Chapter 2 Atoms Molecules Ions Atomic Theory Elements




























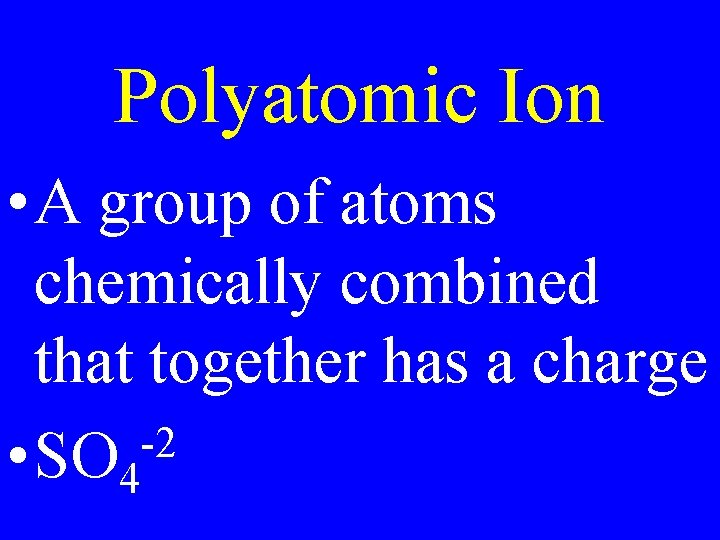







- Slides: 44

Chapter 2 • Atoms, Molecules, & Ions

Atomic Theory • Elements composed of atoms • Atoms can’t be changed • Compounds of multiple atoms • John Dalton

Conservation of Mass • In ordinary chemical reactions, matter can be neither created nor destroyed

Constant Composition • Compounds contain elements that are always in the same proportions

Multiple Proportions • The elements making up a compound will form whole number ratios

Atom • The smallest particle an element can be broken down into and still maintain the identity of the element

Nuclear Atom • Proved by Rutherford & Bohr in the famous gold foil experiments

Atomic Composition • Proton: in the nucleus • Neutron: in the nucleus • Electron: outside the nucleus

Proton • In Nucleus • +1 relative charge • About 1 amu in mass

Neutron • In nucleus • Neutral, zero charge • Mass is about 1 amu

Electron • Outside the nucleus • -1 in relative charge • Negligible mass about 1/2000 amu

Electron Charge • Determined by Robert Milliken in the famous oil droplet experiment

Atomic Number • The number of protons in an element • Z number

Mass Number • The number of protons and neutrons in an atom • A - number

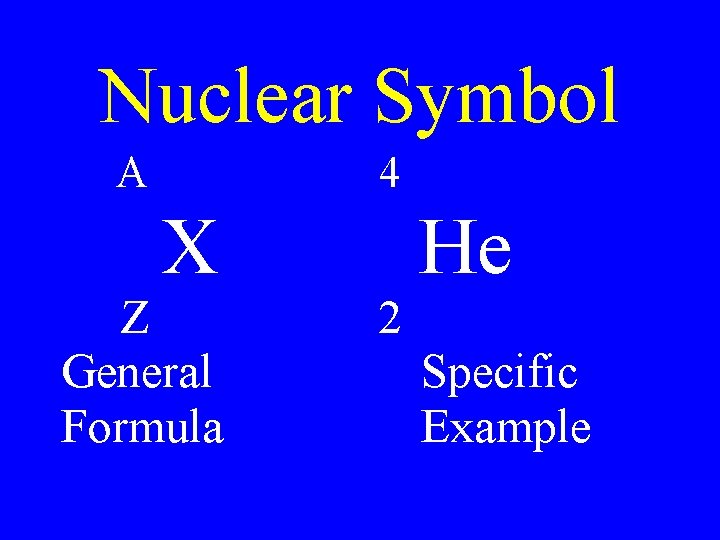
Nuclear Symbol A 4 X Z General Formula 2 He Specific Example

Isotopes • Atoms that contain the same number of protons, but different number of neutrons • Z constant, A variable

Isotopes are useful! • C 14 - dating • Isotopic-tracing

Shorthand for isotopes Instead of full notation: 14 C Write: C-14 6 So, only the Z number (protons + neutrons)

Atomic Mass • All elements have >1 isotope. • The percentage of one isotope is its relative abundance. • Atomic mass = (isotopic mass # 1 multiplied by % of isotope #1) + (isotopic mass # 2 multiplied by % of isotope # 2) + etc… • Usually about 3 or 4 isotopes for a given element is maximum.

Periodic Table • Graphic hierarchy of all the elements. • The order is used to predict size, charge, electronic structure, & reactivity of elements

Periods • Rows which indicate energy level or shell or size of the atoms

Groups or Families • Columns which indicate the number of electrons in the outermost energy level determining charge & reactivity

Metals • Left three quarters of the chart • Lose electrons • Become positive

Nonmetals • Upper right portion • Gain, lose, or share electrons when they react

Metalloids • Along the stair-stepped line from B to At • Share properties of metals & nonmetals

Radioactivity • The spontaneous breakdown of an unstable nucleus

Radioactive Decay • Alpha radiation • Beta radiation • Gamma radiation

Alpha Particle • Helium nucleus • 2 protons & two neutrons • mass = 4 & charge = +2 • Low penetrating power

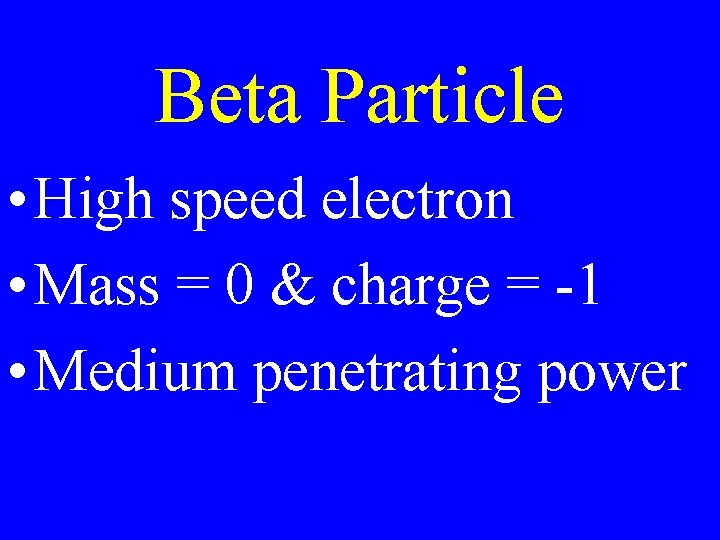
Beta Particle • High speed electron • Mass = 0 & charge = -1 • Medium penetrating power

Gamma Rays • High energy electromagnetic wave • No mass or charge • Very high penetrating power

Compound • A group of atoms that are chemically combined

Molecule • A compound that can exist by itself

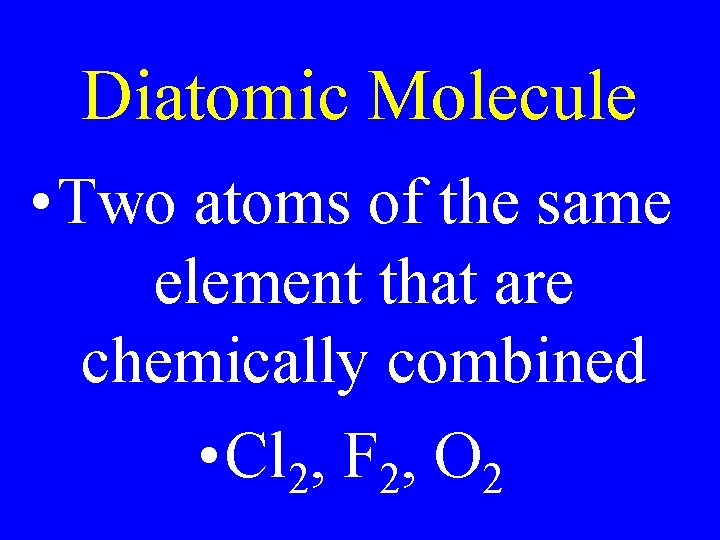
Diatomic Molecule • Two atoms of the same element that are chemically combined • Cl 2, F 2, O 2

Ion • Charged Particle -1 • Cl
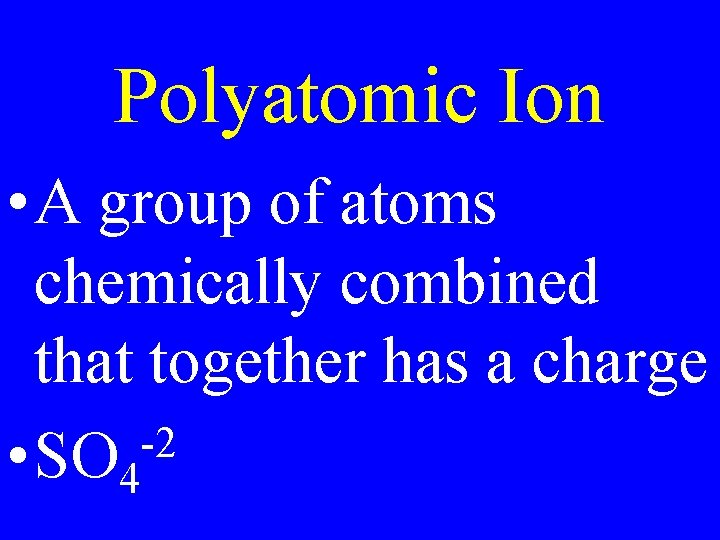
Polyatomic Ion • A group of atoms chemically combined that together has a charge -2 • SO 4

Binary Compound • A compound made up of two elements in any ratio • Na. Cl • Mg 3 P 2

Chemical Formula • A formula that shows the number and kinds of atoms in a compound • Ca. CO 3

Molecular Formula • A formula that shows the number and kinds of atoms in a molecule • C 6 H 12 O 6

Atomic Structure • List & describe three subatomic particles

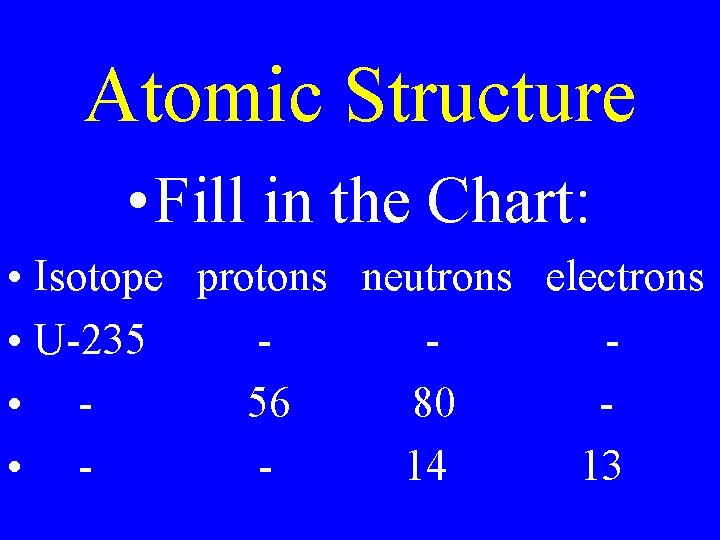
Atomic Structure • Fill in the Chart: • Isotope protons neutrons electrons • U-235 • 56 80 • 14 13

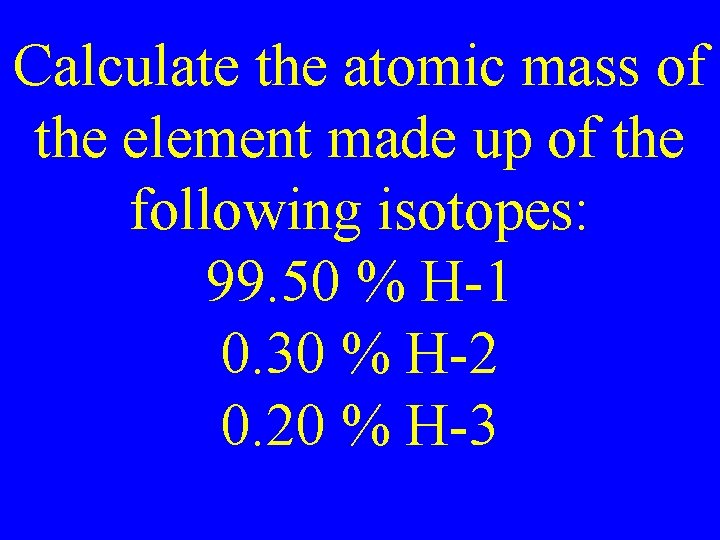
Calculate the atomic mass of the element made up of the following isotopes: 99. 50 % H-1 0. 30 % H-2 0. 20 % H-3

Calculate Pt’s atomic mass : 5. 0 % Pu-242 5. 0 % Pu-243 80. 0 % Pu-244 10. 0 % Pu-245

Determine the number of atoms in each compound • C 6 H 12 O 6 Na. NO 3 • H 3 PO 4 Al 2(SO 4)3

List & describe each of the three types of radiation