Quantum Theory and the Electronic Structure of Atoms



















![Aufbau Principle • Here a few examples. Using the abbreviation [He] for 1 s Aufbau Principle • Here a few examples. Using the abbreviation [He] for 1 s](https://slidetodoc.com/presentation_image_h2/2904220042b61c8f2571044eb8cee3df/image-78.jpg)






- Slides: 91

Quantum Theory and the Electronic Structure of Atoms Chapter 7

Relationship of Quantum Theory and role of electrons • In chemistry, the role of electrons in an atom is understood better by the quantum theory -How many electrons are present in a particular atom -What energies do individual electrons possess -Where in the atom can electrons be found

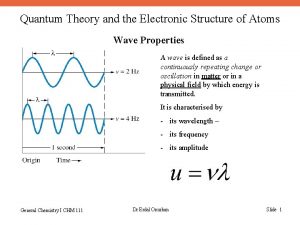
The Wave Nature of Light • A wave can be characterized by its wavelength and frequency. The wavelength, l (lambda), is the distance between any two adjacent identical points of a wave. The frequency, n (nu), of a wave is the number of wavelengths that pass a fixed point in one second.

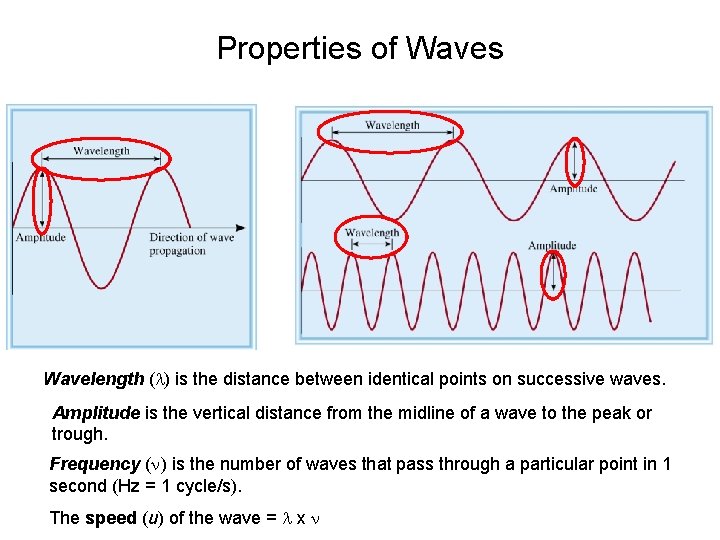
Properties of Waves Wavelength (l) is the distance between identical points on successive waves. Amplitude is the vertical distance from the midline of a wave to the peak or trough. Frequency (n) is the number of waves that pass through a particular point in 1 second (Hz = 1 cycle/s). The speed (u) of the wave = l x n

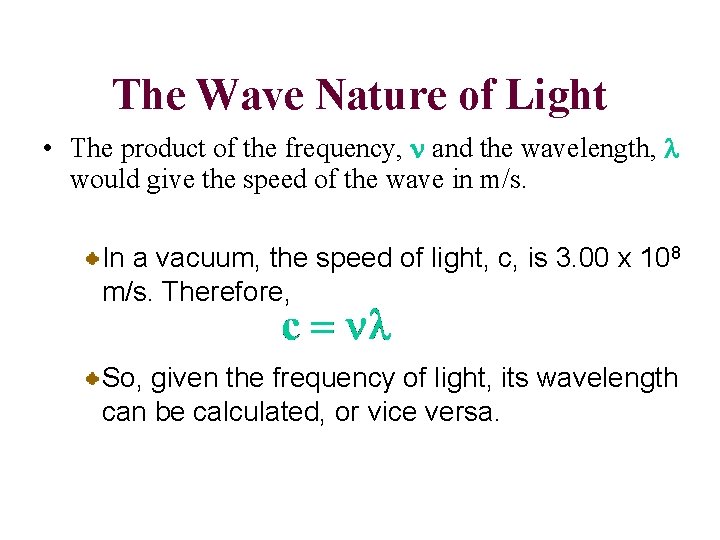
The Wave Nature of Light • The product of the frequency, n and the wavelength, l would give the speed of the wave in m/s. In a vacuum, the speed of light, c, is 3. 00 x 108 m/s. Therefore, So, given the frequency of light, its wavelength can be calculated, or vice versa.

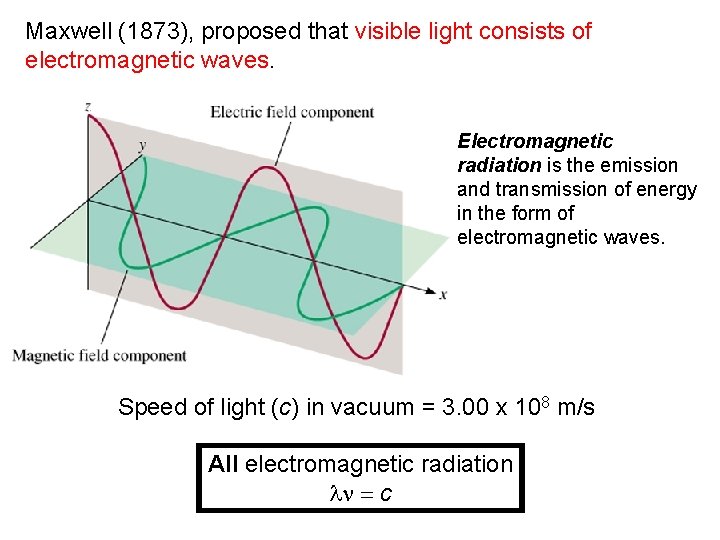
Maxwell (1873), proposed that visible light consists of electromagnetic waves. Electromagnetic radiation is the emission and transmission of energy in the form of electromagnetic waves. Speed of light (c) in vacuum = 3. 00 x 108 m/s All electromagnetic radiation ln = c

The Wave Nature of Light • What is the wavelength of yellow light with a frequency of 5. 09 x 1014 s-1? (Note: s-1, commonly referred to as Hertz (Hz) is defined as “cycles or waves per second”. ) If c = nl, then rearranging, we obtain l = c/n

The Wave Nature of Light • What is the frequency of violet light with a wavelength of 408 nm? If c = nl, then rearranging, we obtain n = c/l.

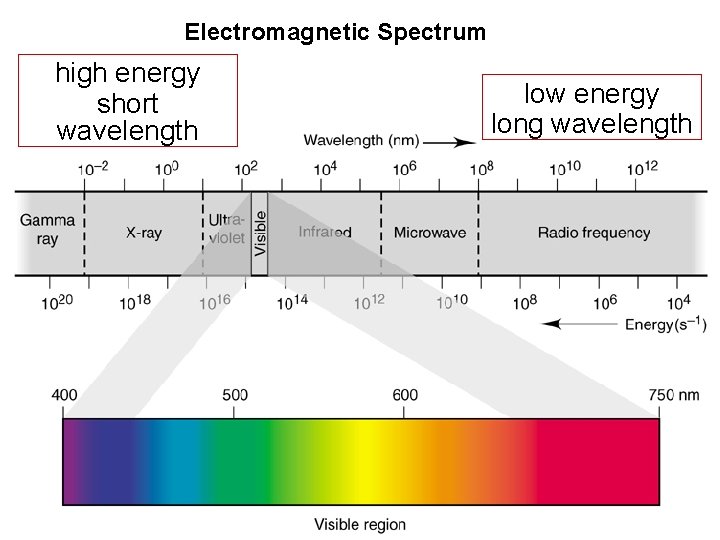
The Wave Nature of Light • The range of frequencies or wavelengths of electromagnetic radiation is called the electromagnetic spectrum. Visible light extends from the violet end of the spectrum at about 400 nm to the red end with wavelengths about 800 nm. Beyond these extremes, electromagnetic radiation is not visible to the human eye.


Electromagnetic Spectrum high energy short wavelength low energy long wavelength

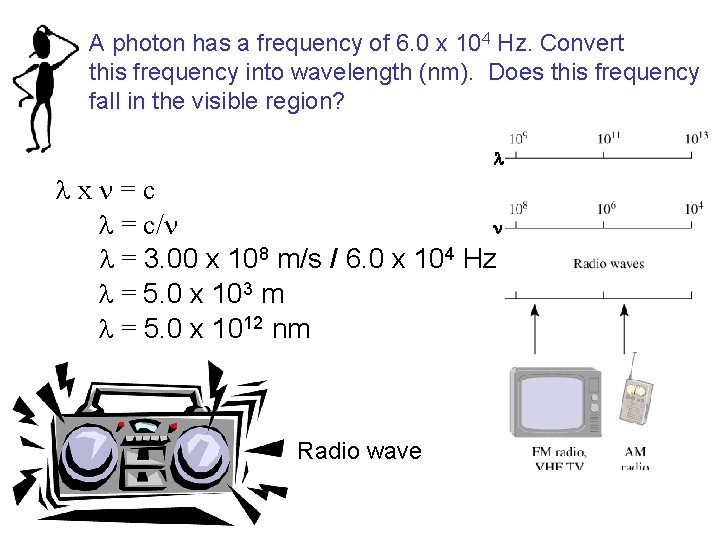
A photon has a frequency of 6. 0 x 104 Hz. Convert this frequency into wavelength (nm). Does this frequency fall in the visible region? l lxn=c n l = c/n l = 3. 00 x 108 m/s / 6. 0 x 104 Hz l = 5. 0 x 103 m l = 5. 0 x 1012 nm Radio wave

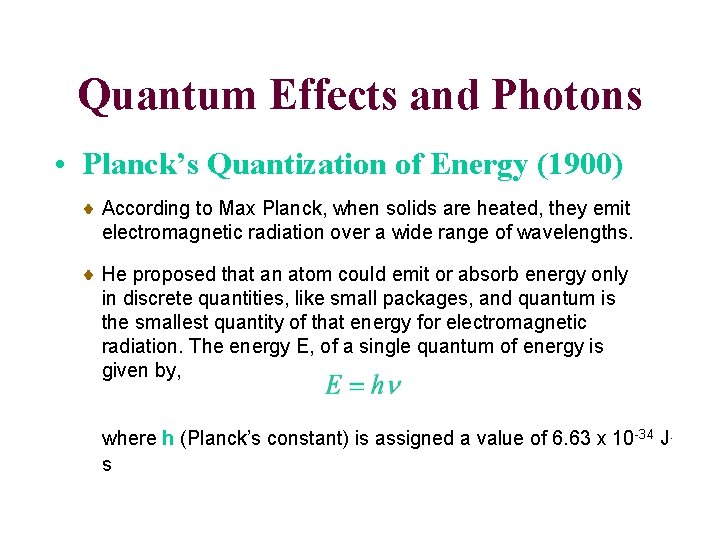
Quantum Effects and Photons • Planck’s Quantization of Energy (1900) According to Max Planck, when solids are heated, they emit electromagnetic radiation over a wide range of wavelengths. He proposed that an atom could emit or absorb energy only in discrete quantities, like small packages, and quantum is the smallest quantity of that energy for electromagnetic radiation. The energy E, of a single quantum of energy is given by, where h (Planck’s constant) is assigned a value of 6. 63 x 10 -34 J. s

Quantum Effects and Photons • By the early part of twentieth century, the wave theory of light seemed to be well entrenched. In 1905, Albert Einstein proposed that light had both wave and particle properties as observed in the photoelectric effect Einstein based this idea on the work of Max Planck.

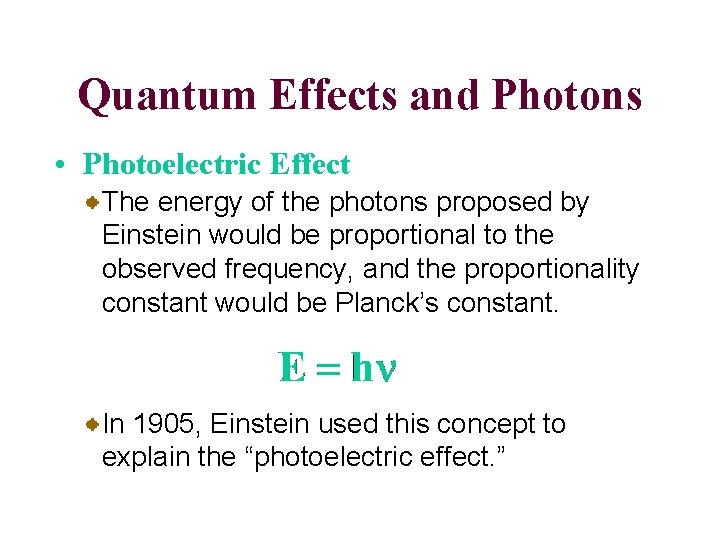
Quantum Effects and Photons • Photoelectric Effect The energy of the photons proposed by Einstein would be proportional to the observed frequency, and the proportionality constant would be Planck’s constant. In 1905, Einstein used this concept to explain the “photoelectric effect. ”

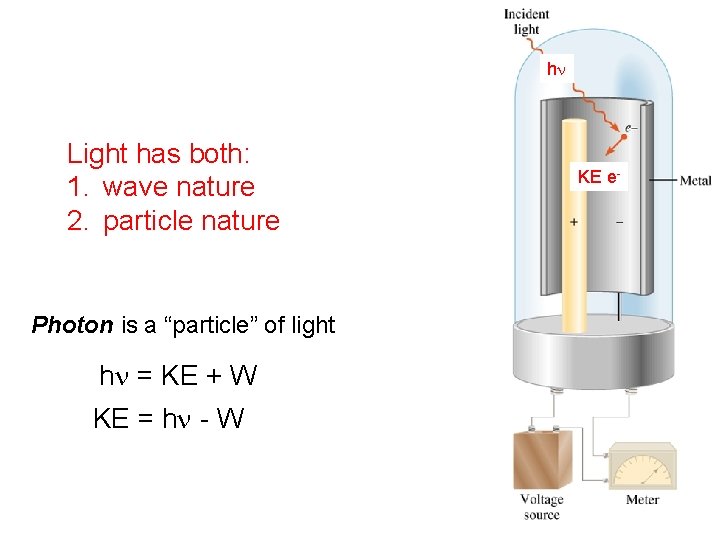
Quantum Effects and Photons • Photoelectric Effect The photoelectric effect is the ejection of electrons from the surface of a metal when light shines on it. Electrons are ejected only if the light exceeds a certain “threshold” frequency. Violet light, for example, will cause potassium to eject electrons, but no amount of red light (which has a lower frequency) has any effect.

Quantum Effects and Photons • Photoelectric Effect Einstein’s assumption that an electron is ejected when struck by a single photon implies that it behaves like a particle. When the photon hits the metal, its energy, hn is taken up by the electron. The photon ceases to exist as a particle; it is said to be “absorbed. ”

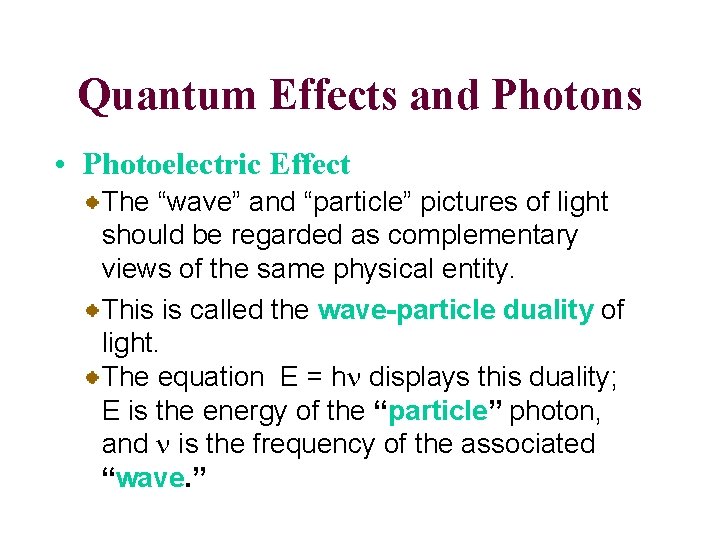
Quantum Effects and Photons • Photoelectric Effect The “wave” and “particle” pictures of light should be regarded as complementary views of the same physical entity. This is called the wave-particle duality of light. The equation E = hn displays this duality; E is the energy of the “particle” photon, and n is the frequency of the associated “wave. ”

hn Light has both: 1. wave nature 2. particle nature Photon is a “particle” of light hn = KE + W KE = hn - W KE e-

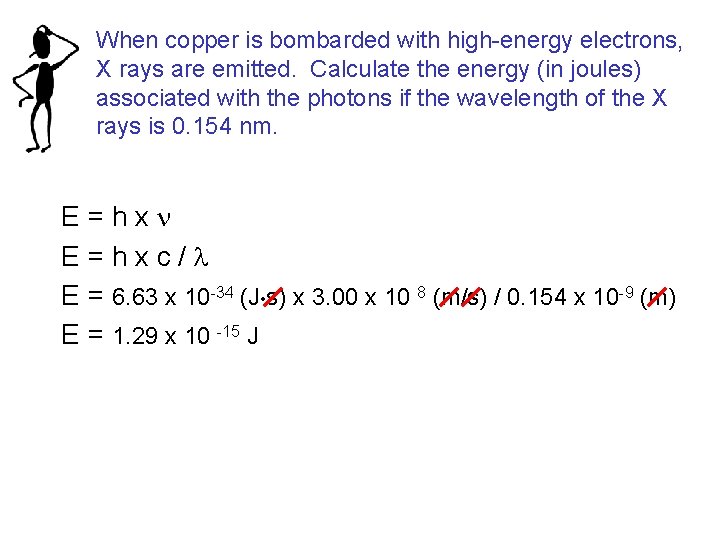
When copper is bombarded with high-energy electrons, X rays are emitted. Calculate the energy (in joules) associated with the photons if the wavelength of the X rays is 0. 154 nm. E=hxn E=hxc/l E = 6. 63 x 10 -34 (J • s) x 3. 00 x 10 8 (m/s) / 0. 154 x 10 -9 (m) E = 1. 29 x 10 -15 J

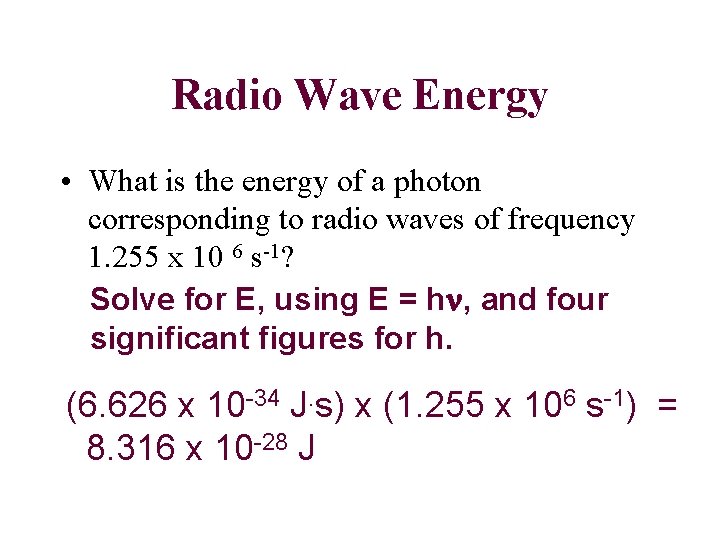
Radio Wave Energy • What is the energy of a photon corresponding to radio waves of frequency 1. 255 x 10 6 s-1? Solve for E, using E = hn, and four significant figures for h. (6. 626 x 10 -34 J. s) x (1. 255 x 106 s-1) = 8. 316 x 10 -28 J

The Bohr Theory of the Hydrogen Atom • Prior to the work of Niels Bohr, the stability of the atom could not be explained using then-current theories. In 1913, using the work of Einstein and Planck, he applied a new theory to the simplest atom, hydrogen. Before looking at Bohr’s theory, we must first examine the “line spectra” of atoms.

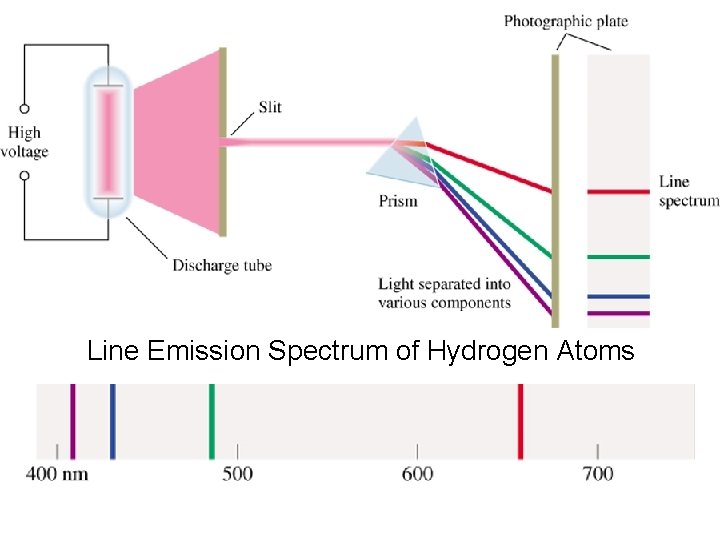
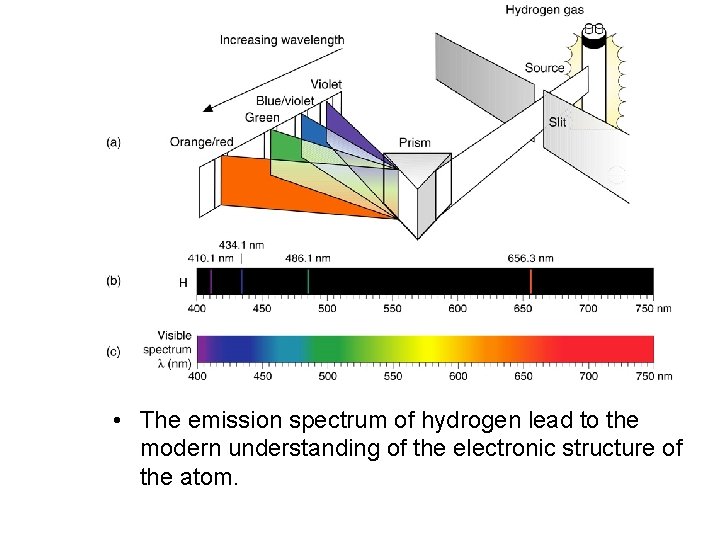
The Bohr Theory of the Hydrogen Atom • Atomic Line Spectra When a heated metal filament emits light, we can use a prism to spread out the light to give a continuous spectrum-that is, a spectrum containing light of all wavelengths. The light emitted by a heated gas, such as hydrogen, results in a line spectrum-a spectrum showing only specific wavelengths of light.

Line Emission Spectrum of Hydrogen Atoms

• • The emission spectrum - light of hydrogen emitted when lead toa the modern substance understanding is excited byofan theenergy electronic source. structure of the atom.


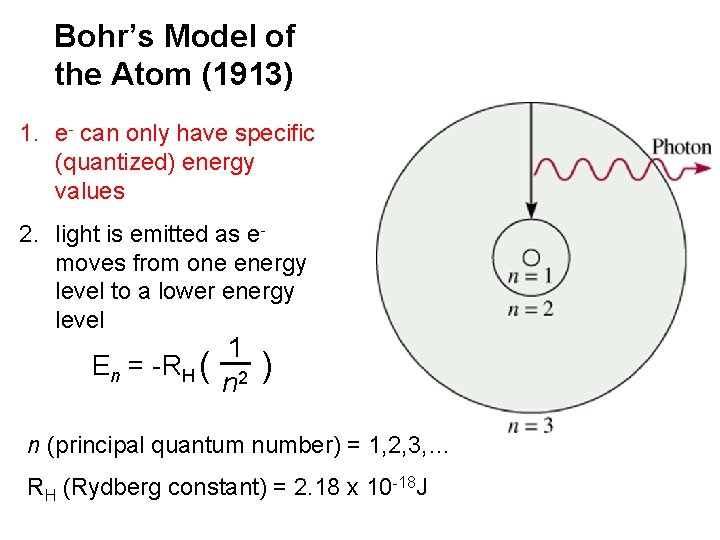
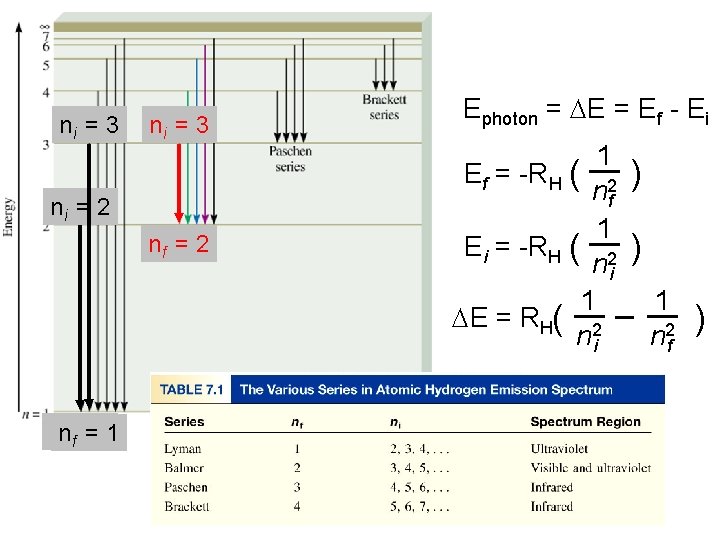
Bohr’s Model of the Atom (1913) 1. e- can only have specific (quantized) energy values 2. light is emitted as emoves from one energy level to a lower energy level En = -RH ( 1 n 2 ) n (principal quantum number) = 1, 2, 3, … RH (Rydberg constant) = 2. 18 x 10 -18 J

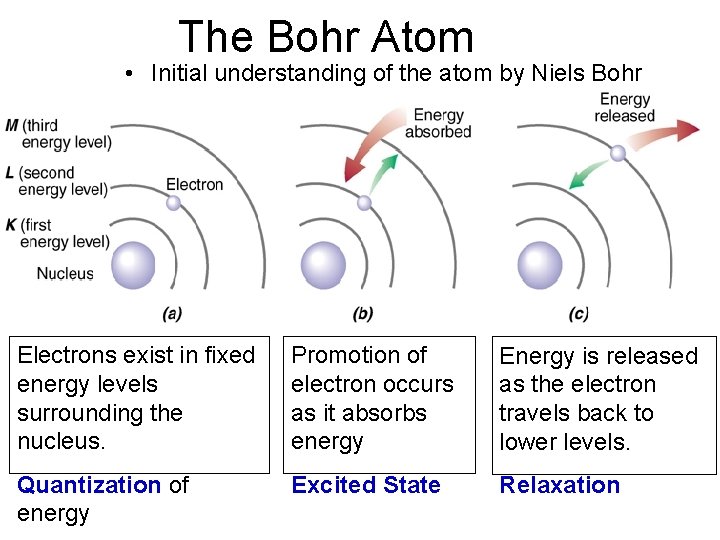
The Bohr Atom • Initial understanding of the atom by Niels Bohr 8 Electrons exist in fixed energy levels surrounding the nucleus. Promotion of electron occurs as it absorbs energy Energy is released as the electron travels back to lower levels. Quantization of energy Excited State Relaxation

• Orbit - what Bohr called the fixed energy levels. • Ground state - the lowest possible energy state.

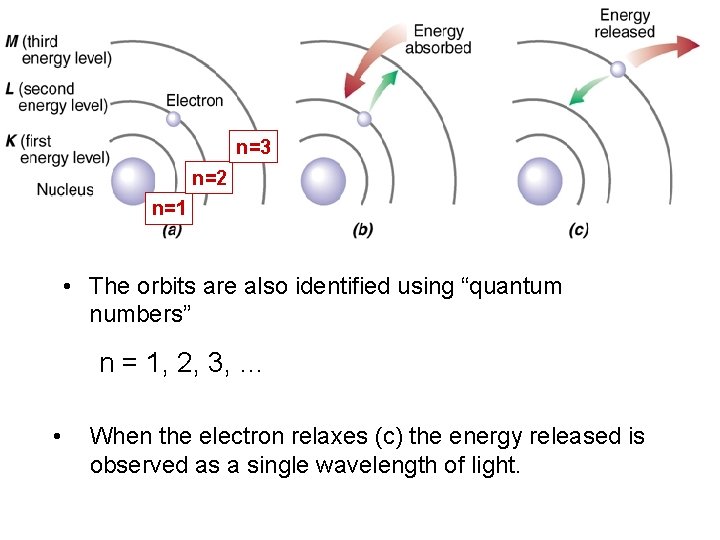
n=3 n=2 n=1 • The orbits are also identified using “quantum numbers” n = 1, 2, 3, … • When the electron relaxes (c) the energy released is observed as a single wavelength of light.

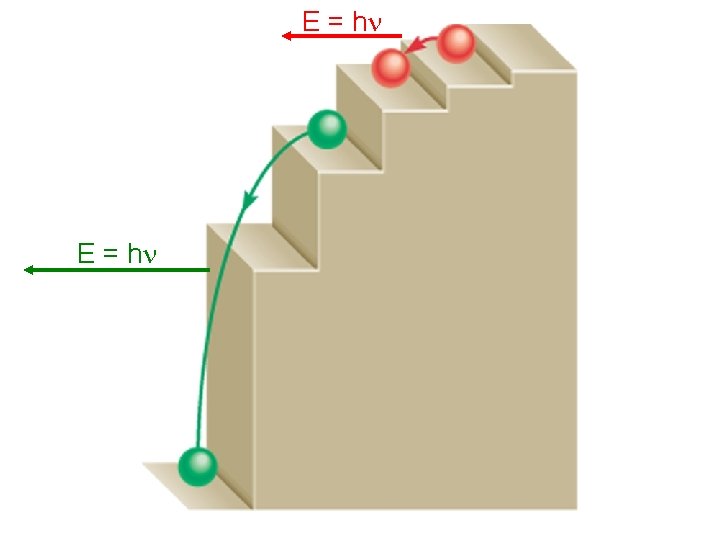
E = hn

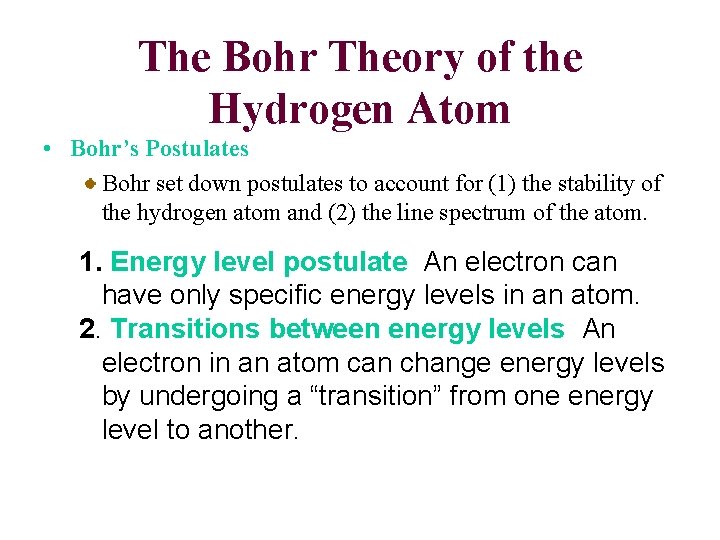
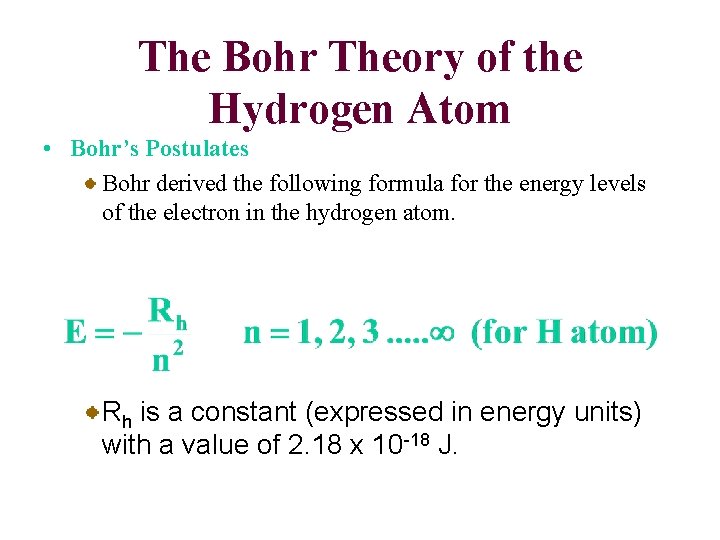
The Bohr Theory of the Hydrogen Atom • Bohr’s Postulates Bohr set down postulates to account for (1) the stability of the hydrogen atom and (2) the line spectrum of the atom. 1. Energy level postulate An electron can have only specific energy levels in an atom. 2. Transitions between energy levels An electron in an atom can change energy levels by undergoing a “transition” from one energy level to another.

The Bohr Theory of the Hydrogen Atom • Bohr’s Postulates Bohr derived the following formula for the energy levels of the electron in the hydrogen atom. Rh is a constant (expressed in energy units) with a value of 2. 18 x 10 -18 J.

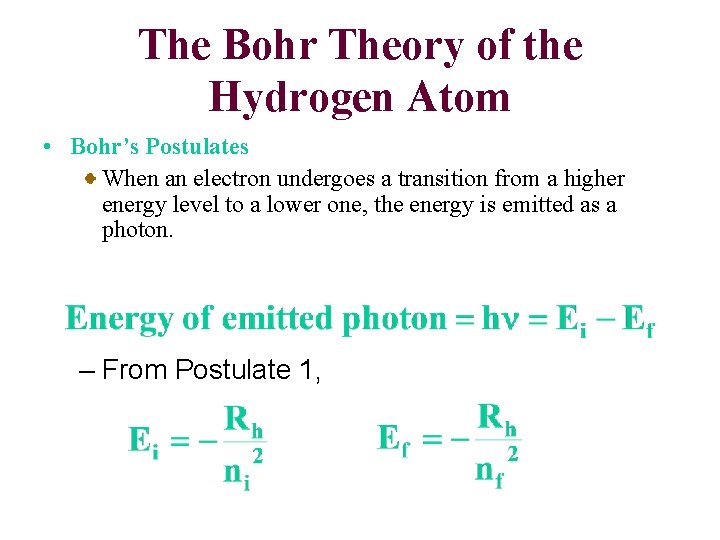
The Bohr Theory of the Hydrogen Atom • Bohr’s Postulates When an electron undergoes a transition from a higher energy level to a lower one, the energy is emitted as a photon. – From Postulate 1,

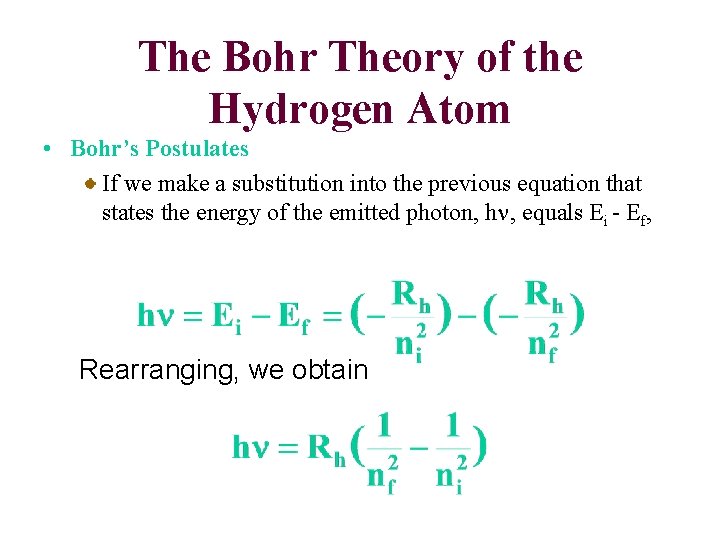
The Bohr Theory of the Hydrogen Atom • Bohr’s Postulates If we make a substitution into the previous equation that states the energy of the emitted photon, hn, equals Ei - Ef, Rearranging, we obtain

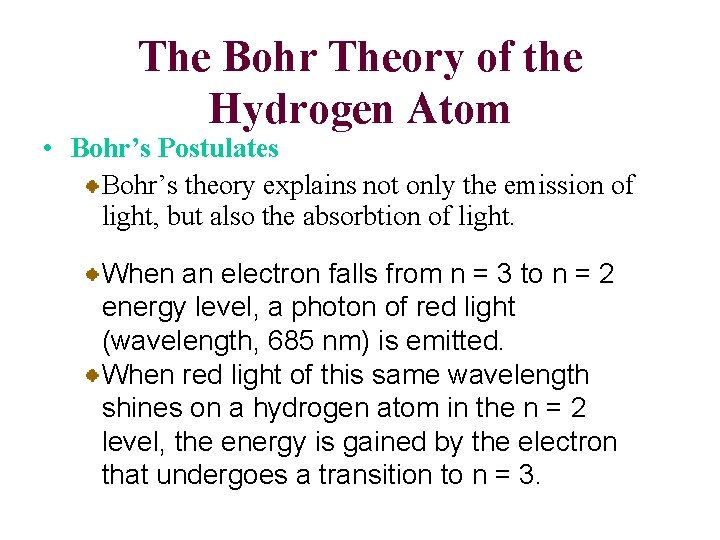
The Bohr Theory of the Hydrogen Atom • Bohr’s Postulates Bohr’s theory explains not only the emission of light, but also the absorbtion of light. When an electron falls from n = 3 to n = 2 energy level, a photon of red light (wavelength, 685 nm) is emitted. When red light of this same wavelength shines on a hydrogen atom in the n = 2 level, the energy is gained by the electron that undergoes a transition to n = 3.

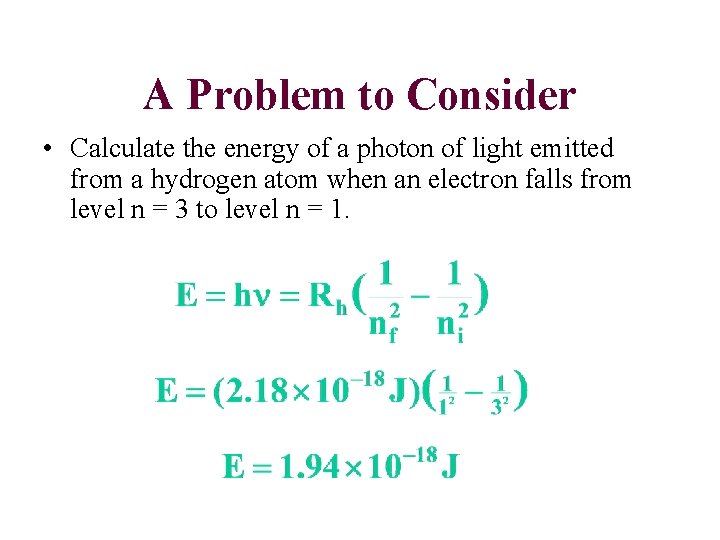
A Problem to Consider • Calculate the energy of a photon of light emitted from a hydrogen atom when an electron falls from level n = 3 to level n = 1.

ni = 3 ni = 2 nf = 2 nnf f==11 Ephoton = DE = Ef - Ei 1 Ef = -RH ( 2 nf 1 Ei = -RH ( 2 ni 1 DE = RH( 2 ni ) ) 1 n 2 f )

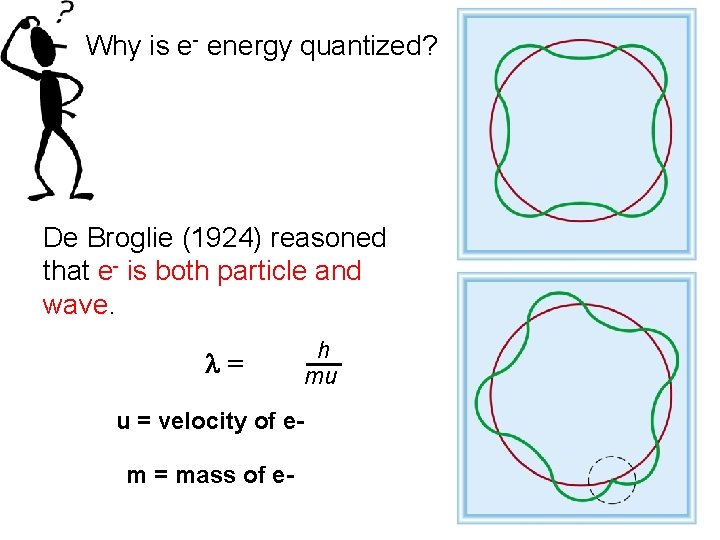
Why is e- energy quantized? De Broglie (1924) reasoned that e- is both particle and wave. l= u = velocity of em = mass of e- h mu

Quantum Mechanics • Bohr’s theory established the concept of atomic energy levels but did not thoroughly explain the “wave-like” behavior of the electron. Current ideas about atomic structure depend on the principles of quantum mechanics, a theory that applies to subatomic particles such as electrons.

Quantum Mechanics • The first clue in the development of quantum theory came with the discovery of the de Broglie relation. In 1923, Louis de Broglie reasoned that if light exhibits particle aspects, perhaps particles of matter show characteristics of waves. He postulated that a particle with mass m and a velocity v has an associated wavelength. The equation l = h/mv is called the de Broglie relation.

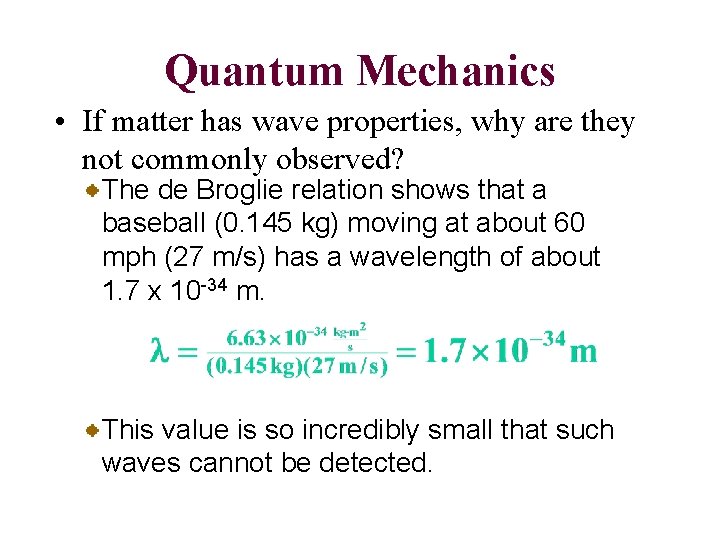
Quantum Mechanics • If matter has wave properties, why are they not commonly observed? The de Broglie relation shows that a baseball (0. 145 kg) moving at about 60 mph (27 m/s) has a wavelength of about 1. 7 x 10 -34 m. This value is so incredibly small that such waves cannot be detected.

Quantum Mechanics • If matter has wave properties, why are they not commonly observed? Electrons have wavelengths on the order of a few picometers (1 pm = 10 -12 m). Under the proper circumstances, the wave character of electrons should be observable.

What is the de Broglie wavelength (in nm) associated with a 2. 5 g Ping-Pong ball traveling at 15. 6 m/s? l = h/mu h in J • s m in kg u in (m/s) l = 6. 63 x 10 -34 kg. m 2 /s (2. 5 x 10 -3 kg x 15. 6 m/s) l = 1. 7 x 10 -32 m = 1. 7 x 10 -23 nm

Chemistry in Action: Electron Microscopy A light microscope uses visible light and an arrangement of lenses to magnify images of small samples. While an electron microscope uses a particle beam of electrons to illuminate the specimen and produce a magnified image and has a greater resolving power.

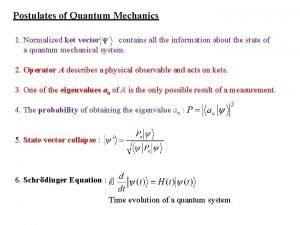
Schrodinger Wave Equation In 1926 Schrodinger wrote an equation that described both the particle and wave nature of the e. Wave function (Y) describes: 1. energy of e- with a given Y 2. probability of finding e- in a volume of space Schrodinger’s equation can only be solved exactly for the hydrogen atom. Must approximate its solution for multi-electron systems.

Quantum Mechanics • Heisenberg’s uncertainty principle it is impossible to know both position and velocity simultaneously. .

Quantum Mechanics • Although we cannot precisely define an electron’s orbit, we can obtain the probability of finding an electron at a given point around the nucleus. Erwin Schrodinger defined this probability in a mathematical expression called a wave function, denoted y (psi). The probability of finding a particle in a region of space is defined by y 2.

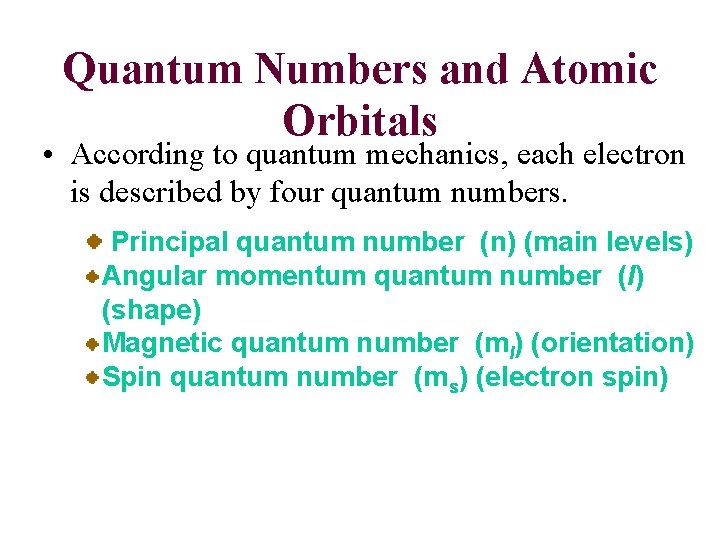
Quantum Numbers and Atomic Orbitals • According to quantum mechanics, each electron is described by four quantum numbers. Principal quantum number (n) (main levels) Angular momentum quantum number (l) (shape) Magnetic quantum number (ml) (orientation) Spin quantum number (ms) (electron spin)

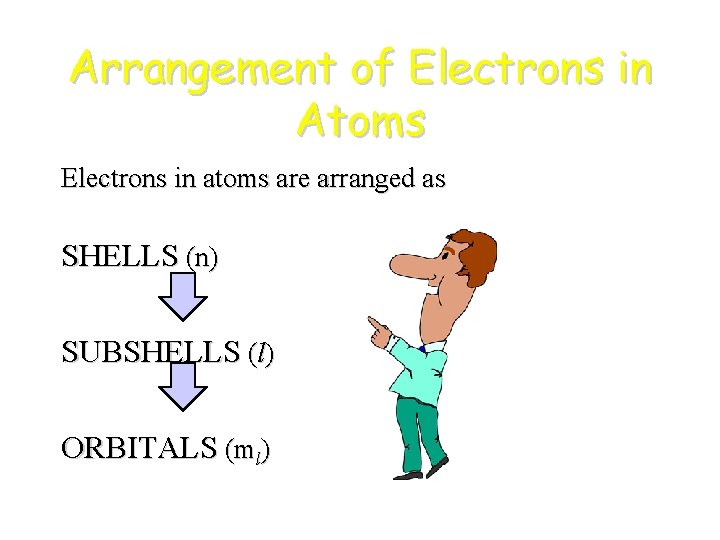
Arrangement of Electrons in Atoms Electrons in atoms are arranged as SHELLS (n) SUBSHELLS (l) ORBITALS (ml)

Arrangement of Electrons in Atoms Each orbital can be assigned no more than 2 electrons! This is tied to the existence of a 4 th quantum number, the electron spin quantum number, ms.

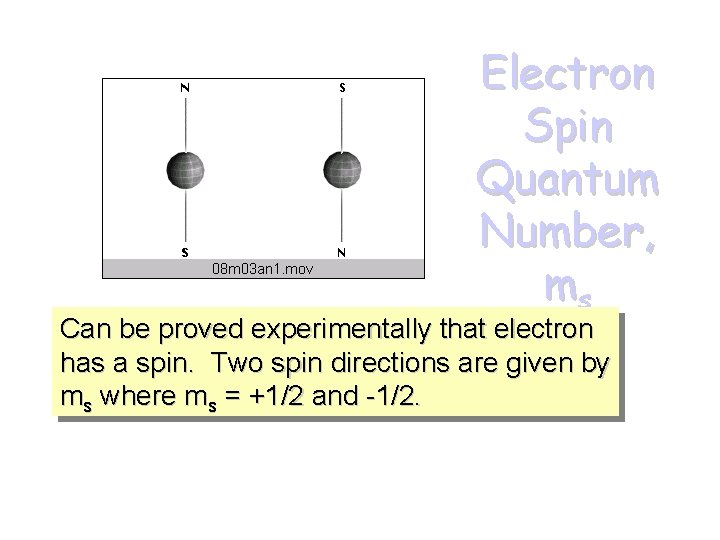
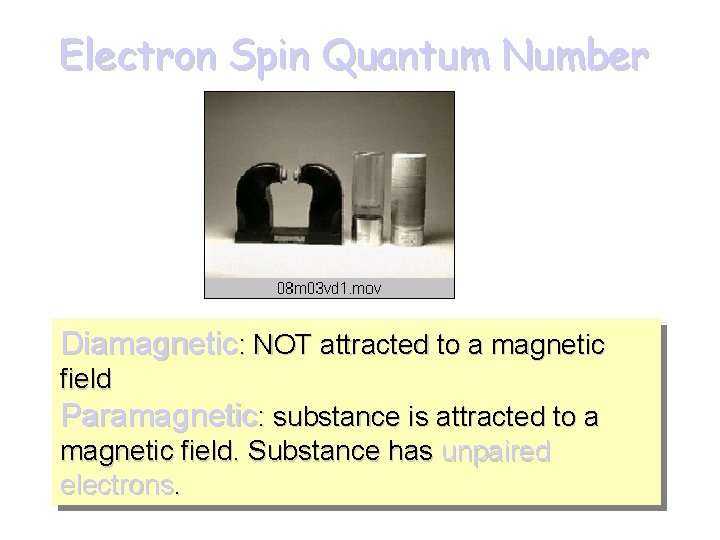
Electron Spin Quantum Number, ms Can be proved experimentally that electron has a spin. Two spin directions are given by ms where ms = +1/2 and -1/2.

Electron Spin Quantum Number Diamagnetic: NOT attracted to a magnetic field Paramagnetic: substance is attracted to a magnetic field. Substance has unpaired electrons.

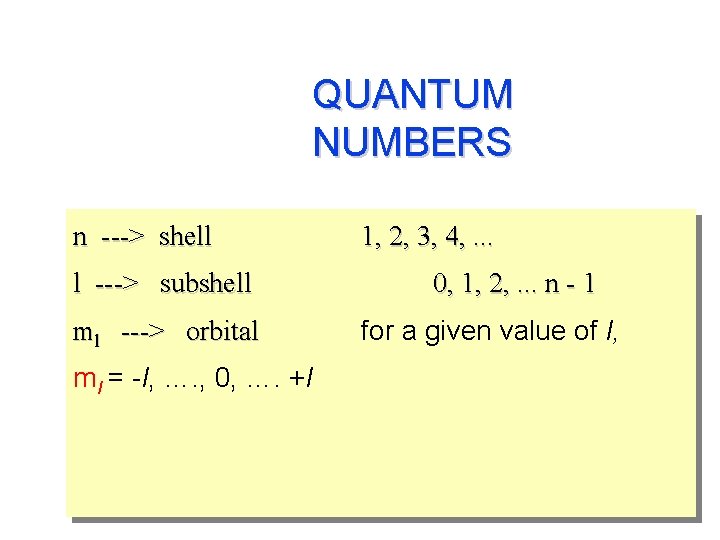
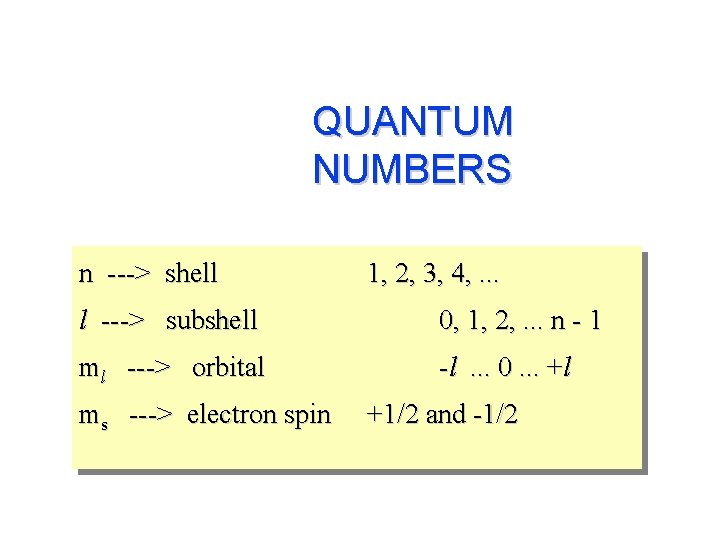
QUANTUM NUMBERS n ---> shell l ---> subshell ml ---> orbital ml = -l, …. , 0, …. +l 1, 2, 3, 4, . . . 0, 1, 2, . . . n - 1 for a given value of l,

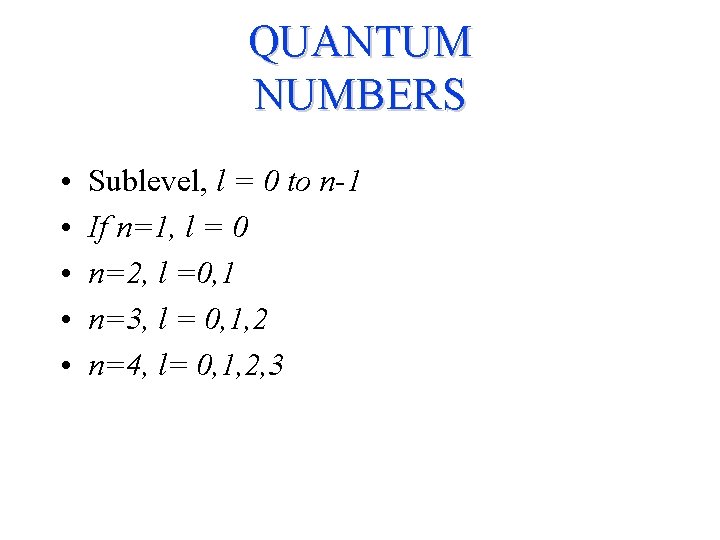
QUANTUM NUMBERS • • • Sublevel, l = 0 to n-1 If n=1, l = 0 n=2, l =0, 1 n=3, l = 0, 1, 2 n=4, l= 0, 1, 2, 3

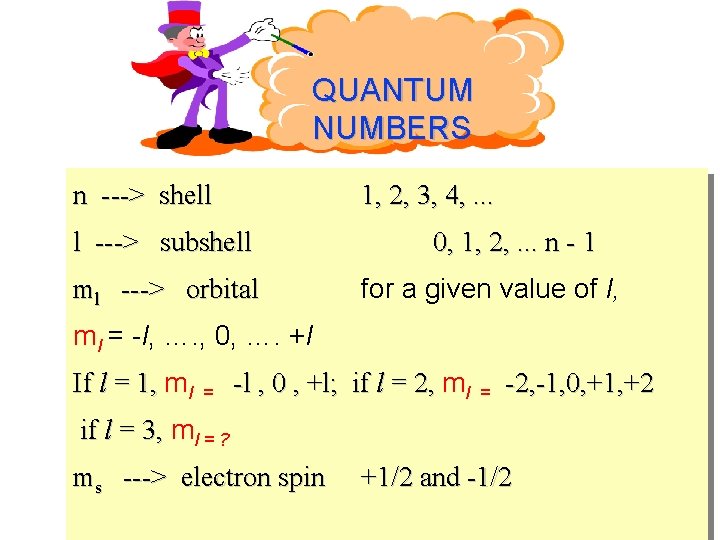
QUANTUM NUMBERS n ---> shell 1, 2, 3, 4, . . . l ---> subshell ml ---> orbital 0, 1, 2, . . . n - 1 for a given value of l, ml = -l, …. , 0, …. +l If l = 1, ml = -l , 0 , +l; if l = 2, ml = -2, -1, 0, +1, +2 if l = 3, ml = ? ms ---> electron spin +1/2 and -1/2

QUANTUM NUMBERS n ---> shell 1, 2, 3, 4, . . . l ---> subshell 0, 1, 2, . . . n - 1 ml ---> orbital -l. . . 0. . . +l ms ---> electron spin +1/2 and -1/2

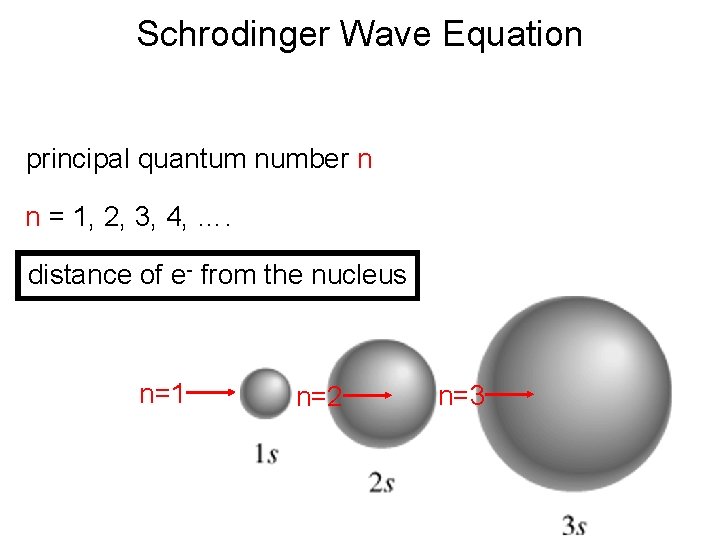
Schrodinger Wave Equation principal quantum number n n = 1, 2, 3, 4, …. distance of e- from the nucleus n=1 n=2 n=3

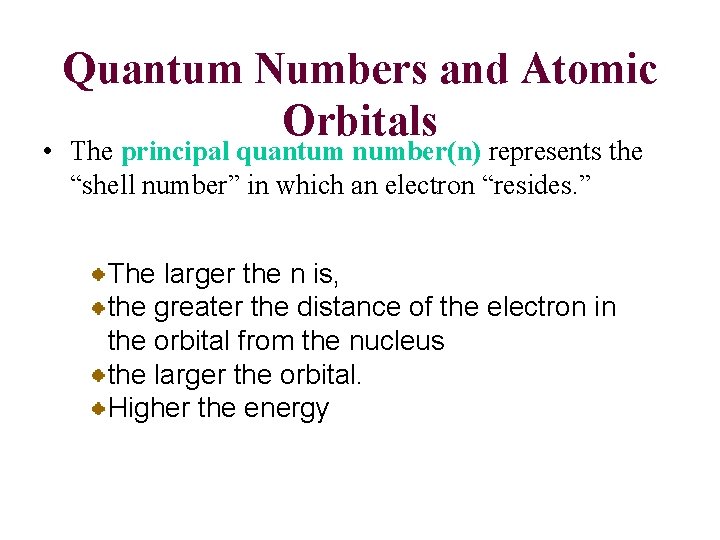
Quantum Numbers and Atomic Orbitals • The principal quantum number(n) represents the “shell number” in which an electron “resides. ” The larger the n is, the greater the distance of the electron in the orbital from the nucleus the larger the orbital. Higher the energy

Electron Density

Quantum Numbers (n, l, ms) n – principal quantum number (main energy level) l - angular momentum quantum number (sublevel) ml – magnetic quantum number ms – electron spin quantum number

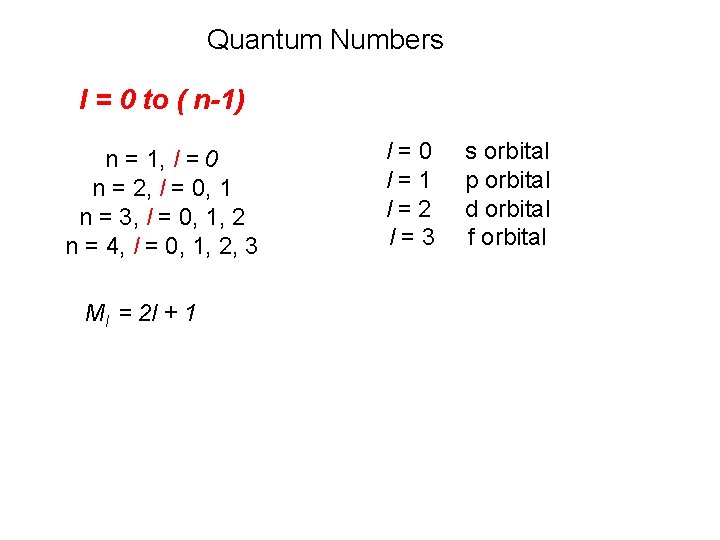
Quantum Numbers l = 0 to ( n-1) n = 1, l = 0 n = 2, l = 0, 1 n = 3, l = 0, 1, 2 n = 4, l = 0, 1, 2, 3 Ml = 2 l + 1 l=0 l=1 l=2 l=3 s orbital p orbital d orbital f orbital

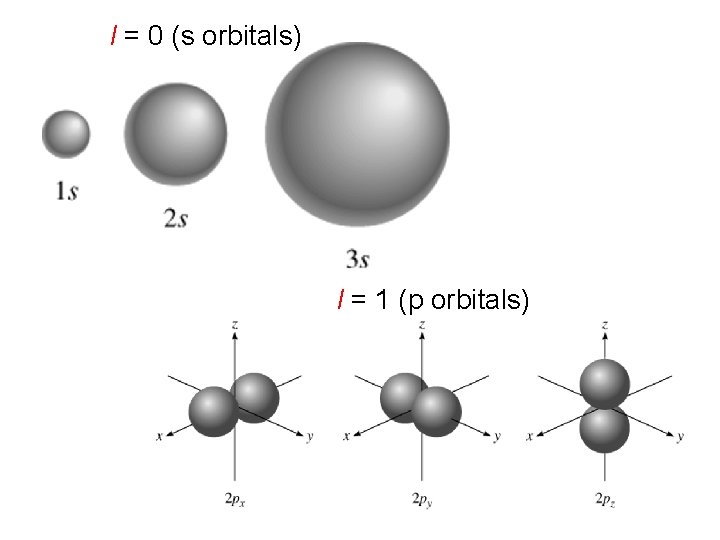
l = 0 (s orbitals) l = 1 (p orbitals)

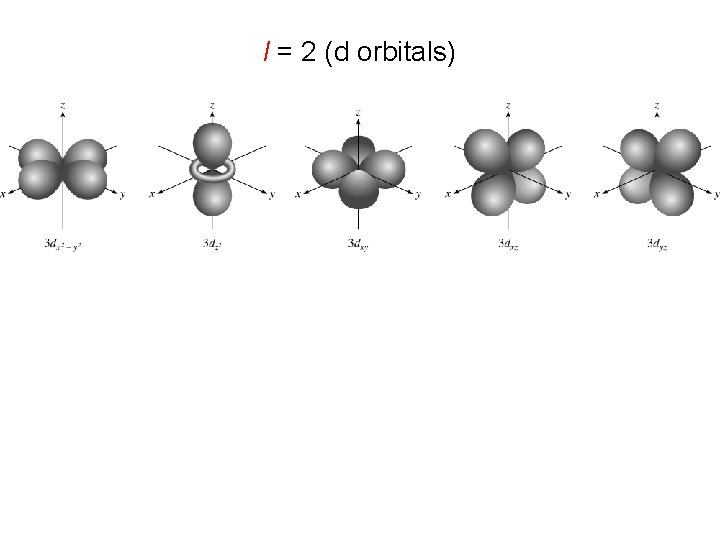
l = 2 (d orbitals)

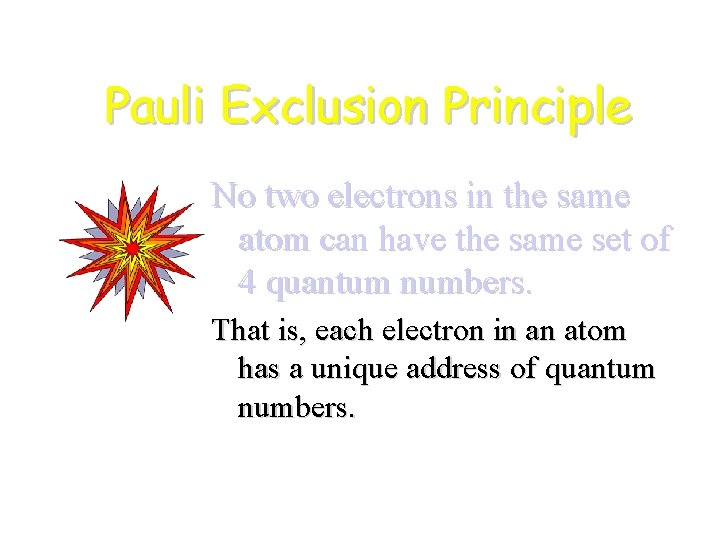
Pauli Exclusion Principle No two electrons in the same atom can have the same set of 4 quantum numbers. That is, each electron in an atom has a unique address of quantum numbers.

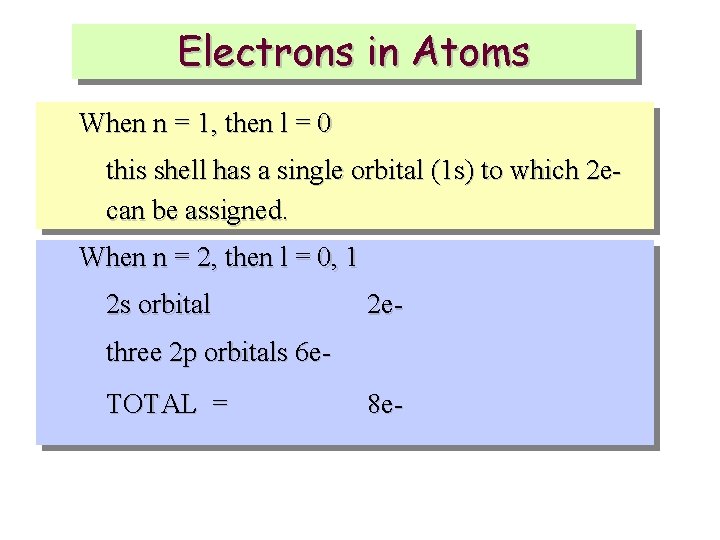
Electrons in Atoms When n = 1, then l = 0 this shell has a single orbital (1 s) to which 2 ecan be assigned. When n = 2, then l = 0, 1 2 s orbital 2 e- three 2 p orbitals 6 e. TOTAL = 8 e-

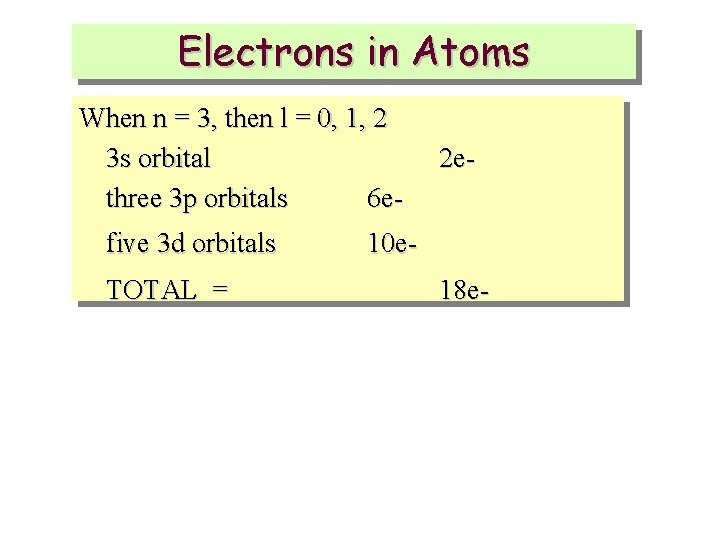
Electrons in Atoms When n = 3, then l = 0, 1, 2 3 s orbital three 3 p orbitals 6 efive 3 d orbitals TOTAL = 2 e- 10 e 18 e-

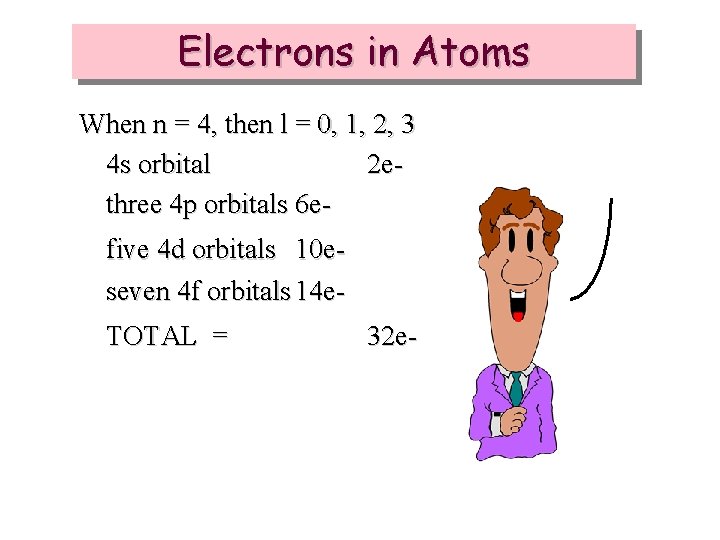
Electrons in Atoms When n = 4, then l = 0, 1, 2, 3 4 s orbital 2 ethree 4 p orbitals 6 efive 4 d orbitals 10 eseven 4 f orbitals 14 e. TOTAL = 32 e-


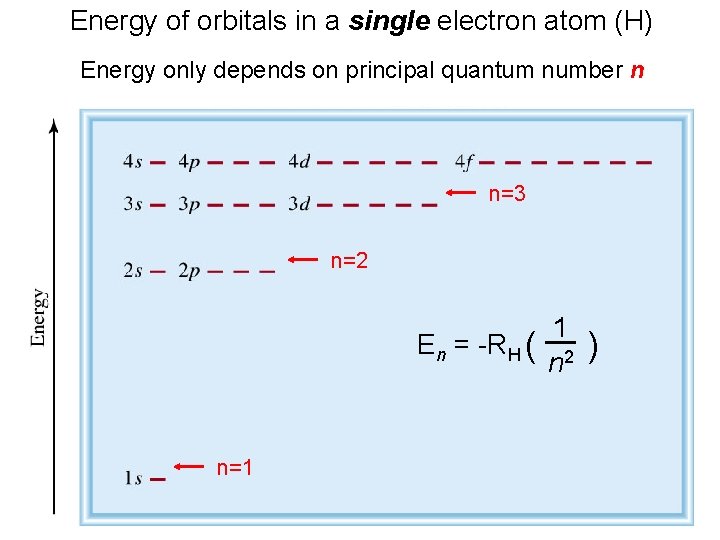
Assigning Electrons to Atoms • Electrons generally assigned to orbitals of successively higher energy. • For H atoms, E = - C(1/n 2). E depends only on n. • For many-electron atoms, energy depends on both n and l.



Energy of orbitals in a single electron atom (H) Energy only depends on principal quantum number n n=3 n=2 En = -RH ( n=1 1 n 2 )

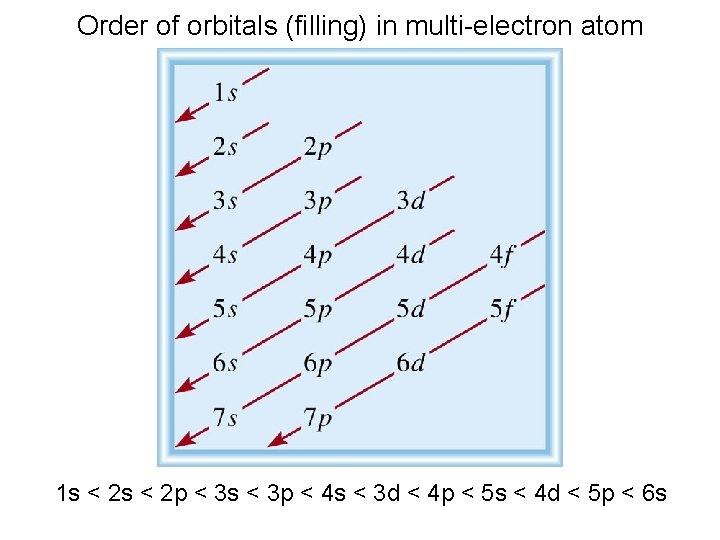
Order of orbitals (filling) in multi-electron atom 1 s < 2 p < 3 s < 3 p < 4 s < 3 d < 4 p < 5 s < 4 d < 5 p < 6 s

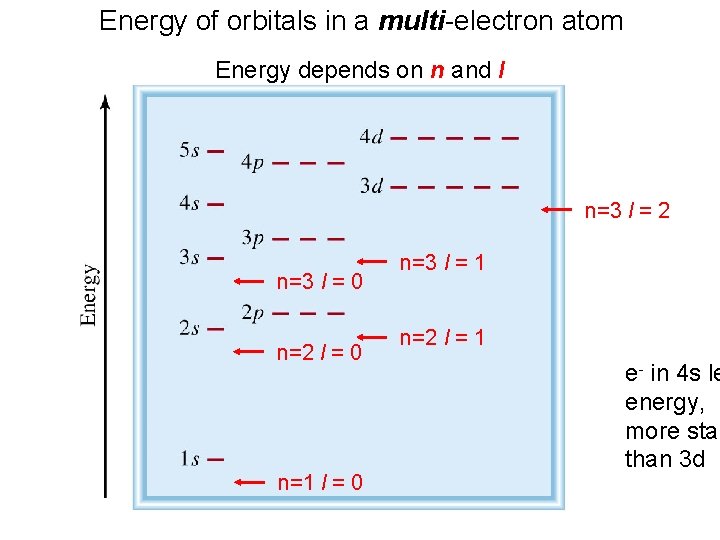
Energy of orbitals in a multi-electron atom Energy depends on n and l n=3 l = 2 n=3 l = 0 n=2 l = 0 n=1 l = 0 n=3 l = 1 n=2 l = 1 e- in 4 s le energy, more stab than 3 d

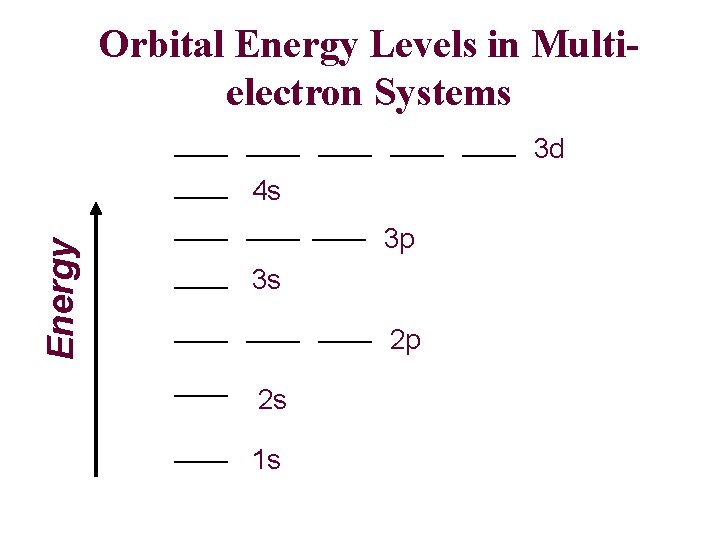
Orbital Energy Levels in Multielectron Systems 3 d Energy 4 s 3 p 3 s 2 p 2 s 1 s

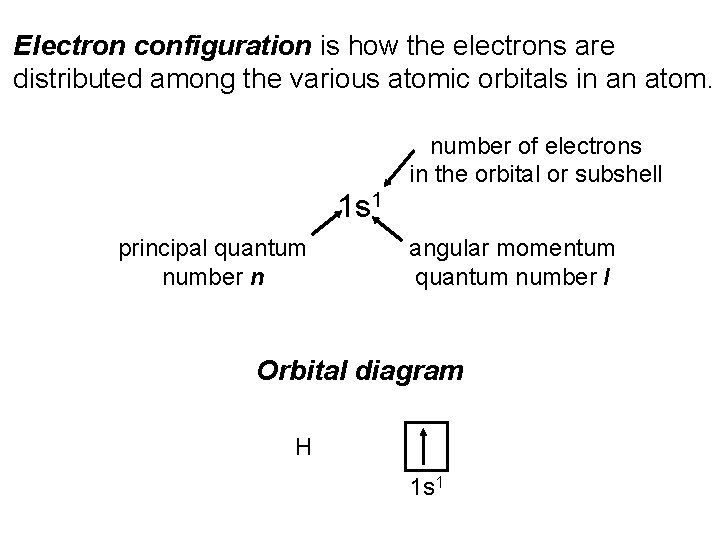
Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom. number of electrons in the orbital or subshell 1 s 1 principal quantum number n angular momentum quantum number l Orbital diagram H 1 s 1
![Aufbau Principle Here a few examples Using the abbreviation He for 1 s Aufbau Principle • Here a few examples. Using the abbreviation [He] for 1 s](https://slidetodoc.com/presentation_image_h2/2904220042b61c8f2571044eb8cee3df/image-78.jpg)
Aufbau Principle • Here a few examples. Using the abbreviation [He] for 1 s 2, the configurations are Z=4 Beryllium 1 s 22 s 2 or [He]2 s 2 Z=3 Lithium 1 s 22 s 1 or [He]2 s 1

Aufbau Principle • With boron (Z=5), the electrons begin filling the 2 p subshell. Z=5 Boron Z=6 Carbon Z=7 Nitrogen Z=8 Oxygen Z=9 Fluorine Z=10 Neon 1 s 22 p 1 1 s 22 p 2 1 s 22 p 3 1 s 22 p 4 1 s 22 p 5 1 s 22 p 6 or or or [He]2 s 22 p 1 [He]2 s 22 p 2 [He]2 s 22 p 3 [He]2 s 22 p 4 [He]2 s 22 p 5 [He]2 s 62 p 6

Aufbau Principle • With sodium (Z = 11), the 3 s sub shell begins to fill. Z=11 Sodium 1 s 22 p 63 s 1 or [Ne]3 s 1 Z=12 Magnesium 1 s 22 p 63 s 2 or [Ne]3 s 2 Then the 3 p sub shell begins to fill. Z=13 Aluminum 1 s 22 p 63 s 23 p 1 or [Ne]3 s 23 p 1 • • Z=18 Argon 1 s 22 p 63 s 23 or [Ne]3 s 23 p 6

Aufbau Principle • The “building up” order corresponds for the most part to increasing energy of the subshells. By filling orbitals of the lowest energy first, you usually get the lowest total energy (“ground state”) of the atom. Now you can see how to reproduce the electron configurations of Table 7. 3 using the Aufbau principle. Remember, the number of electrons in the neutral atom equals the atomic number, Z.

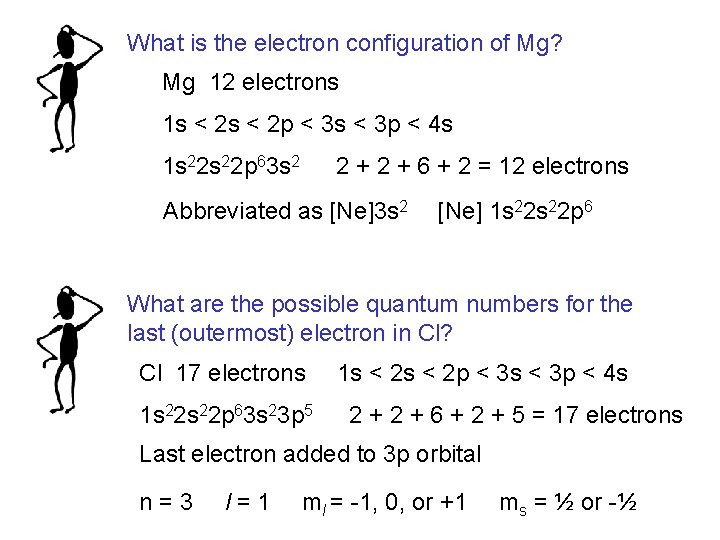
What is the electron configuration of Mg? Mg 12 electrons 1 s < 2 p < 3 s < 3 p < 4 s 1 s 22 p 63 s 2 2 + 6 + 2 = 12 electrons Abbreviated as [Ne]3 s 2 [Ne] 1 s 22 p 6 What are the possible quantum numbers for the last (outermost) electron in Cl? Cl 17 electrons 1 s 22 p 63 s 23 p 5 1 s < 2 p < 3 s < 3 p < 4 s 2 + 6 + 2 + 5 = 17 electrons Last electron added to 3 p orbital n=3 l=1 ml = -1, 0, or +1 ms = ½ or -½

Outermost subshell being filled with electrons

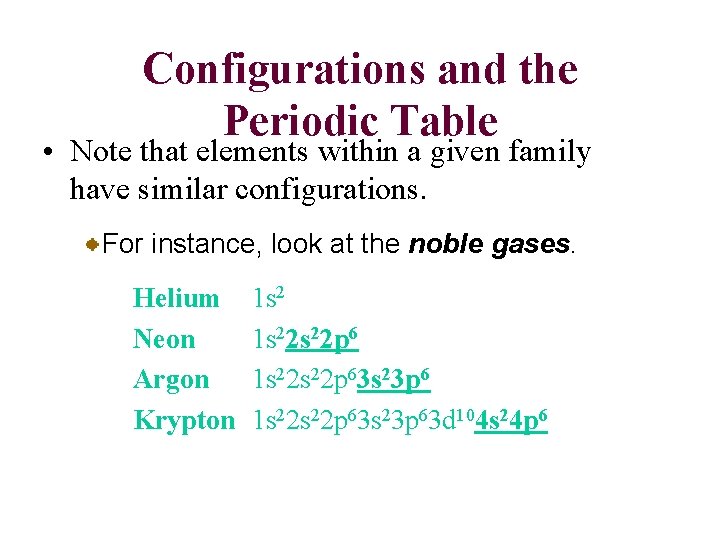
Configurations and the Periodic Table • Note that elements within a given family have similar configurations. For instance, look at the noble gases. Helium Neon Argon Krypton 1 s 22 s 22 p 63 s 23 p 63 d 104 s 24 p 6

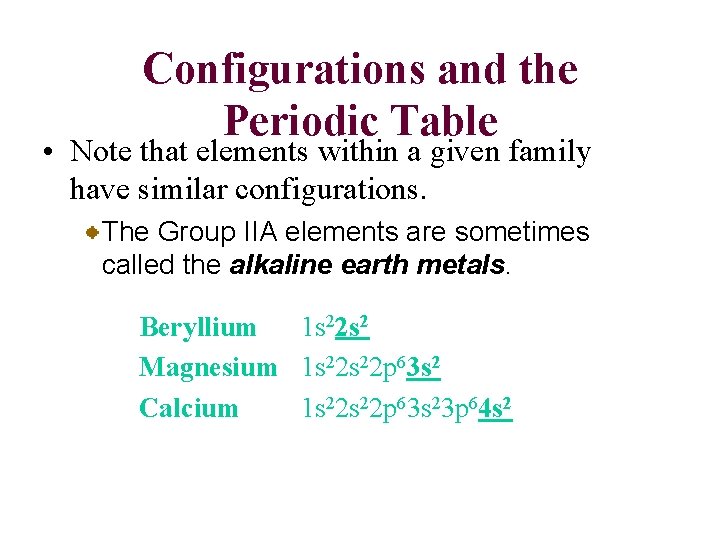
Configurations and the Periodic Table • Note that elements within a given family have similar configurations. The Group IIA elements are sometimes called the alkaline earth metals. Beryllium 1 s 22 s 2 Magnesium 1 s 22 p 63 s 2 Calcium 1 s 22 p 63 s 23 p 64 s 2

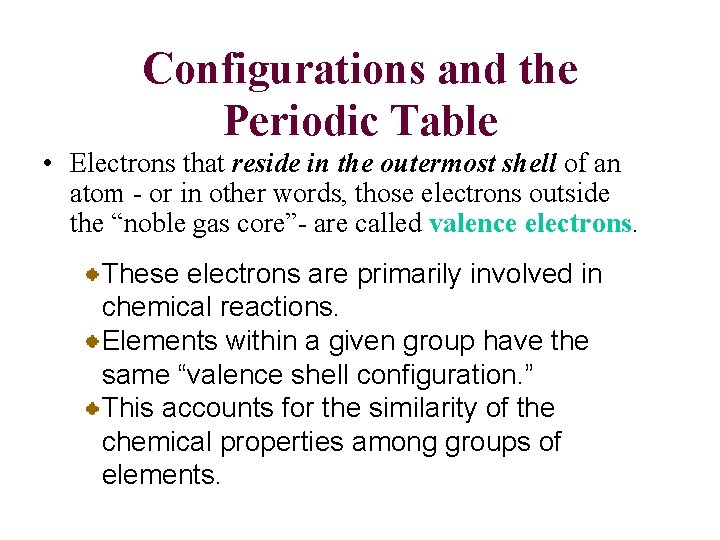
Configurations and the Periodic Table • Electrons that reside in the outermost shell of an atom - or in other words, those electrons outside the “noble gas core”- are called valence electrons. These electrons are primarily involved in chemical reactions. Elements within a given group have the same “valence shell configuration. ” This accounts for the similarity of the chemical properties among groups of elements.

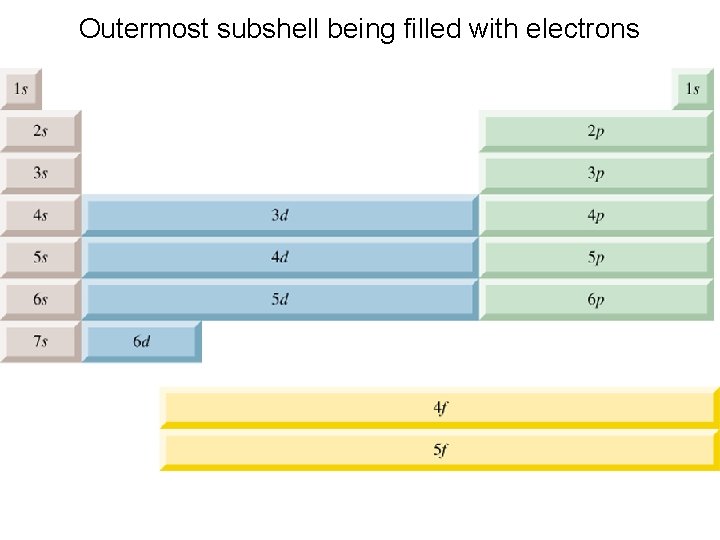
Configurations and the Periodic Table • The following slide illustrates how the periodic table provides a sound way to remember the Aufbau sequence. In many cases you need only the configuration of the outer electrons. You can determine this from their position on the periodic table. The total number of valence electrons for an atom equals its group number.

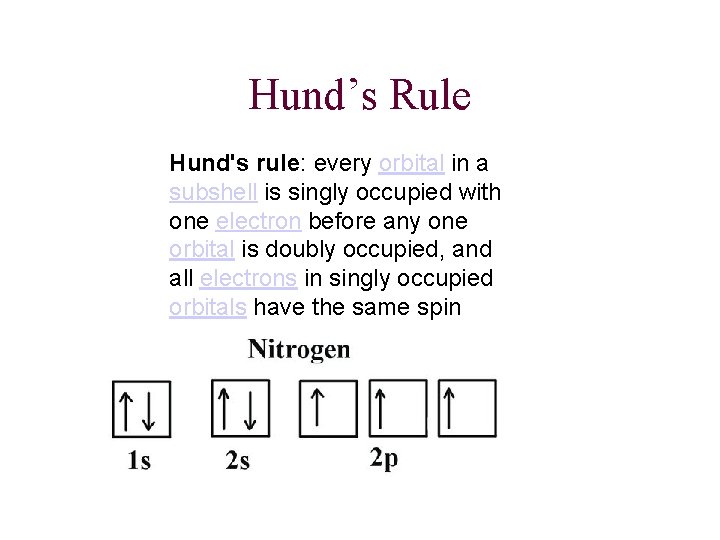
Hund’s Rule Hund's rule: every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin


Shielding Effect The shielding effect describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell For the same n, the shielding effect increases in the electron as, s<p<d<f Electron in s more closer to nucleus than p.

Homework ch-7 • Examples and practice exercises • 7. 8, 7. 16, 7. 20, 7. 28, 7. 34, 7. 48, 7. 56, 7. 58, 7. 61, 7. 65, 7. 69, 7. 70, 7. 72, 7. 74, 7. 76, 7. 78, 7. 79, 7. 89 , 7. 92, 7. 98, 7. 130
 Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms Section 2 quantum theory and the atom
Section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Which of the d orbitals most resembles a pz orbital?
Which of the d orbitals most resembles a pz orbital? At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Origin of quantum mechanics
Origin of quantum mechanics Quantum physics vs mechanics
Quantum physics vs mechanics Electronic news gathering and electronic field production
Electronic news gathering and electronic field production Electronic structure of solid band theory
Electronic structure of solid band theory An electronic is the electronic exchange of money or scrip
An electronic is the electronic exchange of money or scrip Chapter 4 section 2 the structure of atoms
Chapter 4 section 2 the structure of atoms Bjt and fet frequency response
Bjt and fet frequency response Electronic devices and circuit theory
Electronic devices and circuit theory Electronic devices and circuit theory
Electronic devices and circuit theory Load line analysis of transistor
Load line analysis of transistor Electronic devices and circuit theory
Electronic devices and circuit theory Electronic devices and circuit theory
Electronic devices and circuit theory Electronic devices and circuit theory
Electronic devices and circuit theory Quantum shannon theory
Quantum shannon theory Chapter 27 quantum theory answers
Chapter 27 quantum theory answers Quantum game theory
Quantum game theory Sommerfeld quantum theory
Sommerfeld quantum theory Site:slidetodoc.com
Site:slidetodoc.com Fermi temperature formula
Fermi temperature formula Hydrogen wave function
Hydrogen wave function Quantum theory project
Quantum theory project Quantum theory of light
Quantum theory of light Quantum structure
Quantum structure Electronic colonialism theory
Electronic colonialism theory Slidetodoc. com
Slidetodoc. com Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Glasgow thang điểm
Glasgow thang điểm Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số.nguyên tố
Số.nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Relationship between atoms and molecules
Relationship between atoms and molecules Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Compound substance
Compound substance Counting atoms
Counting atoms Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Properties of atoms and the periodic table
Properties of atoms and the periodic table Mit center for bits and atoms
Mit center for bits and atoms Counting atoms and balancing equations
Counting atoms and balancing equations 3bacl2 counting atoms
3bacl2 counting atoms Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Atoms and radioactivity
Atoms and radioactivity Counting atoms and balancing equations worksheet
Counting atoms and balancing equations worksheet Atoms and their isotopes pogil
Atoms and their isotopes pogil Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Atoms molecules and ions
Atoms molecules and ions