Atoms and their Isotopes POGIL Activity Review Atoms

![Key Questions � 1. The atomic number [Z] tells the reader the number of Key Questions � 1. The atomic number [Z] tells the reader the number of](https://slidetodoc.com/presentation_image/1d02b199dc5102e0556fc0d3e5295292/image-3.jpg)





- Slides: 16

Atoms and their Isotopes POGIL Activity Review

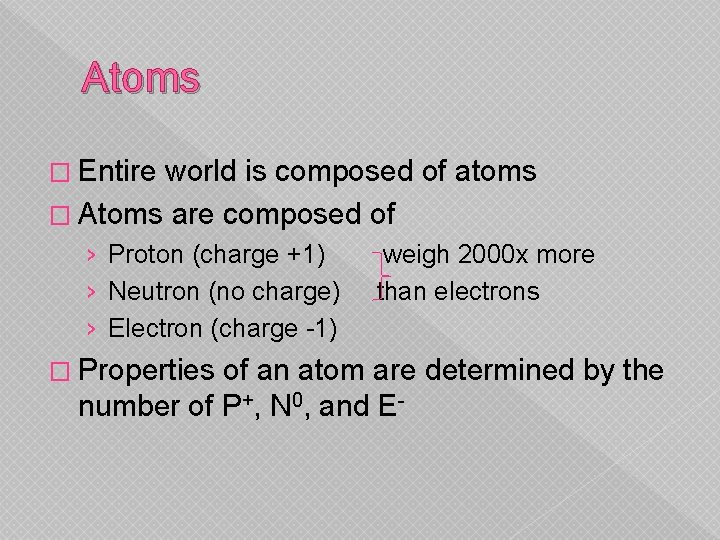
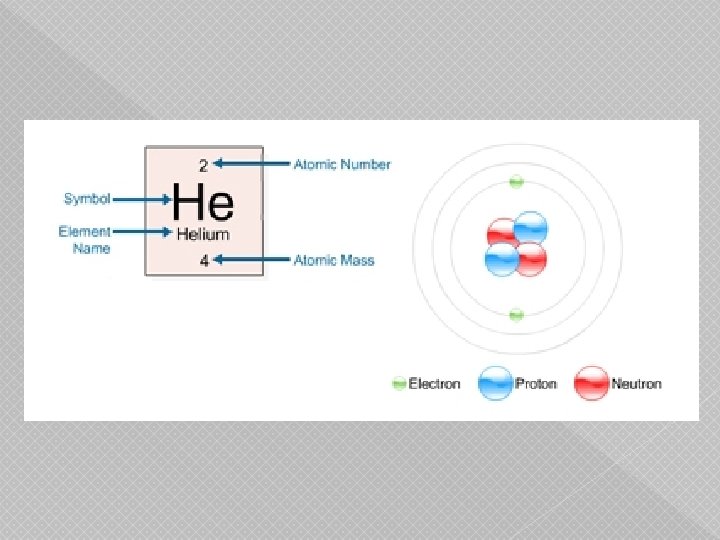
Atoms � Entire world is composed of atoms � Atoms are composed of › Proton (charge +1) › Neutron (no charge) › Electron (charge -1) � Properties weigh 2000 x more than electrons of an atom are determined by the number of P+, N 0, and E-
![Key Questions 1 The atomic number Z tells the reader the number of Key Questions � 1. The atomic number [Z] tells the reader the number of](https://slidetodoc.com/presentation_image/1d02b199dc5102e0556fc0d3e5295292/image-3.jpg)
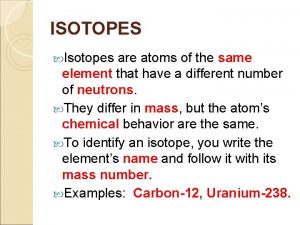
Key Questions � 1. The atomic number [Z] tells the reader the number of protons and electrons in a neutral atom. � 2. The mass number [A] tells the reader the number of protons and neutrons.



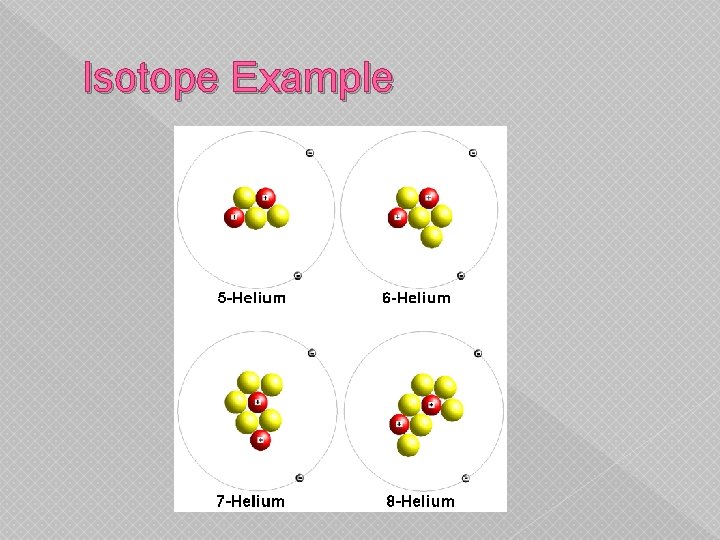
� 3. The number of protons is equal to the number of electrons. � 4. The electrical charge of an atom with an equal number of protons and electrons is neutral or no charge. � 5. The protons and neutrons are located in the nucleus of an atom. � 6. The two sodium isotopes have the same number of protons and electrons. � 7. The two sodium isotopes have different number of neutrons.

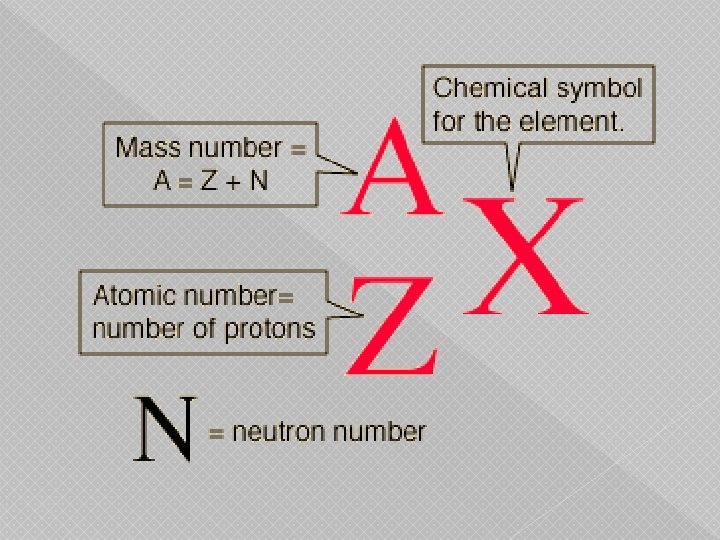
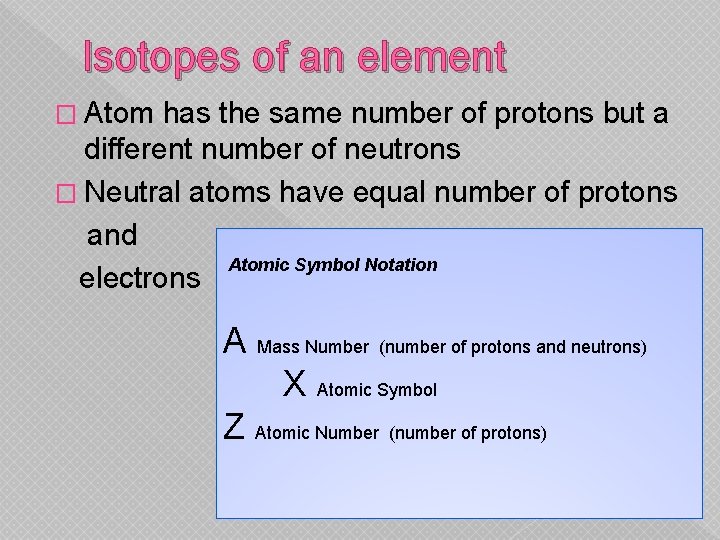
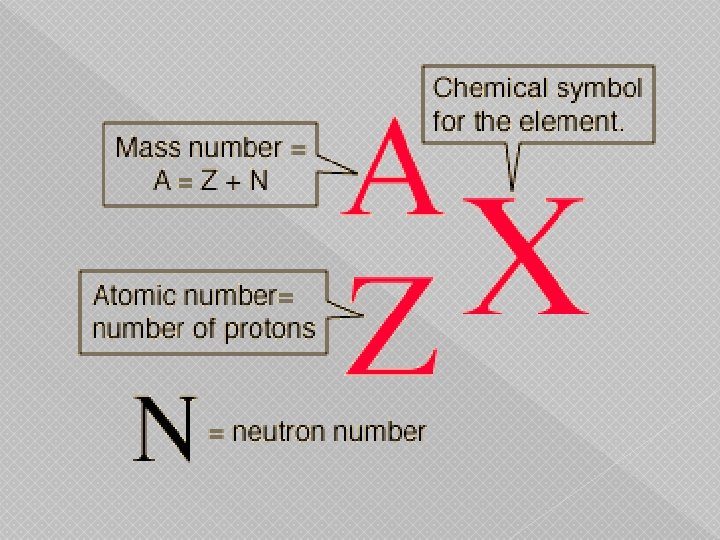
Isotopes of an element � Atom has the same number of protons but a different number of neutrons � Neutral atoms have equal number of protons and Atomic Symbol Notation electrons A Mass Number (number of protons and neutrons) X Atomic Symbol Z Atomic Number (number of protons)

Isotope Example

� 8. The following can distinguish an atom of one element from an atom of another element: › Mass the number of protons and electrons › Atomic number the number of neutrons

Exercises � 1. Similarities include: › They are the same element › They each have 17 protons › They each have 17 electrons � 2. Differences include: › They have different mass numbers. › Cl-35 has 18 neutrons › Cl-37 has 20 neutrons.

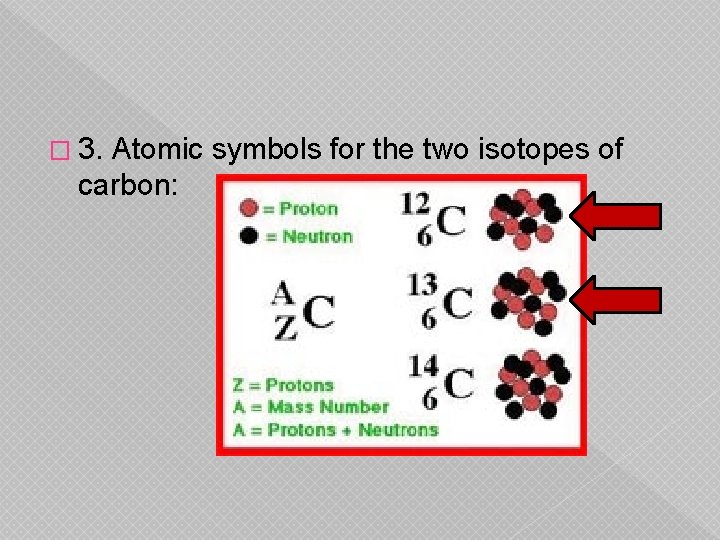
� 3. Atomic symbols for the two isotopes of carbon:


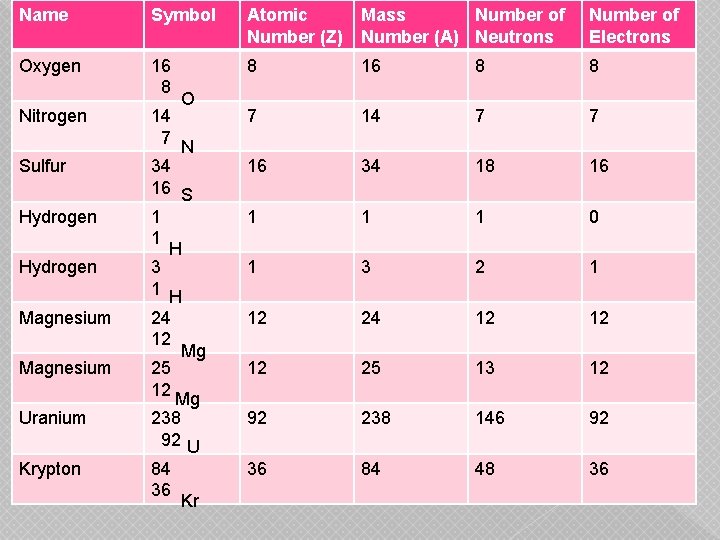
Name Symbol Atomic Number (Z) Mass Number of Number (A) Neutrons Number of Electrons Oxygen 16 8 8 7 14 7 7 34 16 S 1 1 H 3 1 H 16 34 18 16 1 1 1 0 1 3 2 1 24 12 12 12 25 13 12 92 238 146 92 36 84 48 36 Nitrogen Sulfur Hydrogen Magnesium Uranium Krypton 14 7 25 12 O N Mg Mg 238 92 U 84 36 Kr

Problems 1 fm = 1 x 10 -15 m 1 pm = 1 x 10 -12 m � 1. The radius of a Cl nucleus is 4. 0 fm, and the radius of a Cl atom is 100 pm. How many times larger is the diameter of the Cl atom than the diameter of a Cl nucleus? � 2. Two objects that have the same ratio of length.

Problems � 3. Volume = 4/3(pi)*r 3 How many times larger is the volume of the atom than the volume of the nucleus?

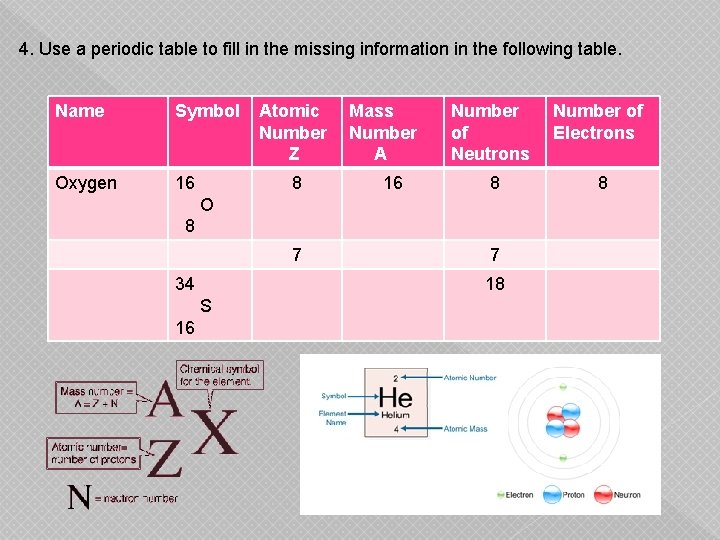
4. Use a periodic table to fill in the missing information in the following table. Name Symbol Oxygen 16 Atomic Number Z 8 Mass Number A 16 Number of Neutrons Number of Electrons 8 8 O 8 7 34 18 S 16 7
 Atoms and their isotopes pogil
Atoms and their isotopes pogil Electrons and light pogil activity 5-1
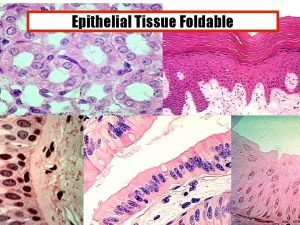
Electrons and light pogil activity 5-1 Histology of epithelial tissue pogil activity
Histology of epithelial tissue pogil activity Periodic table of elements regents
Periodic table of elements regents Isotopes stable and unstable
Isotopes stable and unstable Forms of dna
Forms of dna Significant zeros pogil answers
Significant zeros pogil answers Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Fertile isotopes
Fertile isotopes How to calculate average mass percent
How to calculate average mass percent How to calculate abundance in isotopes
How to calculate abundance in isotopes Atomic isotopes
Atomic isotopes Uses of radioactive isotopes
Uses of radioactive isotopes Rubidium has two common isotopes
Rubidium has two common isotopes Uses of radioactive isotopes in agriculture
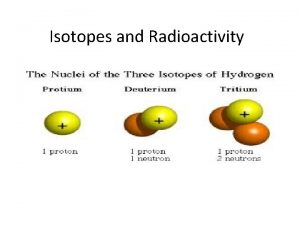
Uses of radioactive isotopes in agriculture Hyphen notation of the three isotopes of hydrogen
Hyphen notation of the three isotopes of hydrogen Mass number of chlorine
Mass number of chlorine