Ions and Ionic Bonding Ions Ions are atoms

Ions and Ionic Bonding

Ions • Ions are atoms or groups of atoms with electrical charges, • There are positive ions or cations, these elements are commonly called metals. • There are negative ions or anions, these elements are commonly called non-metals. • Atoms become ions by losing or gaining electrons.

Where does the Charge Come From? • Lets take Sodium…. • It has 11 positive protons and 11 negative electrons. • These charges cancel each other out and we say that a sodium atom is neutral. • The outer shell of a sodium atom holds only 1 electron, this is not a stable arrangement. • Every atom “prefers” to have a full set of electrons in its outer shell

Where does the Charge Come From? • During chemical reactions, some atoms lose their outer electrons, while others gain one or more electrons to get a full set of electrons in their outer shell. • Atoms with 1, 2 or 3 electrons in their outer shell tend to lose their electrons to become positive ions or cations • Atoms with 5, 6 or 7 electrons in their outer shell tend to gain electrons to become negative ions or anions
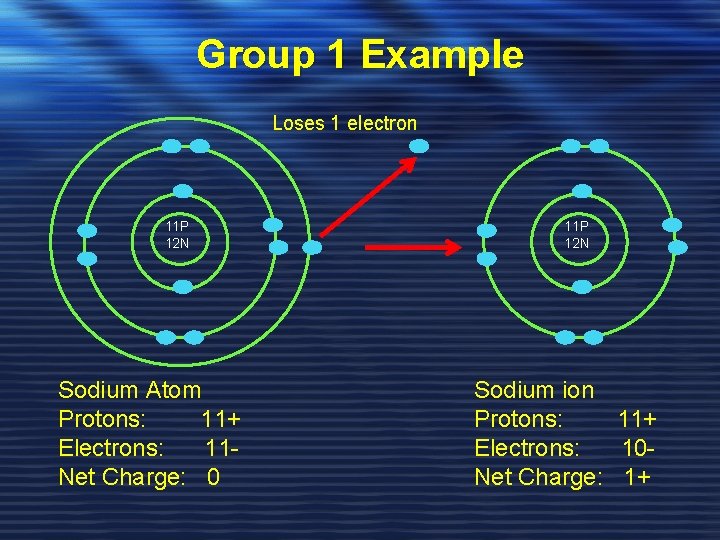
Group 1 Example Loses 1 electron 11 P 12 N Sodium Atom Protons: 11+ Electrons: 11 Net Charge: 0 11 P 12 N Sodium ion Protons: 11+ Electrons: 10 Net Charge: 1+
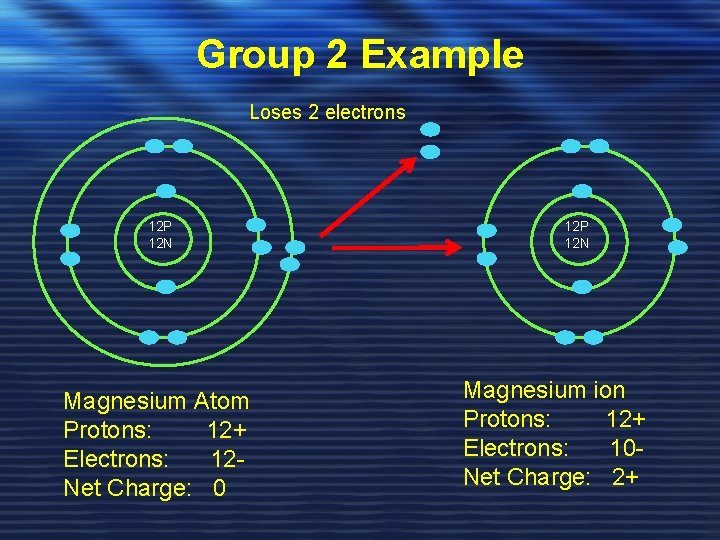
Group 2 Example Loses 2 electrons 12 P 12 N Magnesium Atom Protons: 12+ Electrons: 12 Net Charge: 0 12 P 12 N Magnesium ion Protons: 12+ Electrons: 10 Net Charge: 2+
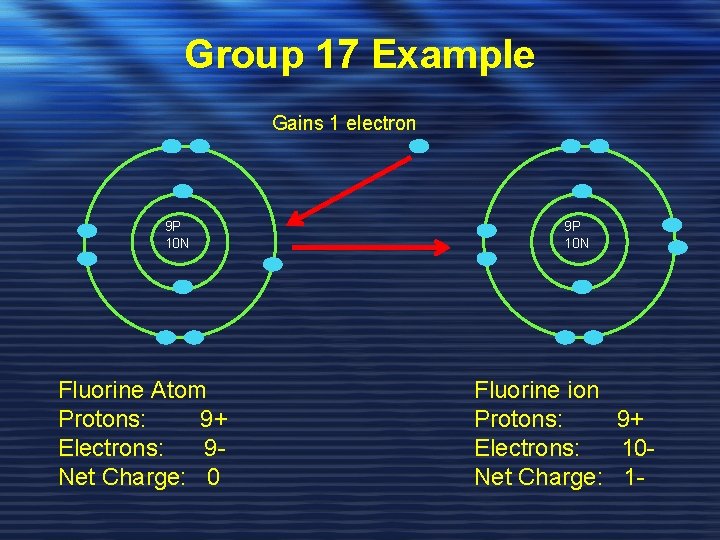
Group 17 Example Gains 1 electron 9 P 10 N Fluorine Atom Protons: 9+ Electrons: 9 Net Charge: 0 9 P 10 N Fluorine ion Protons: 9+ Electrons: 10 Net Charge: 1 -
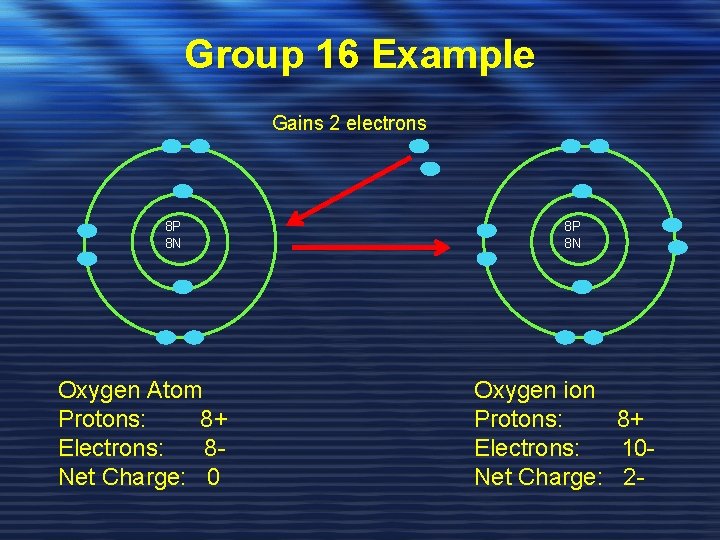
Group 16 Example Gains 2 electrons 8 P 8 N Oxygen Atom Protons: 8+ Electrons: 8 Net Charge: 0 8 P 8 N Oxygen ion Protons: 8+ Electrons: 10 Net Charge: 2 -

Ion Formation • When non-metal atoms become negative ions, their names change. • Chlorine atoms become chloride ions • Bromine atoms become bromide ions • Group 1 metals become +1 ions • Group 2 metals become +2 ions • Group 3/13 elements become +3 ions • Group 7/17 elements become -1 ions • Group 6/16 elements become -2 ions • Group 5/15 elements become -3 ions

Transition Metals and Compound Ions • Transition elements can form more than 1 type of ion. • You need to learn these ones. • Compound ions contain more than one type of atom chemically joined.
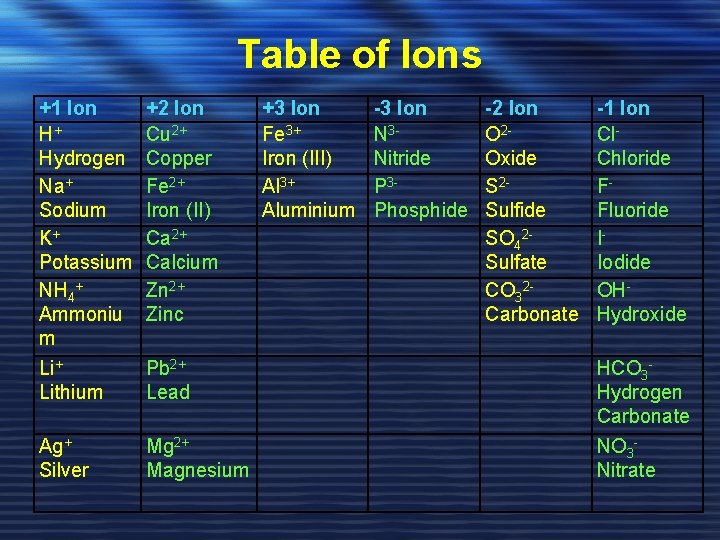
Table of Ions +1 Ion H+ Hydrogen Na+ Sodium K+ Potassium NH 4+ Ammoniu m +2 Ion Cu 2+ Copper Fe 2+ Iron (II) Ca 2+ Calcium Zn 2+ Zinc +3 Ion Fe 3+ Iron (III) Al 3+ Aluminium -3 Ion N 3 Nitride P 3 Phosphide -2 Ion O 2 Oxide S 2 Sulfide SO 42 Sulfate CO 32 Carbonate -1 Ion Cl. Chloride FFluoride IIodide OHHydroxide Li+ Lithium Pb 2+ Lead HCO 3 Hydrogen Carbonate Ag+ Silver Mg 2+ Magnesium NO 3 Nitrate
- Slides: 11