WHY Why are gases gas at room temp












- Slides: 29

WHY? • Why are gases gas at room temp and not liquid or solid? • Why are liquids liquid and not gases or solid? • Why are solids solid and not gases or liquid?

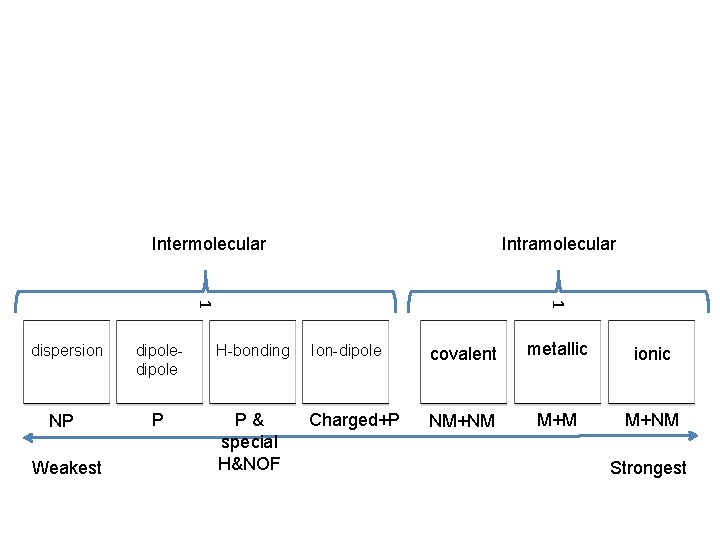
Forces of Attraction Inter vs. Intra


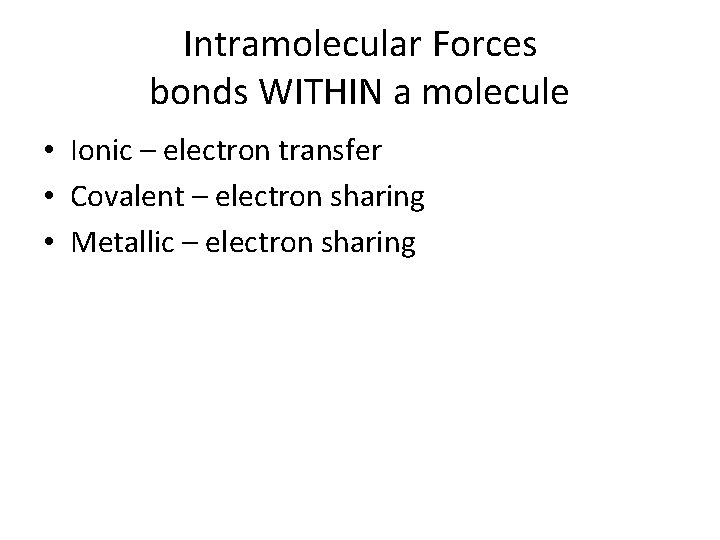
Intramolecular Forces bonds WITHIN a molecule • Ionic – electron transfer • Covalent – electron sharing • Metallic – electron sharing


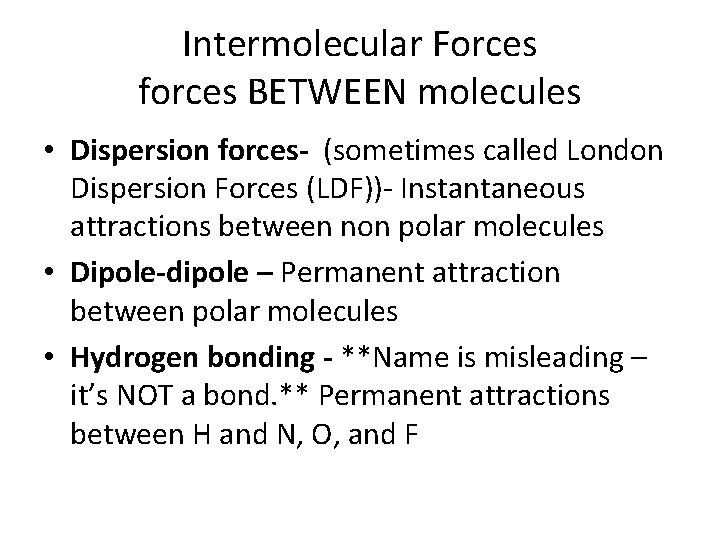
Intermolecular Forces forces BETWEEN molecules • Dispersion forces- (sometimes called London Dispersion Forces (LDF))- Instantaneous attractions between non polar molecules • Dipole-dipole – Permanent attraction between polar molecules • Hydrogen bonding - **Name is misleading – it’s NOT a bond. ** Permanent attractions between H and N, O, and F

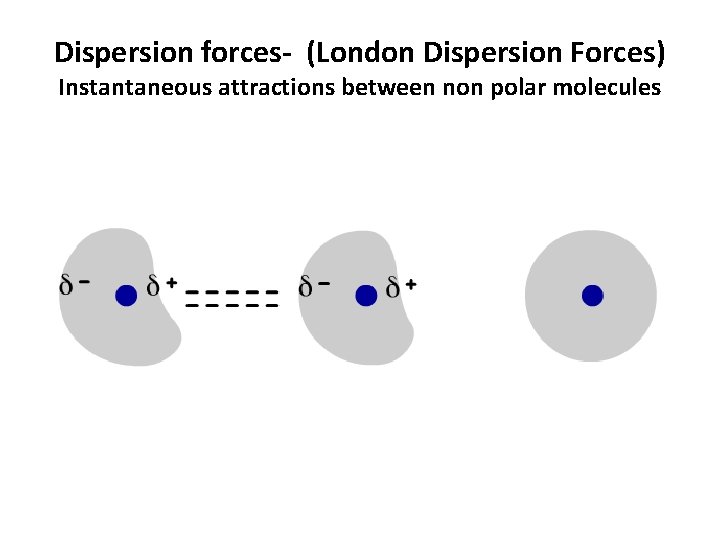
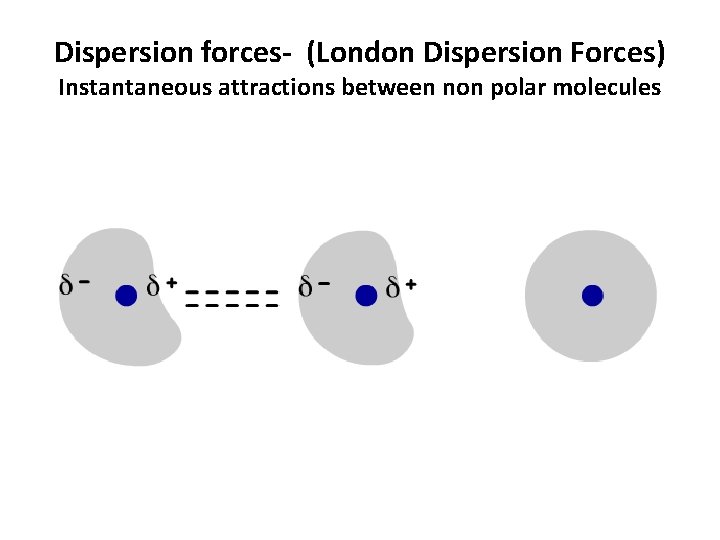
Dispersion forces- (London Dispersion Forces) Instantaneous attractions between non polar molecules

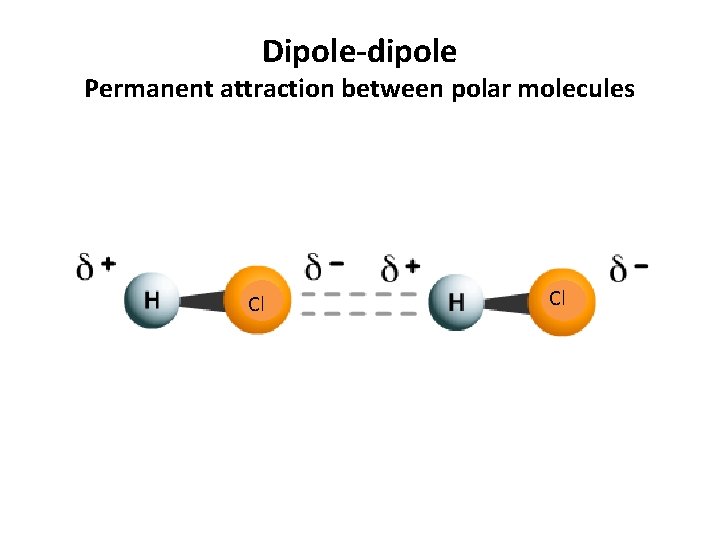
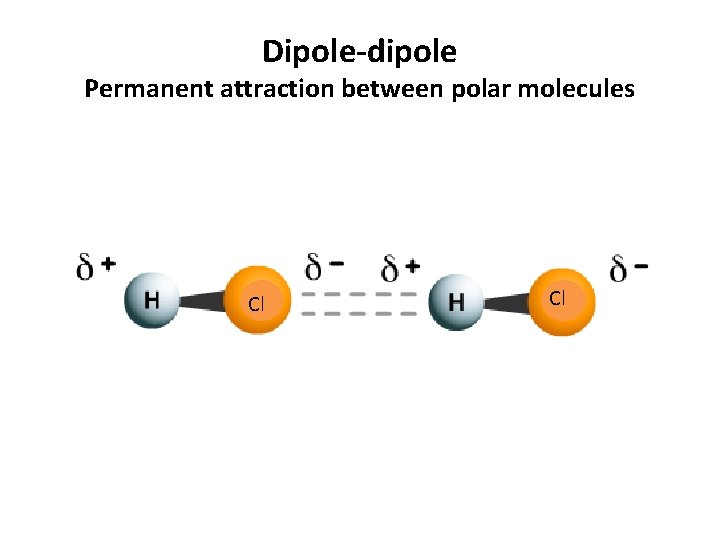
Dipole-dipole Permanent attraction between polar molecules Cl Cl

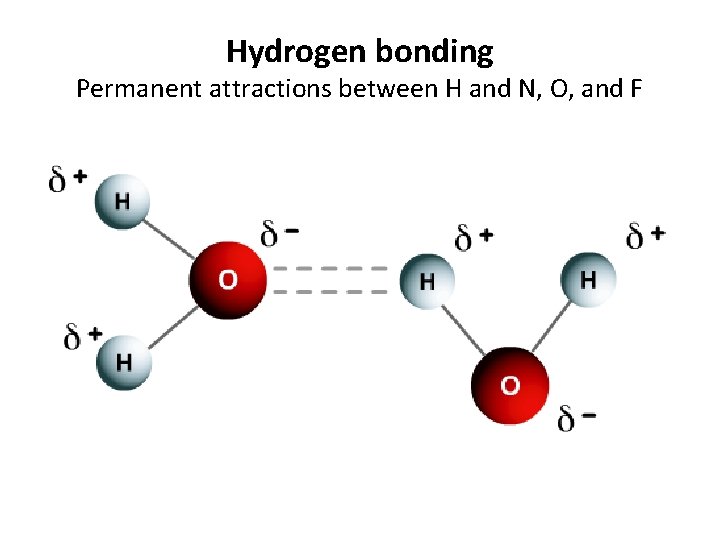
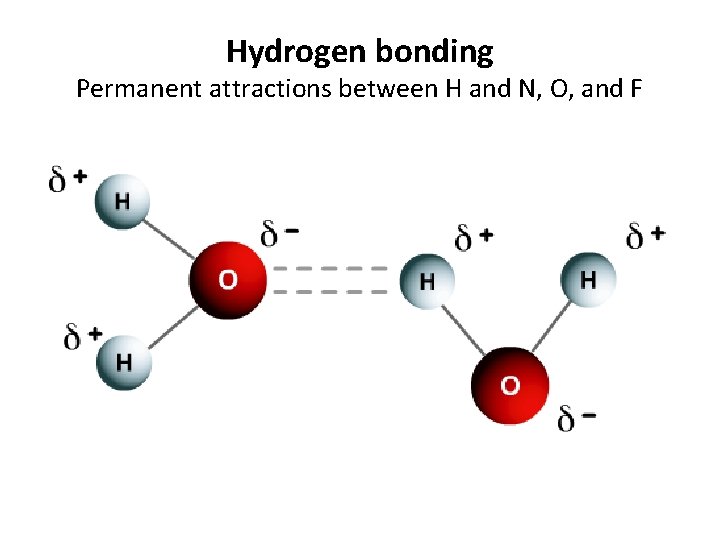
Hydrogen bonding Permanent attractions between H and N, O, and F

Dispersion forces- (London Dispersion Forces) Instantaneous attractions between non polar molecules

Dispersion forces- (induced dipole-induced dipole) Instantaneous attractions between non polar molecules • Formation: Electron clouds shift to form egg-shaped clouds with partially positive and partially negative ends. The clouds only form for an instant before rearranging. Thus, they are called instantaneous dipoles. Dispersion forces are formed by the brief attraction between oppositely charged ends of these dipoles, before they disappear. • Occurs in: ALL particles • Strength: Strength increases as mass increases. When mass increases, there are more electrons, which results in a larger dipole and consequently a stronger attractive force between the dipoles. *Weakest of the intermolecular forces.

Dipole-dipole Permanent attraction between polar molecules Cl Cl

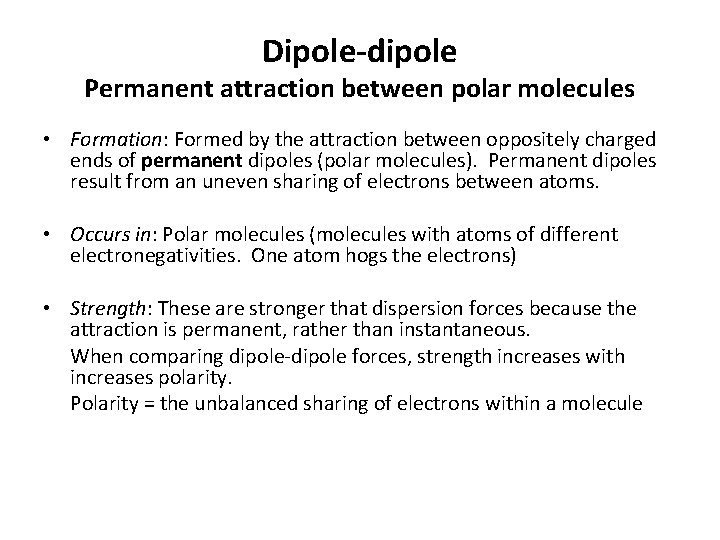
Dipole-dipole Permanent attraction between polar molecules • Formation: Formed by the attraction between oppositely charged ends of permanent dipoles (polar molecules). Permanent dipoles result from an uneven sharing of electrons between atoms. • Occurs in: Polar molecules (molecules with atoms of different electronegativities. One atom hogs the electrons) • Strength: These are stronger that dispersion forces because the attraction is permanent, rather than instantaneous. When comparing dipole-dipole forces, strength increases with increases polarity. Polarity = the unbalanced sharing of electrons within a molecule

Hydrogen bonding Permanent attractions between H and N, O, and F

Hydrogen bonding Permanent attractions between H and N, O, and F • Formation: Special form of dipole-dipole, where a hydrogen atom in one molecule is attracted to the lone electrons of oxygen, Fluorine, or Nitrogen in another water molecule. Hydrogen is “cool by association. ” • Occurs: Mainly in water, but with anything where H is attatched and can be attracted to another NOF • Strength: This is the strongest intermolecular force. Ex. CH 4 gets close to SF 6 Ex. WATER

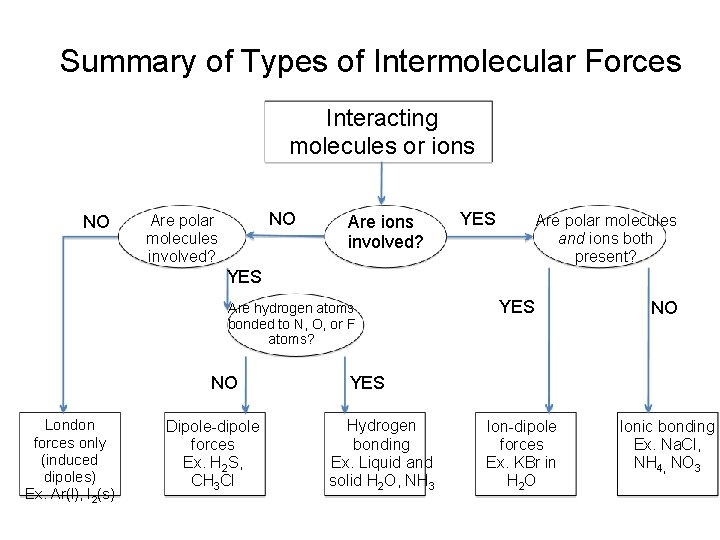
Summary of Types of Intermolecular Forces Interacting molecules or ions NO NO Are polar molecules involved? Are ions involved? YES Are polar molecules and ions both present? YES Are hydrogen atoms bonded to N, O, or F atoms? NO London forces only (induced dipoles) Ex. Ar(l), I 2(s) Dipole-dipole forces Ex. H 2 S, CH 3 Cl YES NO YES Hydrogen bonding Ex. Liquid and solid H 2 O, NH 3 Ion-dipole forces Ex. KBr in H 2 O Ionic bonding Ex. Na. Cl, NH 4, NO 3

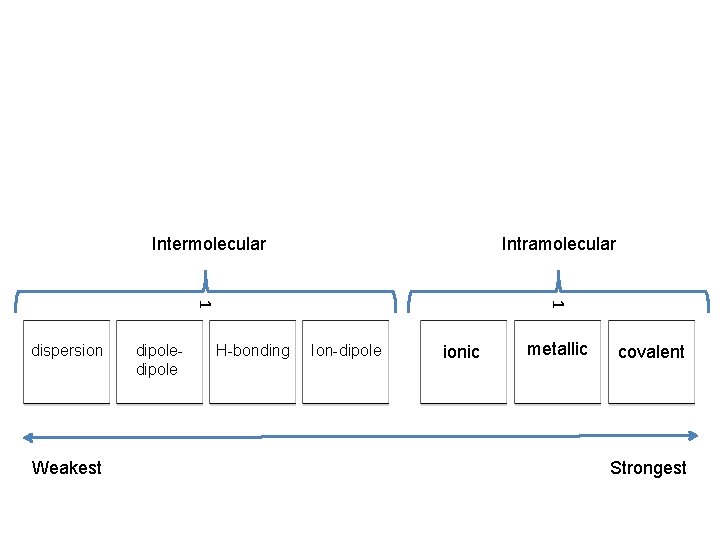
Intermolecular Intramolecular Weakest dipole H-bonding 1 1 dispersion Ion-dipole ionic metallic covalent Strongest

Intermolecular Intramolecular NP Weakest 1 1 dispersion dipole H-bonding Ion-dipole covalent metallic ionic P P & special H&NOF Charged+P NM+NM Strongest

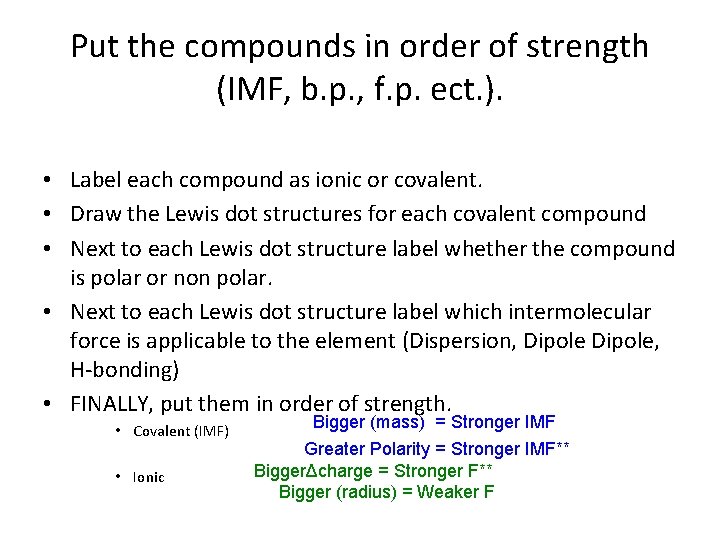
Put the compounds in order of strength (IMF, b. p. , f. p. ect. ). • Label each compound as ionic or covalent. • Draw the Lewis dot structures for each covalent compound • Next to each Lewis dot structure label whether the compound is polar or non polar. • Next to each Lewis dot structure label which intermolecular force is applicable to the element (Dispersion, Dipole, H-bonding) • FINALLY, put them in order of strength. • Covalent (IMF) • Ionic Bigger (mass) = Stronger IMF Greater Polarity = Stronger IMF** BiggerΔcharge = Stronger F** Bigger (radius) = Weaker F

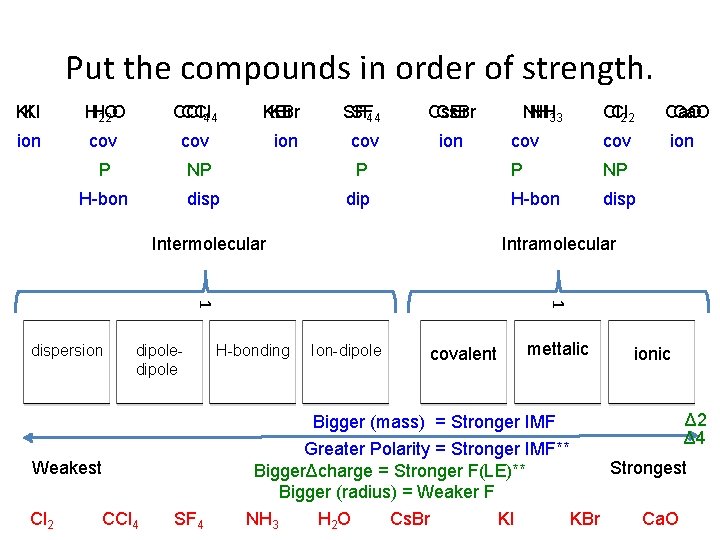
Put the compounds in order of strength. KIKI HH 2 O CCl 4 4 KBr SF KBr SF 4 Cs. Br NH Cs. Br NH 3 Cl 2 2 Ca. O 2 O 4 3 Cl ion cov ion P NP H-bon disp cov ion cov P P NP dip H-bon disp Intermolecular Intramolecular dipole 1 1 dispersion H-bonding Ion-dipole covalent mettalic Bigger (mass) = Stronger IMF Weakest Cl 2 CCl 4 SF 4 ionic Δ 2 Δ 4 Greater Polarity = Stronger IMF** Strongest BiggerΔcharge = Stronger F(LE)** Bigger (radius) = Weaker F NH 3 H 2 O Cs. Br KI KBr Ca. O

WHY? WHAT DOES IT MEAN? • Understanding intermolecular forces helps explain ‘macroscopic’ observations such as: – Solids, liquids or gases at certain temperatures – Boiling point – Freezing point – Viscosity – Surface tension – Capillary action

Liquids Characteristics • Particles are semi-ordered. • A liquid will take the shape of its container. • Can be compressed with an enormous amount of pressure. • Classified as a fluid because it has the ability to flow. Properties • Viscosity – a measure of the resistance of a liquid to flow. • Surface Tension – an inward force, (from IMFs) that tends to minimize the surface area of a liquid; causes the surface to behave as if it were a thin skin • Capillary Action – occurs when the adhesive forces between two different molecules are stronger than one molecule’s cohesive forces.

Properties Liquids • Viscosity – a measure of the resistance of a liquid to flow. – stronger IMF = higher viscosity ex: H 2 SO 4, – bigger particle = higher viscosity ex: molasses, honey (big starch molecules) – lower temperature = higher viscosity • slows molecules down, doesn’t flow as easily • Surface Tension – an inward force, (from IMFs) that tends to minimize the surface area of a liquid; causes the surface to behave as if it were a thin skin – Water has a high surface tension because its molecules experience hydrogen bonding. – Surfactants lower the surface tension of water • Capillary Action – occurs when the adhesive forces between two different molecules are stronger than one molecule’s cohesive forces. – Cohesion – force of attraction between identical molecules – Adhesion – force of attraction between molecules that are different

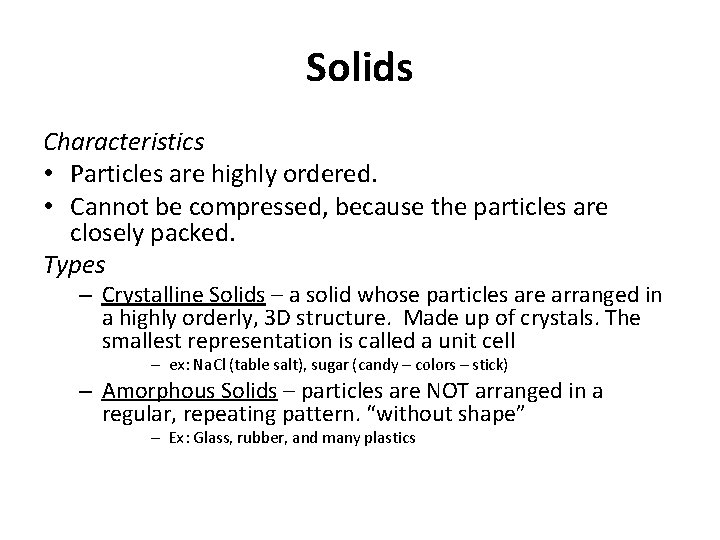
Solids Characteristics • Particles are highly ordered. • Cannot be compressed, because the particles are closely packed. Types – Crystalline Solids – a solid whose particles are arranged in a highly orderly, 3 D structure. Made up of crystals. The smallest representation is called a unit cell – ex: Na. Cl (table salt), sugar (candy – colors – stick) – Amorphous Solids – particles are NOT arranged in a regular, repeating pattern. “without shape” – Ex: Glass, rubber, and many plastics

Gases • It is easy to compress gases because of all empty space • Gases completely fill their containers • Different gases can move through each other quite rapidly – mix easily (Called diffusion) • Gases exert pressure • Pressure – amount of molecules hitting the side of the container per area per time, depends on temp

Gases A gas consists of very small particles which have mass. The distance between gas particles is great. Gas particles are in constant, rapid, random motion. Collisions are perfectly elastic (they bounce off each other). • Average kinetic energy of a gas depends only on temperature. • Gas particles exert no attractive or repulsive forces on one another. • •

Phase Changes • • • Freezing Melting Vaporization (boiling) Condensation Sublimation Deposition

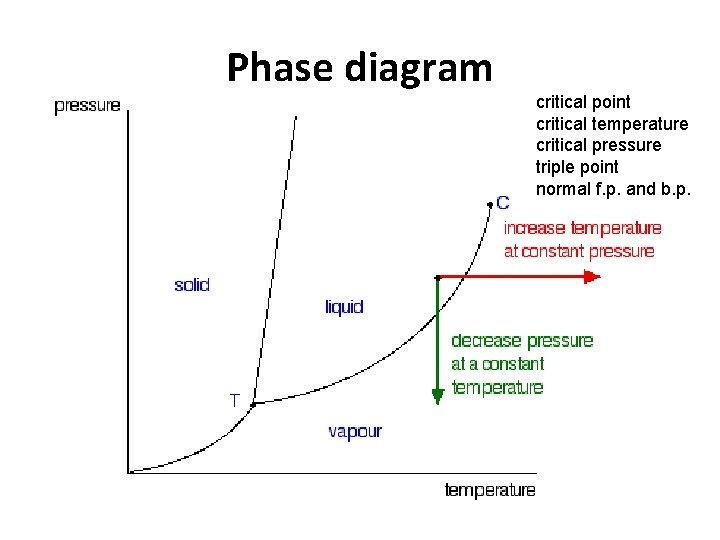
Phase diagram critical point critical temperature critical pressure triple point normal f. p. and b. p.

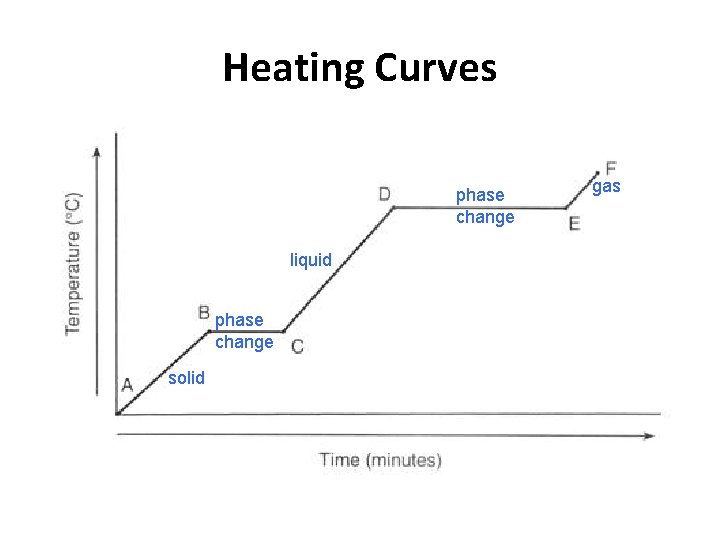
Heating Curves phase change liquid phase change solid gas
 Antigentest åre
Antigentest åre Baripity
Baripity Hey hey bye bye
Hey hey bye bye Bedroom downstairs living room upstairs
Bedroom downstairs living room upstairs To room nineteen theme
To room nineteen theme Hotel.hotelno=room.hotelno(hotel room)
Hotel.hotelno=room.hotelno(hotel room) Refrigerator part names
Refrigerator part names Sub cooling chart
Sub cooling chart What is the temp of the mesosphere
What is the temp of the mesosphere Vasoconstriction and temperature
Vasoconstriction and temperature High temp hydrogen attack
High temp hydrogen attack Rutgers sea water temps mid atlantic
Rutgers sea water temps mid atlantic Color09112004
Color09112004 Etr florida tag
Etr florida tag Simdis d6352
Simdis d6352 Capit latin root
Capit latin root China seamless square pipe
China seamless square pipe Rankin temp
Rankin temp Node temp = tail.getprev()
Node temp = tail.getprev() Urban sprawl
Urban sprawl I became a system chapter 29
I became a system chapter 29 Uranus belt
Uranus belt Temp tap
Temp tap Temp程式
Temp程式 Stratosphere height
Stratosphere height Galveston bay water temp
Galveston bay water temp Fret assay
Fret assay Function of economizer in boiler
Function of economizer in boiler Aural temp
Aural temp Tropopause fold
Tropopause fold