2 1 Atoms Ions and Molecules KEY CONCEPT









- Slides: 43

2. 1 Atoms, Ions, and Molecules KEY CONCEPT All living things are based on atoms and their interactions.

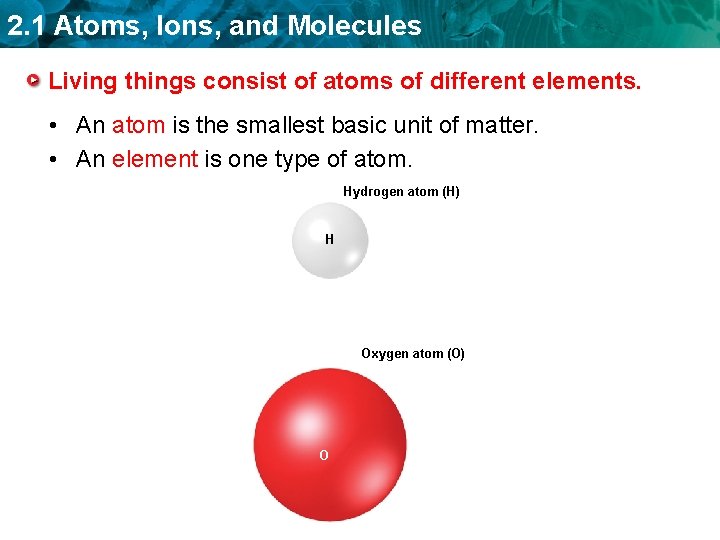
2. 1 Atoms, Ions, and Molecules Living things consist of atoms of different elements. • An atom is the smallest basic unit of matter. • An element is one type of atom. Hydrogen atom (H) H Oxygen atom (O) O

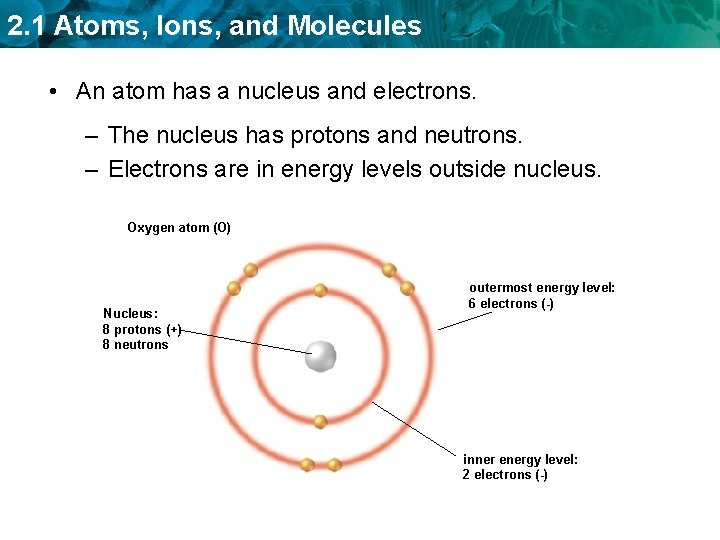
2. 1 Atoms, Ions, and Molecules • An atom has a nucleus and electrons. – The nucleus has protons and neutrons. – Electrons are in energy levels outside nucleus. Oxygen atom (O) Nucleus: 8 protons (+) 8 neutrons outermost energy level: 6 electrons (-) inner energy level: 2 electrons (-)

2. 1 Atoms, Ions, and Molecules • A compound is made of atoms of different elements bonded together. – water (H 2 O) – carbon dioxide (CO 2) – many other carbon-based compounds in living things

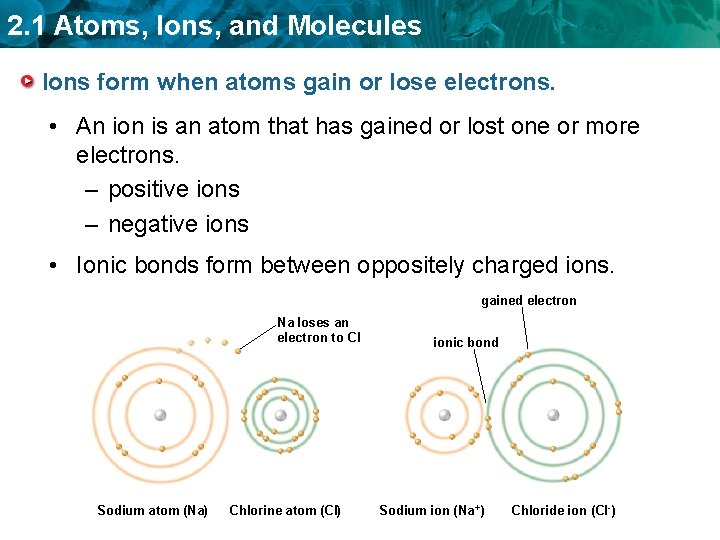
2. 1 Atoms, Ions, and Molecules Ions form when atoms gain or lose electrons. • An ion is an atom that has gained or lost one or more electrons. – positive ions – negative ions • Ionic bonds form between oppositely charged ions. gained electron Na loses an electron to CI Sodium atom (Na) Chlorine atom (CI) ionic bond Sodium ion (Na +) Chloride ion (CI -)

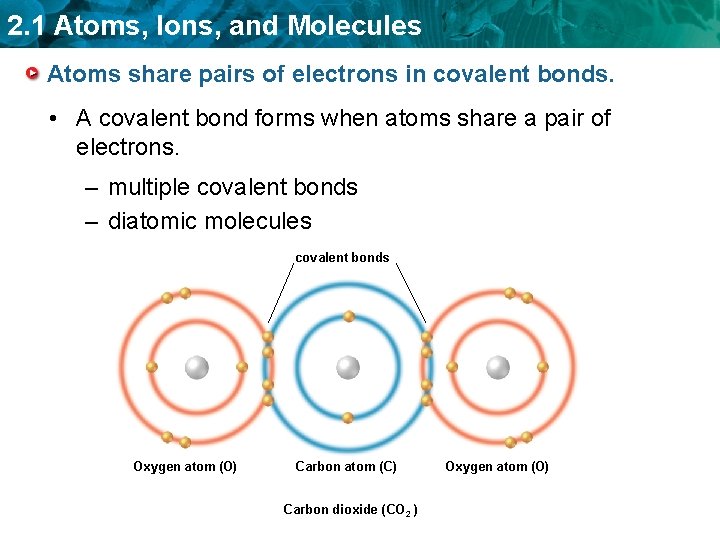
2. 1 Atoms, Ions, and Molecules Atoms share pairs of electrons in covalent bonds. • A covalent bond forms when atoms share a pair of electrons. – multiple covalent bonds – diatomic molecules covalent bonds Oxygen atom (O) Carbon atom (C) Carbon dioxide (CO 2 ) Oxygen atom (O)

2. 2 Properties of Water KEY CONCEPT Water’s unique properties allow life to exist on Earth.

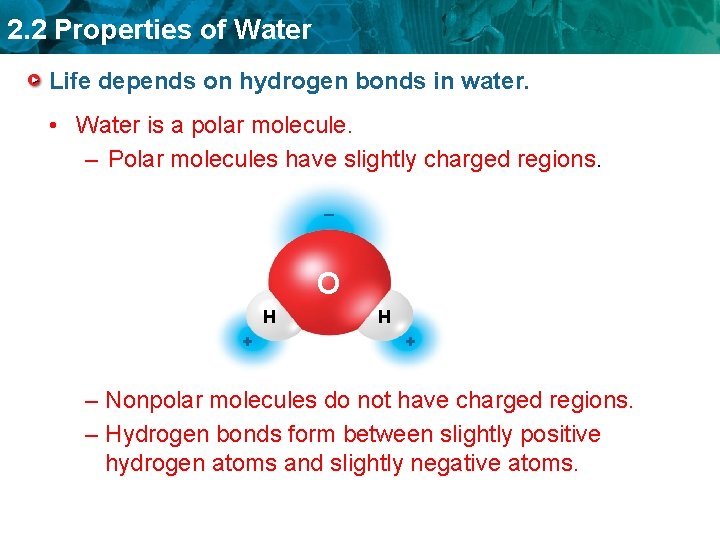
2. 2 Properties of Water Life depends on hydrogen bonds in water. • Water is a polar molecule. – Polar molecules have slightly charged regions. _ O H + – Nonpolar molecules do not have charged regions. – Hydrogen bonds form between slightly positive hydrogen atoms and slightly negative atoms.

2. 2 Properties of Water • Hydrogen bonds are responsible for three important properties of water. – high specific heat – cohesion – adhesion

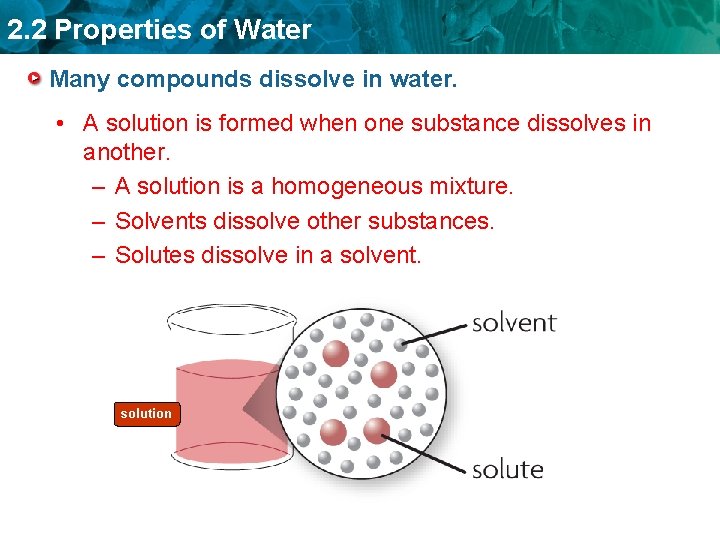
2. 2 Properties of Water Many compounds dissolve in water. • A solution is formed when one substance dissolves in another. – A solution is a homogeneous mixture. – Solvents dissolve other substances. – Solutes dissolve in a solvent. solution

2. 2 Properties of Water • “Like dissolves like. ” – Polar solvents dissolve polar solutes. – Nonpolar solvents dissolve nonpolar solutes. – Polar substances and nonpolar substances generally remain separate.

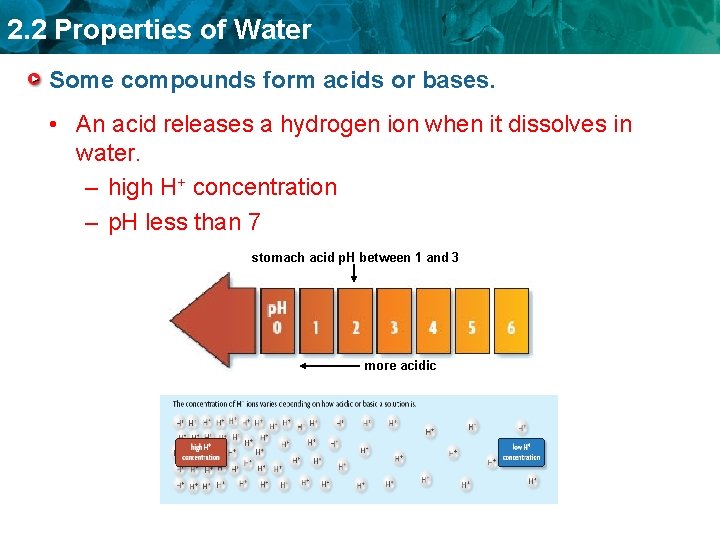
2. 2 Properties of Water Some compounds form acids or bases. • An acid releases a hydrogen ion when it dissolves in water. – high H+ concentration – p. H less than 7 stomach acid p. H between 1 and 3 more acidic

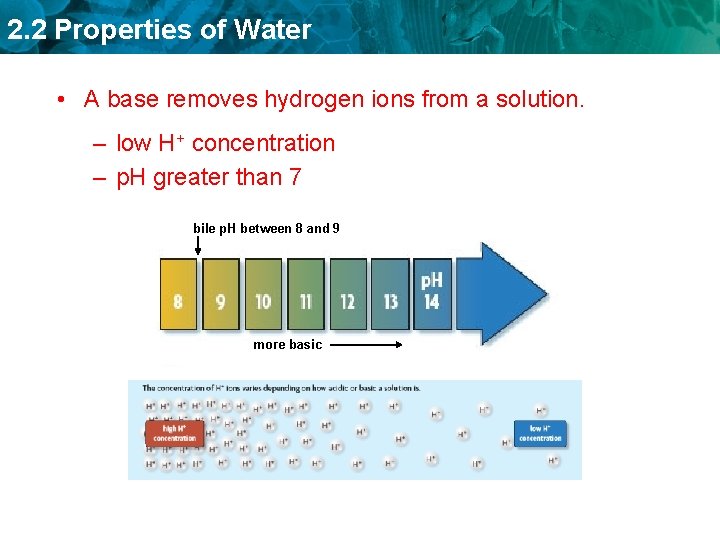
2. 2 Properties of Water • A base removes hydrogen ions from a solution. – low H+ concentration – p. H greater than 7 bile p. H between 8 and 9 more basic

2. 2 Properties of Water • A neutral solution has a p. H of 7. pure water p. H 7

2. 3 Carbon-Based Molecules KEY CONCEPT Carbon-based molecules are the foundation of life.

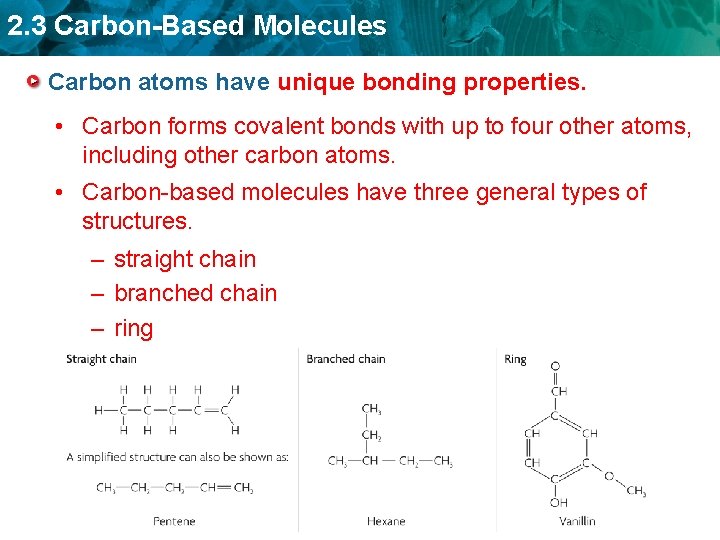
2. 3 Carbon-Based Molecules Carbon atoms have unique bonding properties. • Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. • Carbon-based molecules have three general types of structures. – straight chain – branched chain – ring

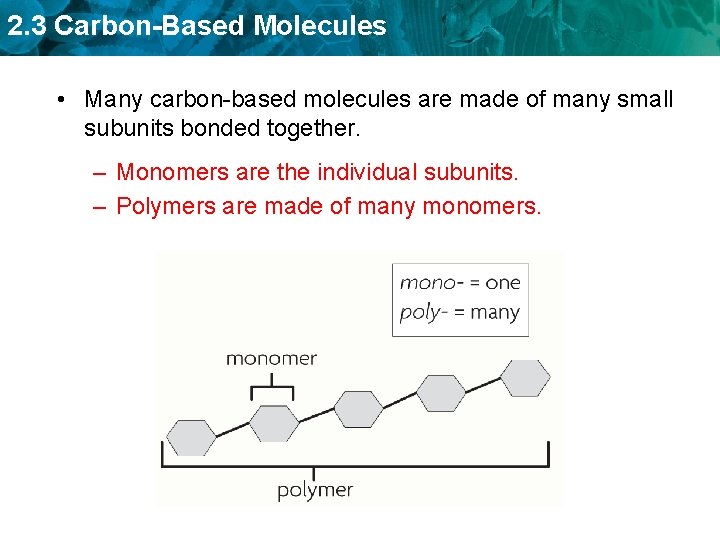
2. 3 Carbon-Based Molecules • Many carbon-based molecules are made of many small subunits bonded together. – Monomers are the individual subunits. – Polymers are made of many monomers.

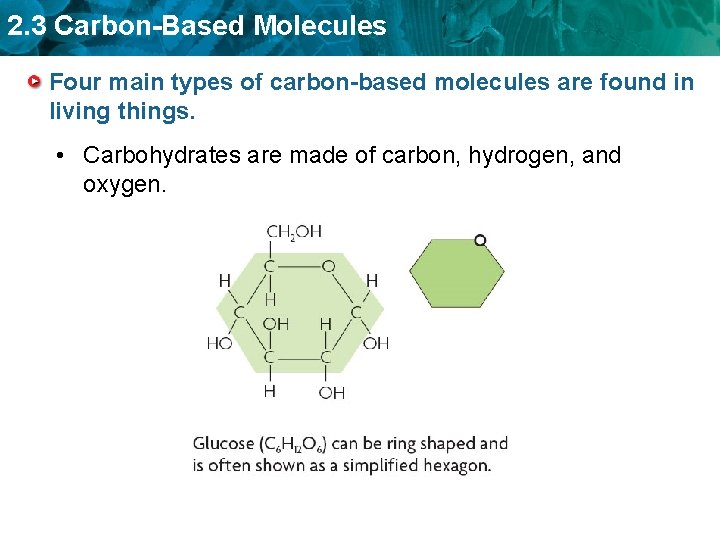
2. 3 Carbon-Based Molecules Four main types of carbon-based molecules are found in living things. • Carbohydrates are made of carbon, hydrogen, and oxygen.

2. 3 Carbon-Based Molecules Four main types of carbon-based molecules are found in living things. • Carbohydrates are made of carbon, hydrogen, and oxygen. – Carbohydrates include sugars and starches. – Monosaccharides are simple sugars. – Polysaccharides include starches, cellulose, and glycogen.

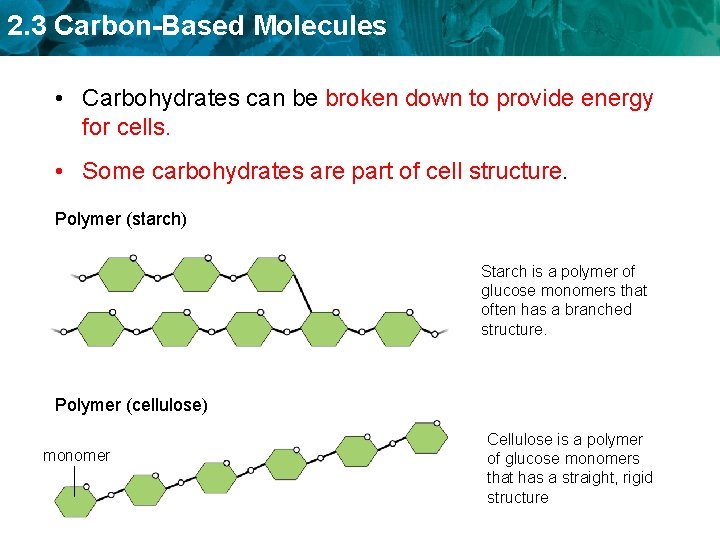
2. 3 Carbon-Based Molecules • Carbohydrates can be broken down to provide energy for cells. • Some carbohydrates are part of cell structure. Polymer (starch) Starch is a polymer of glucose monomers that often has a branched structure. Polymer (cellulose) monomer Cellulose is a polymer of glucose monomers that has a straight, rigid structure

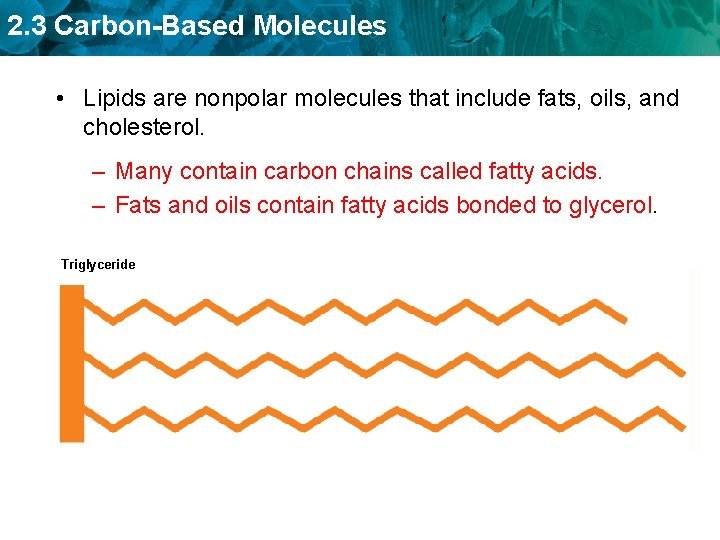
2. 3 Carbon-Based Molecules • Lipids are nonpolar molecules that include fats, oils, and cholesterol. – Many contain carbon chains called fatty acids. – Fats and oils contain fatty acids bonded to glycerol. Triglyceride

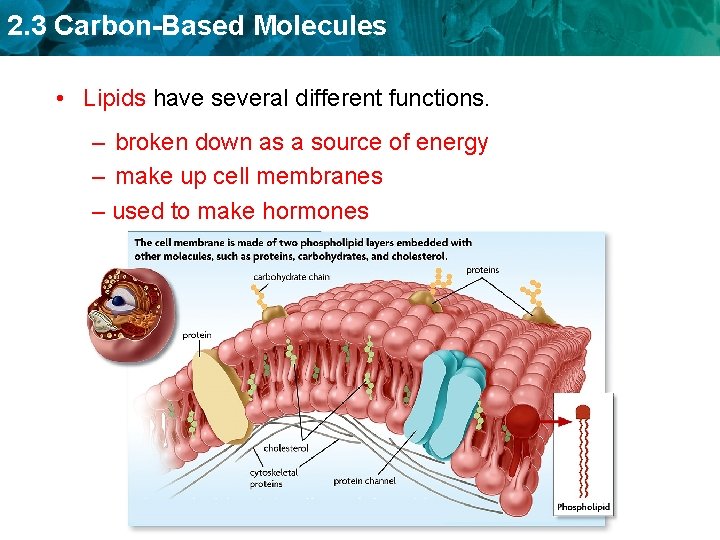
2. 3 Carbon-Based Molecules • Lipids have several different functions. – broken down as a source of energy – make up cell membranes – used to make hormones

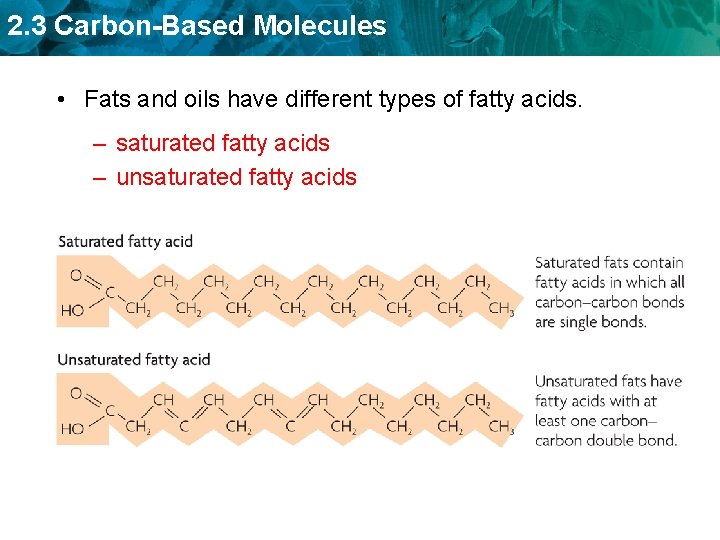
2. 3 Carbon-Based Molecules • Fats and oils have different types of fatty acids. – saturated fatty acids – unsaturated fatty acids

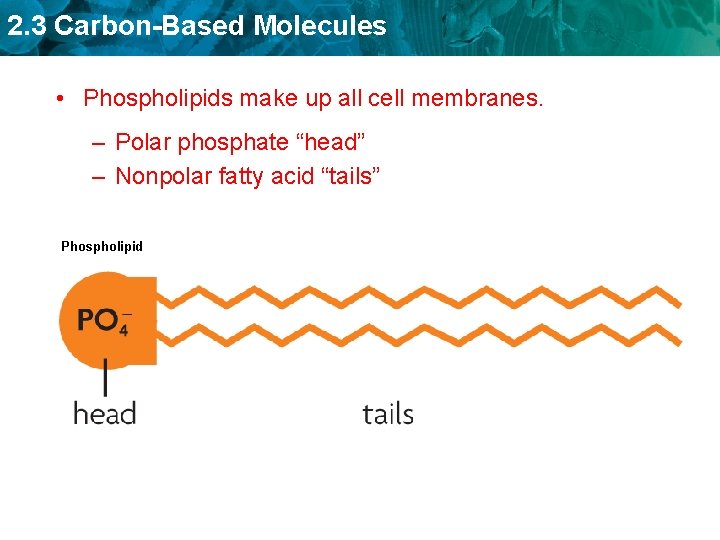
2. 3 Carbon-Based Molecules • Phospholipids make up all cell membranes. – Polar phosphate “head” – Nonpolar fatty acid “tails” Phospholipid

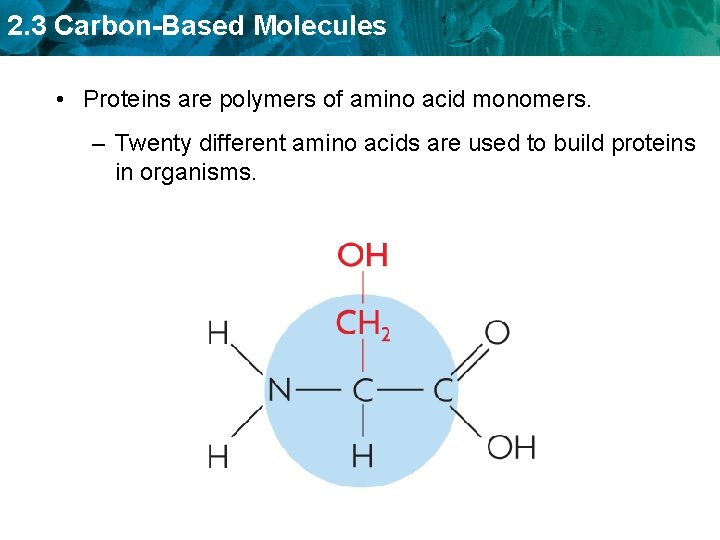
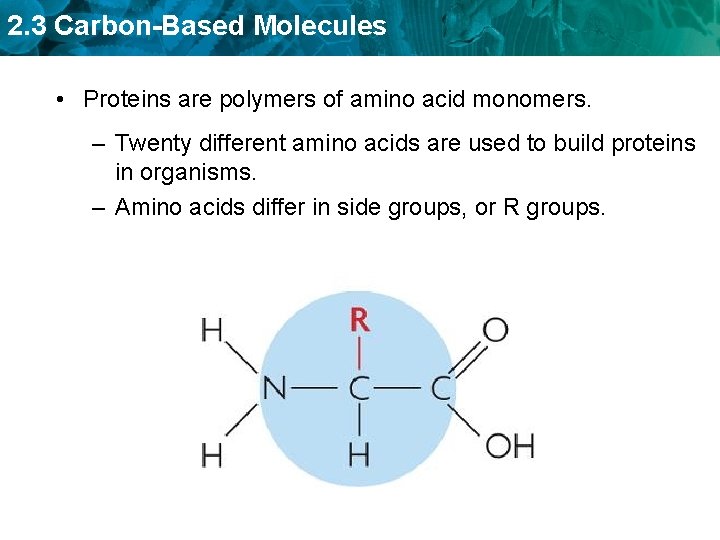
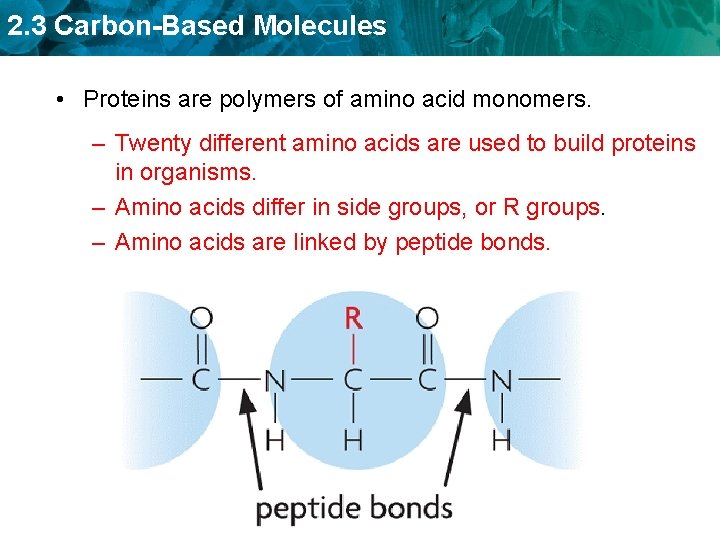
2. 3 Carbon-Based Molecules • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms.

2. 3 Carbon-Based Molecules • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms. – Amino acids differ in side groups, or R groups.

2. 3 Carbon-Based Molecules • Proteins are polymers of amino acid monomers. – Twenty different amino acids are used to build proteins in organisms. – Amino acids differ in side groups, or R groups. – Amino acids are linked by peptide bonds.

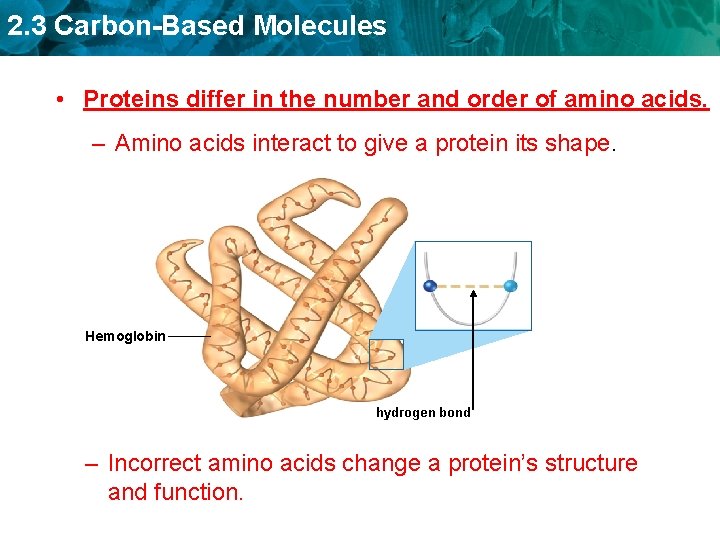
2. 3 Carbon-Based Molecules • Proteins differ in the number and order of amino acids. – Amino acids interact to give a protein its shape. Hemoglobin hydrogen bond – Incorrect amino acids change a protein’s structure and function.

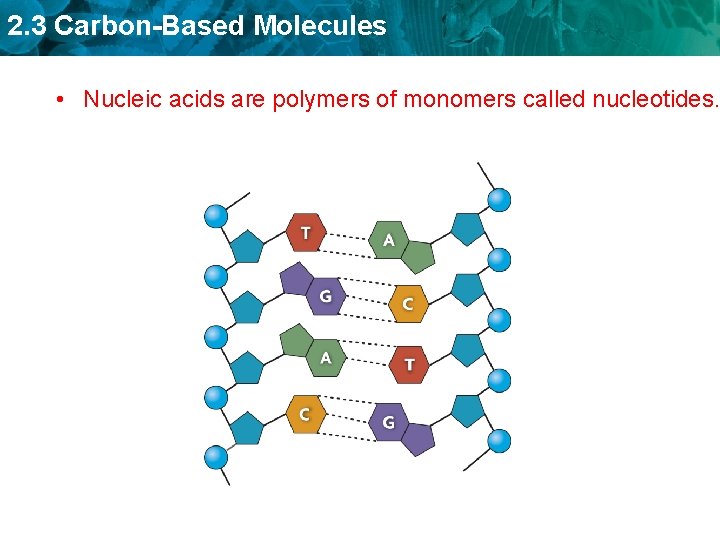
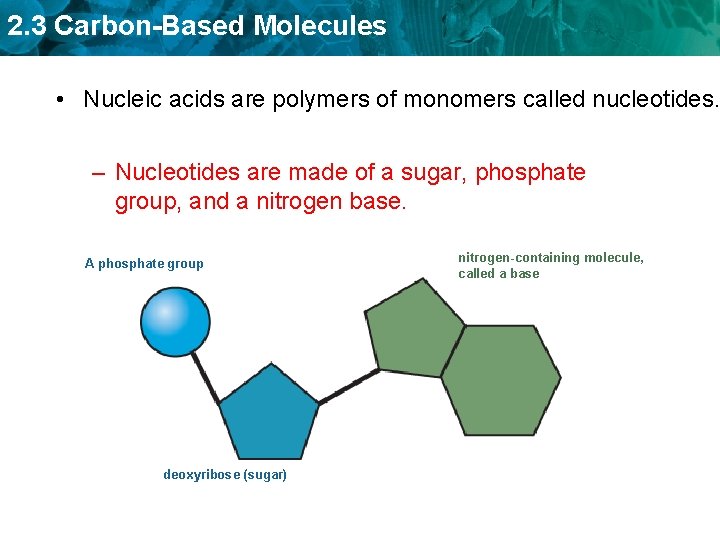
2. 3 Carbon-Based Molecules • Nucleic acids are polymers of monomers called nucleotides.

2. 3 Carbon-Based Molecules • Nucleic acids are polymers of monomers called nucleotides. – Nucleotides are made of a sugar, phosphate group, and a nitrogen base. A phosphate group deoxyribose (sugar) nitrogen-containing molecule, called a base

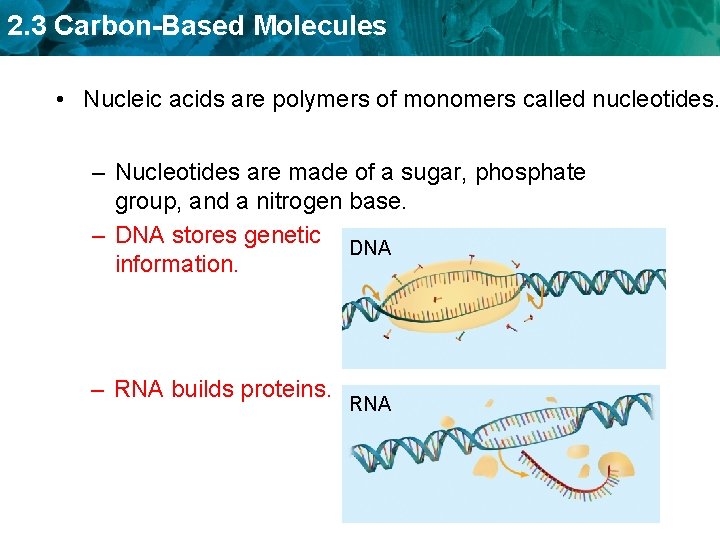
2. 3 Carbon-Based Molecules • Nucleic acids are polymers of monomers called nucleotides. – Nucleotides are made of a sugar, phosphate group, and a nitrogen base. – DNA stores genetic DNA information. – RNA builds proteins. RNA

2. 4 Chemical Reactions KEY CONCEPT Life depends on chemical reactions.

2. 4 Chemical Reactions Bonds break and form during chemical reactions. • Chemical reactions change substances into different ones by breaking and forming chemical bonds. – Reactants are changed during a chemical reaction. – Products are made by a chemical reaction.

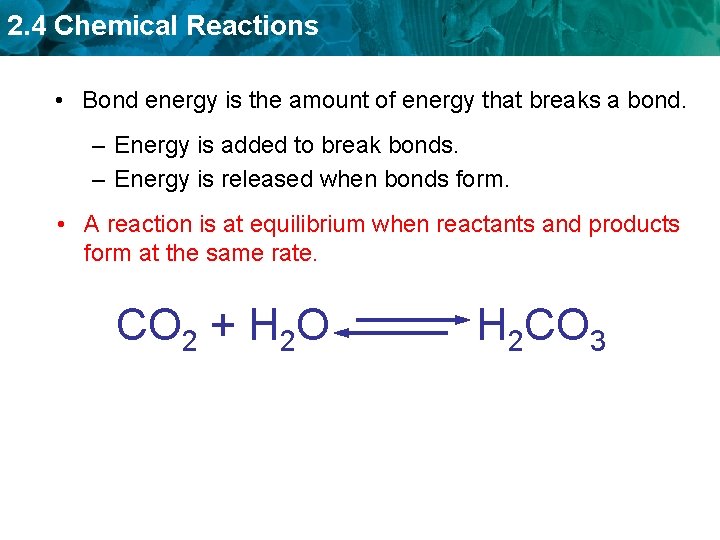
2. 4 Chemical Reactions • Bond energy is the amount of energy that breaks a bond. – Energy is added to break bonds. – Energy is released when bonds form. • A reaction is at equilibrium when reactants and products form at the same rate. CO 2 + H 2 O H 2 CO 3

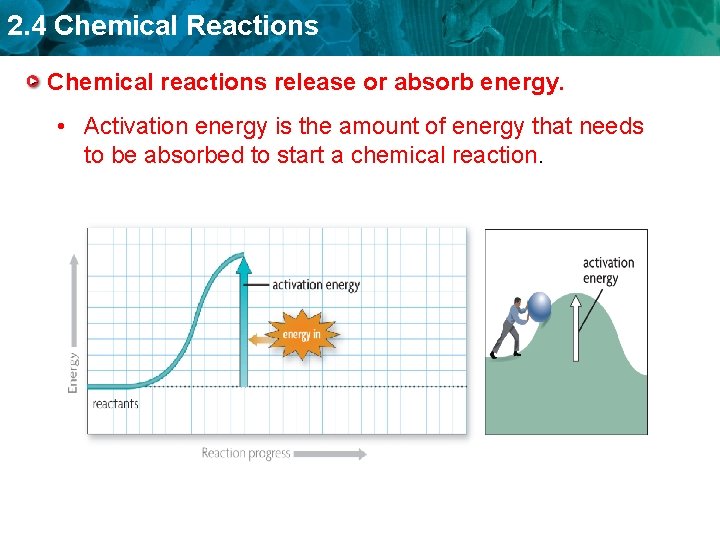
2. 4 Chemical Reactions Chemical reactions release or absorb energy. • Activation energy is the amount of energy that needs to be absorbed to start a chemical reaction.

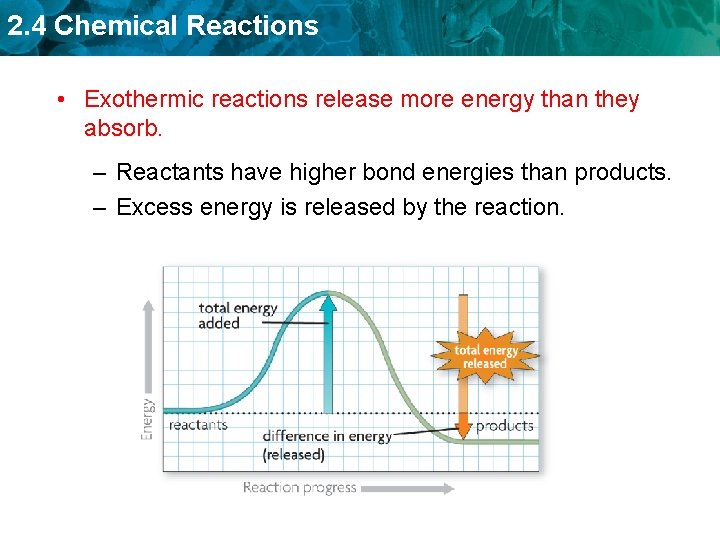
2. 4 Chemical Reactions • Exothermic reactions release more energy than they absorb. – Reactants have higher bond energies than products. – Excess energy is released by the reaction.

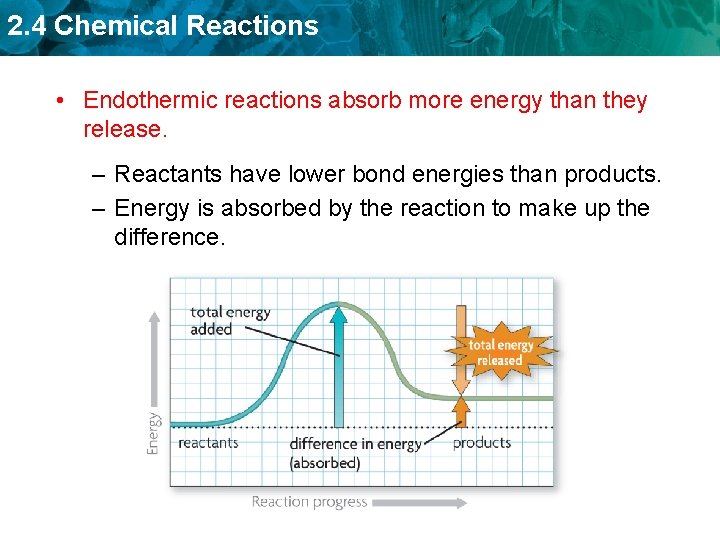
2. 4 Chemical Reactions • Endothermic reactions absorb more energy than they release. – Reactants have lower bond energies than products. – Energy is absorbed by the reaction to make up the difference.

2. 5 Enzymes KEY CONCEPT Enzymes are catalysts for chemical reactions in living things.

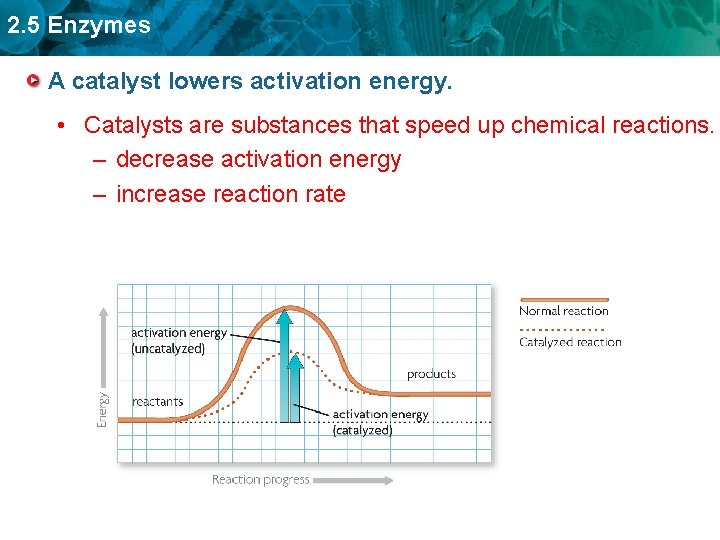
2. 5 Enzymes A catalyst lowers activation energy. • Catalysts are substances that speed up chemical reactions. – decrease activation energy – increase reaction rate

2. 5 Enzymes allow chemical reactions to occur under tightly controlled conditions. • Enzymes are catalysts in living things. – Enzymes are needed for almost all processes. – Most enzymes are proteins.

2. 5 Enzymes • Disruptions in homeostasis can prevent enzymes from functioning. – Enzymes function best in a small range of conditions. – Changes in temperature and p. H can break hydrogen bonds. – An enzyme’s function depends on its structure.

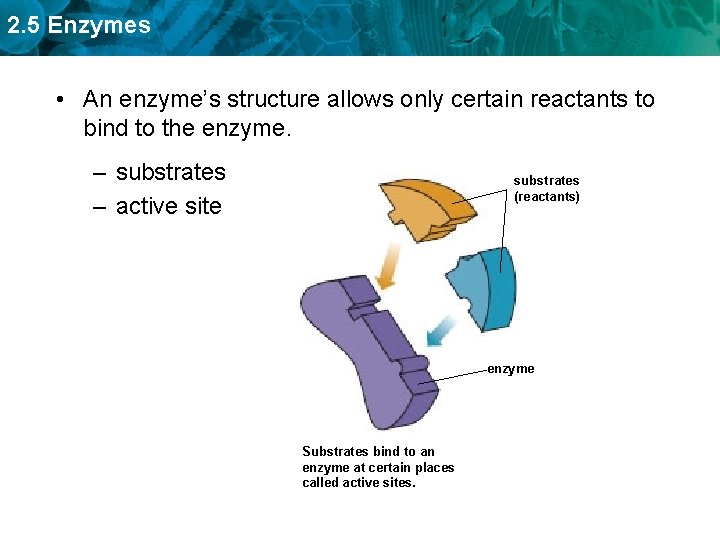
2. 5 Enzymes • An enzyme’s structure allows only certain reactants to bind to the enzyme. – substrates – active site substrates (reactants) enzyme Substrates bind to an enzyme at certain places called active sites.

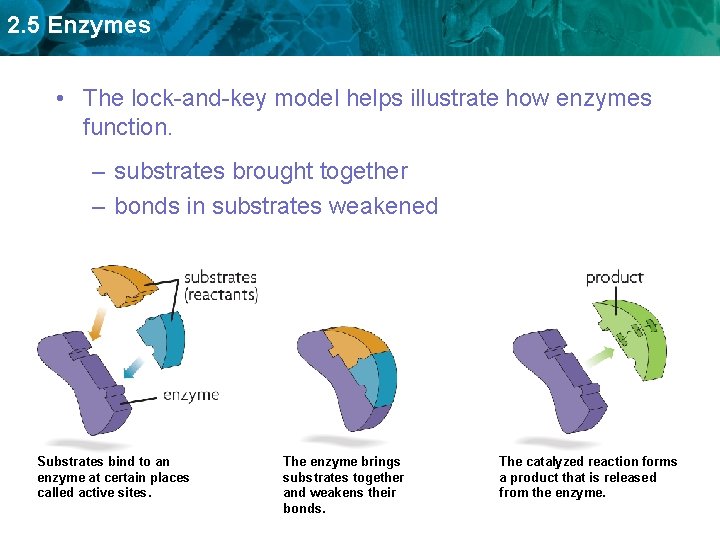
2. 5 Enzymes • The lock-and-key model helps illustrate how enzymes function. – substrates brought together – bonds in substrates weakened Substrates bind to an enzyme at certain places called active sites. The enzyme brings substrates together and weakens their bonds. The catalyzed reaction forms a product that is released from the enzyme.