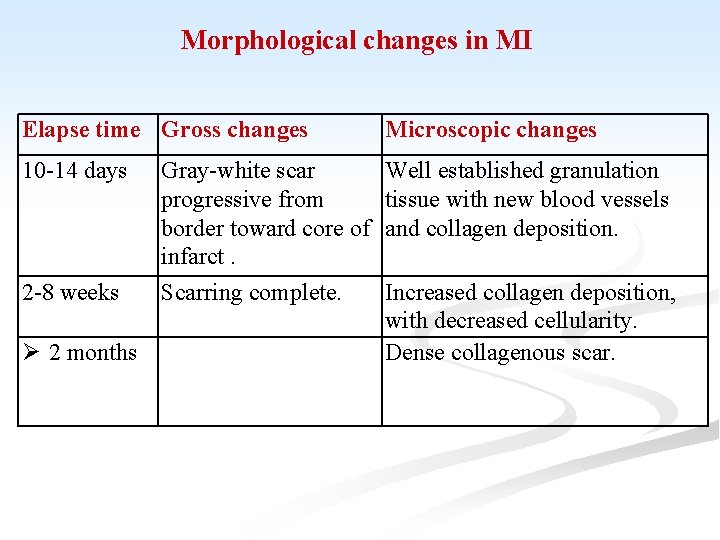
Morphological changes in MI Elapse time Gross changes Microscopic changes 10 -14 days Well established granulation tissue with new blood vessels and collagen deposition. 2 -8 weeks 2 months Gray-white scar progressive from border toward core of infarct. Scarring complete. Increased collagen deposition, with decreased cellularity. Dense collagenous scar.

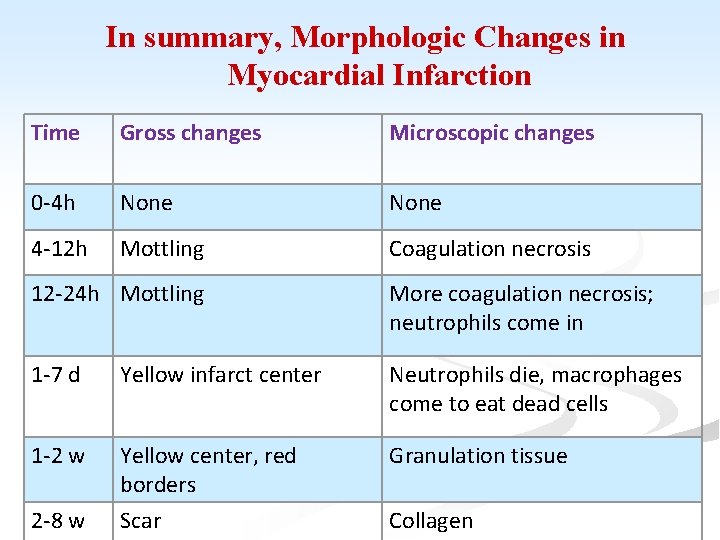
In summary, Morphologic Changes in Myocardial Infarction Time Gross changes Microscopic changes 0 -4 h None 4 -12 h Mottling Coagulation necrosis 12 -24 h Mottling More coagulation necrosis; neutrophils come in 1 -7 d Yellow infarct center Neutrophils die, macrophages come to eat dead cells 1 -2 w Yellow center, red borders Scar Granulation tissue 2 -8 w Collagen

n Size and rate of development of MI depend on: 1. The size and distribution of the affected vessel. 2. The rate of development and duration of occlusion. 3. Metabolic demands of the myocardium (affected by blood pressure and heart rate). 4. Extent of collateral circulation.

n Clinical feature and course: n The dominant symptom of MI is severe retrosternal pain, that does not relived by rest or vasodilators and persists for at least one or several hours. n It is usually accompanied by nausea, vomiting, sweating, weakness and prostration. n These early features are usually dramatic, but there is a spectrum of severity of symptoms. n In some cases MI is “silent” with little or no

n With the first few hours there is usually mild fever, moderate neutrophil leucocytosis, and characteristic ECG changes. n Necrosis of the myocardium is followed by release of cellular enzymes with consequent rise in their serum levels. n There is typical rise and gradual fall (troponin) or more rapid rise and fall (CKMB); biochemical markers of myocardial necrosis. n Serum creatinine phosphokinase (CPK), and its isoenzyme (MB) rises in 2 -3 hours, peaks about 36 hours, and is diagnostic of

n Serum glutamic-oxaloacetic aminotransferase (SGOT) rises in 6 -8 hours, peaks at about 36 hours, and returns to normal usually within a week. n Lactic dehydrogenase (LDH-5 iso-enzyme) rises and peaks slightly later. n Acute MI is confirmed clinically by pathological Q waves on the ECG together with appropriate enzyme changes. n Electrocardiographic changes indicative of ischemia (ST segment elevation or depression).

n Outcome of myocardial infarction: n The mortality of heart attacks is 30 -50% with 50% of all deaths occurring within the first hour. n About 30 -50% of patients with MI die, mainly from VF, during the first week. n The “in hospital” mortality varies with the age of the patient, and the size of the infarct, but overall is about 15%. n The subsequent mortality in the first year and succeeding years is 10% and 5% respectively.

Complications of MI: n 1. Arrhythmias: VF is the most common cause of death in MI. n Primary VF occurs in the first 24 hours after infarction (usually the first hour). It is responsible for most sudden deaths. n Secondary VF occurs some days later and is associated with extensive infarction. n The occurrence of frequent premature ventricular beats after the first month, indicates a particular liability to VF. n By involving the conducting system, MI may also cause various grades of heart block and other arrhythmias.

2. Cardiac failure: n Extensive infarction of LV can cause acute HF. n If MI progresses to cardiogenic shock mortality rate is 80%. n MI also predisposes to chronic HF which may develop at any time after infarction. n It indicates bad prognosis.

3. Mural thrombosis: n Release of tissue thromboplastin from damaged muscle following acute MI, together with damage to the endocardium and localized stasis of blood, predispose to mural venticular thrombosis. n This occurs in 30% of cases at autopsy. n Thrombus is eventually organized in patients who survive. n Systemic emboli may result but are less frequent than would be expected.

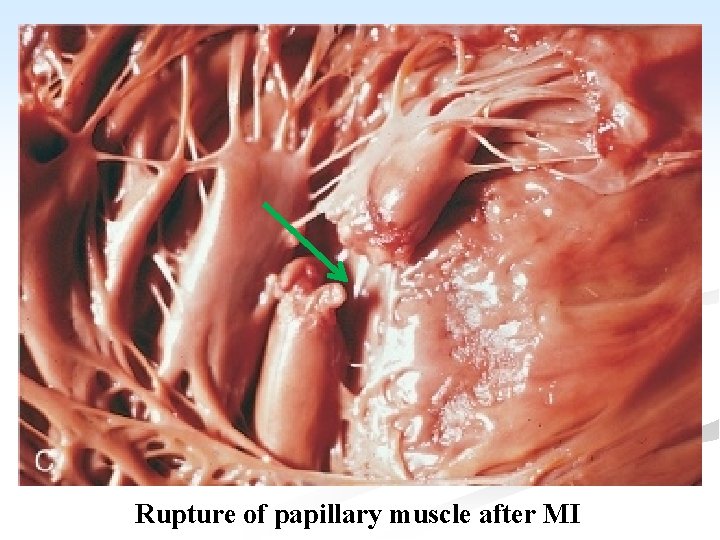
4. Venous thrombosis: Systemic venous thrombosis affecting the leg veins in up to 30% of cases, but fatal pulmonary embolism is an uncommon cause of death in MI. n 5. Rupture of infarcted myocardium: n It occurs in 5% of cases. The rupture occurs in the wall of the LV and causes hemopericardium and death from cardiac tamponade. n Rupture of either the intervenrliclar septum or papillary muscle may also occur

Rupture of papillary muscle after MI

6. Cardiac aneurysm: n The healing LV infarction may stretch to form cardiac aneurysm. n This occurs in 12 -15% of long term survivors. n Laminated thrombus tend to form in the cavity, and may cause embolism. n The aneurysm impairs ventricular function, causing cardiac failure.

7. Angina pectoris: In some patients angina dates from the time of MI because occlusion of a major coronary artery render the surrounding areas of the myocardium chronically ischemic. n 8. Recurrence of infarction: n Individuals who have had MI are prone to infarction because of the underlying coronary artery diseases. n Cigarette smoking greatly ↑ the risk of this recurrence.

9. Post-infarction Dressler’s syndrome: n The patients of MI may develop pericardial and pleural effusions. n Raised ESR, fever and leucocytosis develop up to 10 weeks following infarction. n Raised titer of myocardial antibodies, and resolution of symptoms following corticosteroid therapy suggesting an autoimmune nature.

II. Subendocardial MI: n Less than 10% of cases of MI. It may be focal but when subendocardial MI affects most or all of the circumference of LV it is called “global infarction”. n The major coronary arteries are usually severely narrowed by atheroma. In 75% of cases, there is no thrombotic occlusion. n The immediate cause is failure of perfusion due to hypotension precipitated by coronary artery occlusion.

n Combination of ventricular hypertrophy and atheroma → diffuse subendocardial infarction. n In LV hypertrophy due to aortic valve diseases, subendocardial infarction occurs even without serious coronary artery disease. n Subendocardial infarction is not complicated by pericarditis, nor by rupture of the ventricular wall or septum; otherwise its clinical features are those of regional MI. n Organization of dead muscle → subendocardial scarring.

n Other effects of IHD: n Sudden death: n IHD is commonest cause of sudden cardiac death. n There may be a history of angina, previous infarction, or chest pain immediately before death, but sometimes there is no warning symptoms. n At autopsy there is usually severe atheroma, with or without old organized thrombotic occlusion. n IHD is the commonest cause of various

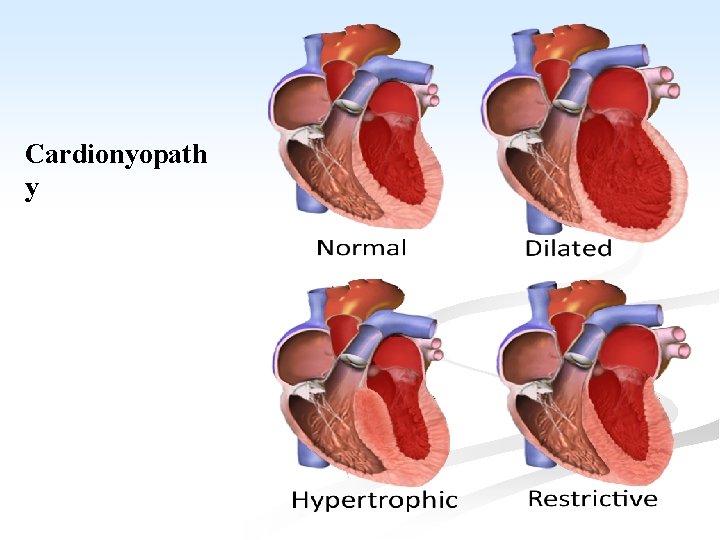
CARDIOMYOPATHY n A heterogenous group of disorders, in which there is chronic myocardial dysfunction of uncertain cause. n Cardiomyopathy is classified as hypertrophic, dilated and restrictive. n They may be further divided into, primary types of unknown etiology, and confined to the myocardium and secondary types which occur with some systemic disorders.

A. Primary Cardiomyopathy: the condition includes: I. Hypertrophic cardiomyopathy (HCM): n An autosomal dominant disorder characterized by massive hypertrophy of the LV, especially of the inter-venticular septum, but with no dilatation. n Most cases are caused by point mutations in one of the genes encoding proteins forming the contractile apparatus. n Symptoms may occur at any age and sudden death is common.

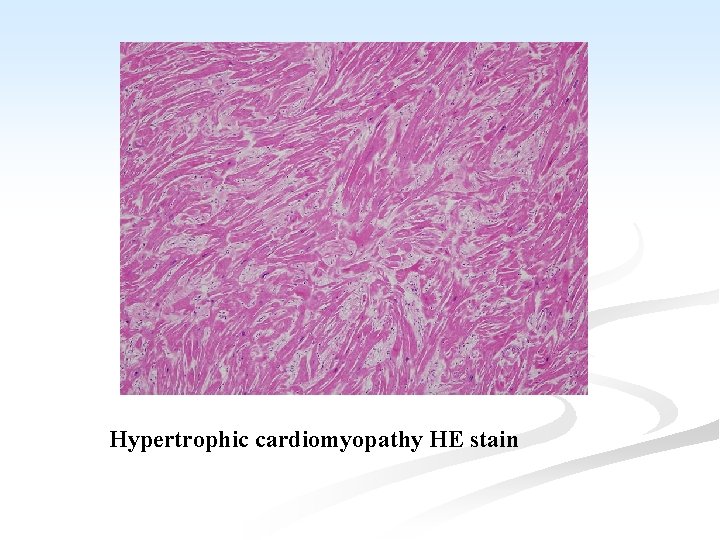
n Function is affected by: A. ↓ compliance of the LV which interferes with diastolic filling. B. the asymmetrical hypertrophy of the septum, which may obstruct the outflow from the LV. C. mitral regurgitation due to distortion of the ventricle by the asymmetrical hypertrophy. n Microscopiclly: n Hypertrophy of the cardiac muscle, accompanied by interstitial fibrosis.

Hypertrophic cardiomyopathy HE stain

II. n n Dilated (congestive) cardiomyopathy (DCM): Characterized by progressive cardiac dilatation and contractile systolic dysfunction usually with concurrent hypertrophy. Patients present with unexplained congestive HF. n DCM may shows familial aggregation, and hereditary basis in 20 -50% of cases. n Coxsackie virus B can occasionally be detected in the myocardium from late stage DCM patients.

n Some cases are associated with alcohol abuse. n Peripartum cardiomyopathy: occurs late in gestation or postpartum. n Iron overload: due to hereditary hemochromatosis may be blamed.

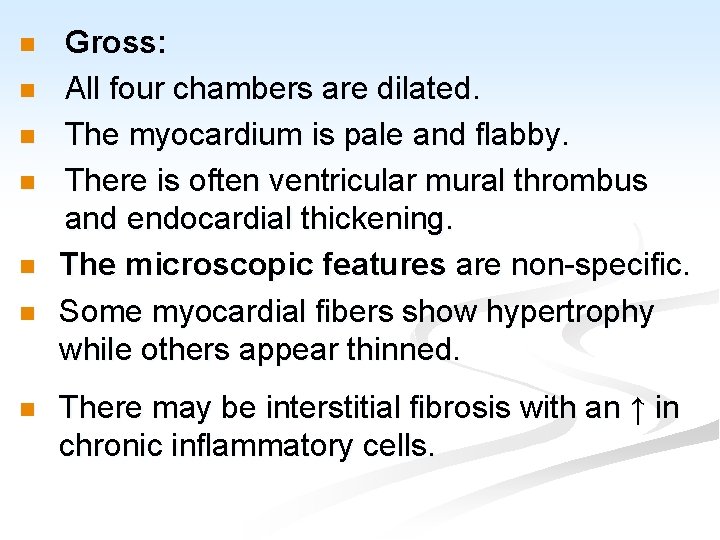
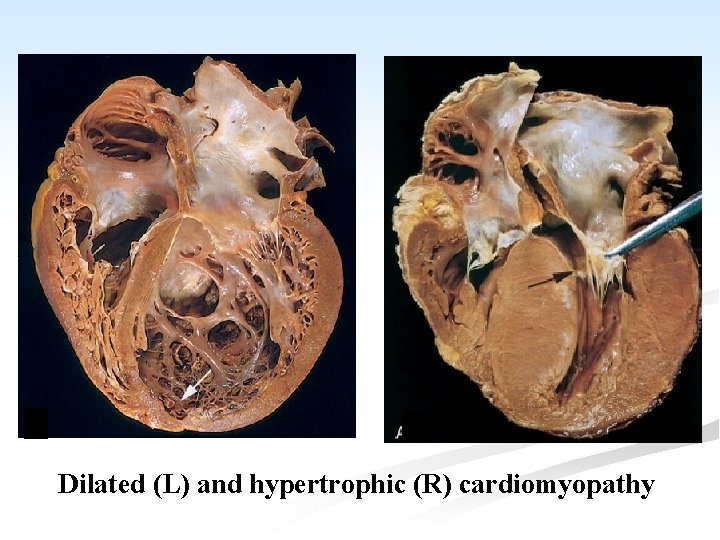
n n n n Gross: All four chambers are dilated. The myocardium is pale and flabby. There is often ventricular mural thrombus and endocardial thickening. The microscopic features are non-specific. Some myocardial fibers show hypertrophy while others appear thinned. There may be interstitial fibrosis with an ↑ in chronic inflammatory cells.

Dilated (L) and hypertrophic (R) cardiomyopathy

III. Restrictive (oblitrative) cardiomyopathy: n Characterized by ↓ in cardiac compliance resulting in impaired venricular filling during diastole. n The wall is stiff. n This is the least common form of cardiomyopathy. n It is of unknown etiology and includes two conditions. A. endomyocardial fibrosis.

n They are characterized by abnormal rigidity mainly due to gross endocardial thickening without myocardial disease. n The rigidity interferes with the filling and emptying of the affected chambers so that progressive diastolic HF results.

Cardionyopath y

B. Secondary cardiomyopathy: n Hypertrophy in association with (a) Toxic conditions e. g. alcohol, co…etc. (b) Metabolic diseases e. g. hyperthyroidism (c) Storage diseases e. g. glycogen storage disease (d) Infiltrative diseases e. g. leukemia. (e) Vitamin deficiency e. g, beri.

MYOCARDITIS n Definition: n It is an inflammation of the myocardium, which occurs as a result of: a) Direct involvement by the causal agents. b) Toxin-mediated injury. A local hypersensitivity reaction. The commonest causes are viral. Toxoplasma affects the myocardium in neonates, and in immuno-compromised patients. c) n n

Viral interstitial myocarditis: n The commonest cause of myocarditis. n It may complicate viral diseases as coxsackie A and B virus, influenza virus, EBV, herpes virus, poliomyelitis virus, and viral hepatitis. n It is usually mild and complete recovery is the role but it may be severe with fatal cardiac failure. n In some cases of congestive cardiomyopathy, a previous history of viral infection may be elicited. 1.

Asymptomatic cardiac involvement is believed to occur in many common viral illnesses; influenza, chickenpox, measles and mumps, and may be responsible for some of the sudden deaths during strenuous exercise in patients suffering from apparently trivial upper respiratory tract infection. n Histologically: n The interstitial tissue shows edema, and diffuse infiltration by lymphocytes, plasma cells and macrophages, and sometimes eosinophils. n Focal necrosis of muscle fibers. n

2. n n n Suppurative myocarditis: It is caused by pyogenic bacteria in cases of septicemia and pyemia. It also occurs as a serious complication of acute bacterial endocarditis. Staphylococcus aureus give rise to localized abscesses, while streptococcal pyogenes causes a spreading infection with extensive necrosis and hemorrhage. Focal suppurations occur in the interstitial tissue of the heart. The muscle fibers show degenerations and necrosis.

3. Toxic myocarditis: n It was a major manifestation of diphtheria. n A similar appearances, may be seen in pneumococcal pneumonia, typhoid fever. n It is also caused by chemical toxins as carbon monoxide, arsenic and phosphorus. n Microscopically: n There are numerous small foci of coagulative necrosis which are surrounded by macrophages and lymphocytes, although PML may be also present. n The necrotic muscle fibers undergo absorption and

4. n n Hypersensitivity reactions: Myocarditis is one of the most serious features of rheumatic fever. It may also complicate rheumatoid arthritis, and SLE. 5. Granulomatous myocarditis: n Granuloma are found in the myocardium in cases of TB myocarditis, syphilitic myocarditis and sarcoidosis.

DISEASES OF THE PERICARDIUM

PERICARDITIS n Clinical features of acute pericarditis include fever, tachycardia, chest pain, although it may be clinically silent. n If the volume of exudate in the sac is small as in early or mild pericarditis, pain results from rubbing together of the inflamed, roughened pericardium. n It is sharp and stabbing, and accompanied by an audible friction rub synchronous with the heart beat. n If fluid is more abundant, it separates the pericardial layers and the heart sounds

n Morphologically: n There are the usual features of acute inflammation of the serosal lining. n Active hyperemia, inflammatory edema, and emigration of leucocytes occur in the pericardium. n Exudates accumulates in the pericardial sac and fibrin is deposited on its surface. n These changes may be mild and brief, with accumulation of clear or slightly turbid fluid and deposition of fine layer of fibrin on the

n If the cause of a greater severity, there is turbid, blood stained or purulent exudates, and formation of thick layers of fibrin on the surfaces. n A fine deposit of fibrin on the pericardial surfaces undergoes lysis. n A thick layer is removed by organization, with consequent fibrous thickening and adhesion of the two layers of the pericardium, and if severe, constrictive pericarditis may result.

n Types of pericarditis: v Viral and idiopathic pericarditis: n A mild pericarditis occurs almost always in young adults. n It subsides within 2 weeks, but in 25 -30% of cases there may be recurrences. v Bacterial pericarditis: n It may complicate septicemia, pyemia, bacterial pneumonia, empyema, bronchogenic or esophageal carcinoma spreading to the outside. n Pyogenic bacteria are almost always responsible; staphylococcal, streptoccoci, and hemophilus

v Tuberculous pericarditis: n It is common in chronic pulmonary TB. n The route of infection is lymphatic, or direct spread from infected pleura. n There is an abundant turbid or blood stained exudate. n Tubercles may be seen on the pericardial surfaces. n It may progress to fibro-oblitrative calcification of the pericardial sac.

v Other causes of acute pericarditis: n Mild pericarditis may develop in the first week of acute MI. n A mild acute diffuse persistent pericarditis may occur as a part of post-infarction syndrome, or poscardiotomy (Dressler’s syndrome). An autoimmune hypersensitivity reaction are triggered by injury to the heart is suggested. n Acute pericarditis occurs also in cases of SLE and rheumatoid arthritis. n Fibrinous pericarditis is also a feature of uremia, and is related to the level of blood urea and relieved spontaneously by dialysis.

v Constrictive pericarditis: n It is characterized by obliteration of the pericardial sac by a thick layer of dense fibrous tissue, which sometimes becomes calcified. n It results from prolonged pyogenic or from TB pericarditis. It also occur as a complication of rheumatoid arthritis. In many cases the etiology is unknown. n The fibrous tissue interferes with the filling of the heart. This may be aggravated by constriction of the great veins, as they enter the atria. n The clinical picture is that of progressive

n Hydropericardium: n Hydropericardium is the accumulation of clear serous fluid (transudate) in the pericardial sac, as a part of generalized edema (cardiac, renal and nutritional). n The lesion causes chronic HF. n Hemopericardium: n Blood accumulation in the pericardial sac. n Rapid blood accumulation in the pericardium causes acute HF.

n Causes: 1. Rupture of recent or healed cardiac infarct. 2. Rupture of cardiac aneurysm. 3. Rupture of aortic aneurysm. 4. Chest injuries and penetrating wounds of the heart. 5. Scurvy and hemorrhagic blood diseases. 6. Malignant tumors in the pericardium.

Thank you