Atoms molecules and ions Daltons Atomic Theory 1808



- Slides: 24
Atoms, molecules and ions

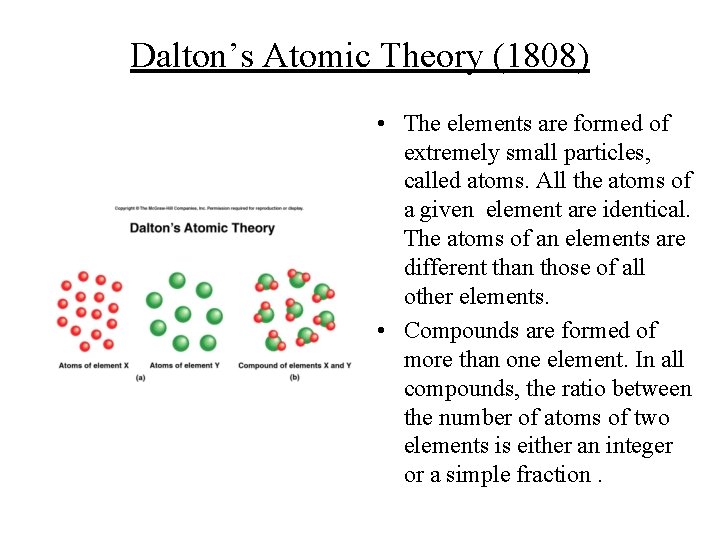
Dalton’s Atomic Theory (1808) • The elements are formed of extremely small particles, called atoms. All the atoms of a given element are identical. The atoms of an elements are different than those of all other elements. • Compounds are formed of more than one element. In all compounds, the ratio between the number of atoms of two elements is either an integer or a simple fraction.

Dalton’s Atomic Theory (1808) • A chemical reaction is the separation, combination, or rearrangement of atoms; it causes neither the destruction nor the creation of atoms. • N. B. Dalton did not know the structure of the atom (i. e. , electron, proton, neutron, nucleus, etc. ) - Dalton imagined an atom was small and indivisible

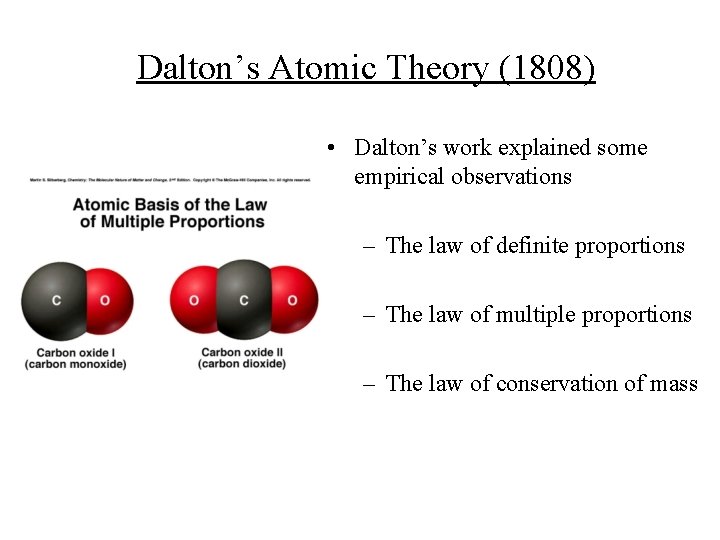
Dalton’s Atomic Theory (1808) • Dalton’s work explained some empirical observations – The law of definite proportions – The law of multiple proportions – The law of conservation of mass

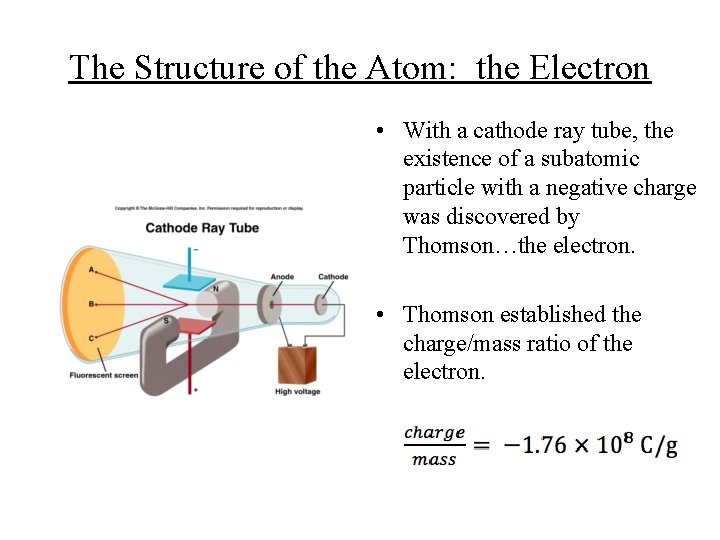
The Structure of the Atom: the Electron • With a cathode ray tube, the existence of a subatomic particle with a negative charge was discovered by Thomson…the electron. • Thomson established the charge/mass ratio of the electron.

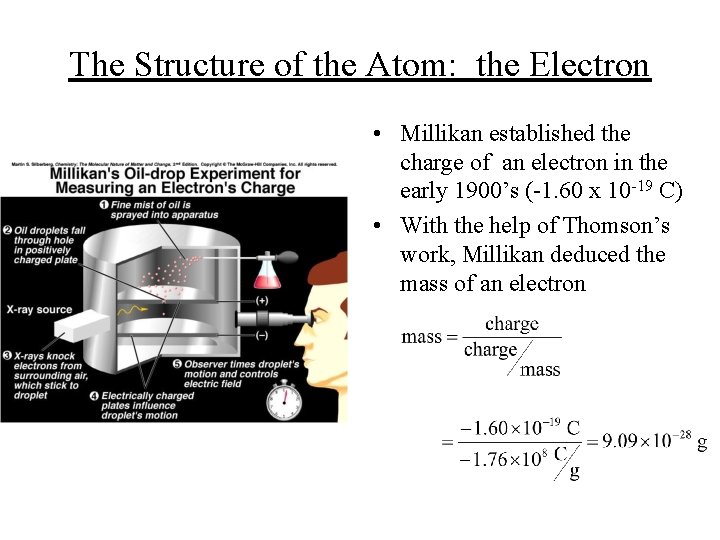
The Structure of the Atom: the Electron • Millikan established the charge of an electron in the early 1900’s (-1. 60 x 10 -19 C) • With the help of Thomson’s work, Millikan deduced the mass of an electron

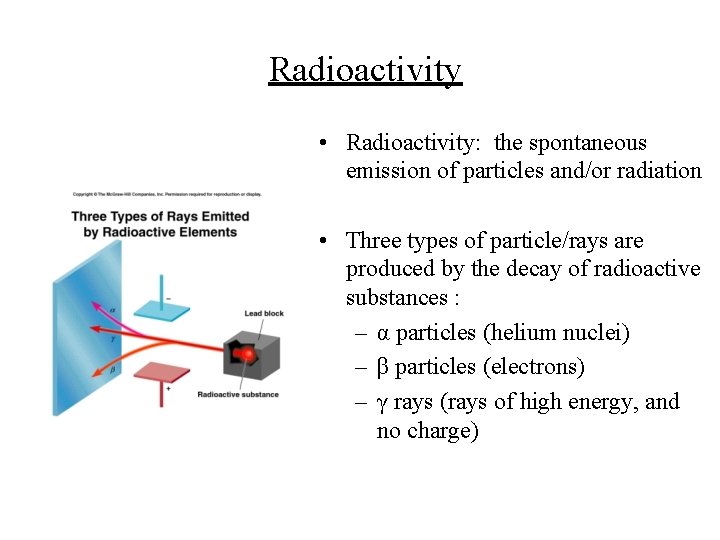
Radioactivity • Radioactivity: the spontaneous emission of particles and/or radiation • Three types of particle/rays are produced by the decay of radioactive substances : – α particles (helium nuclei) – β particles (electrons) – γ rays (rays of high energy, and no charge)

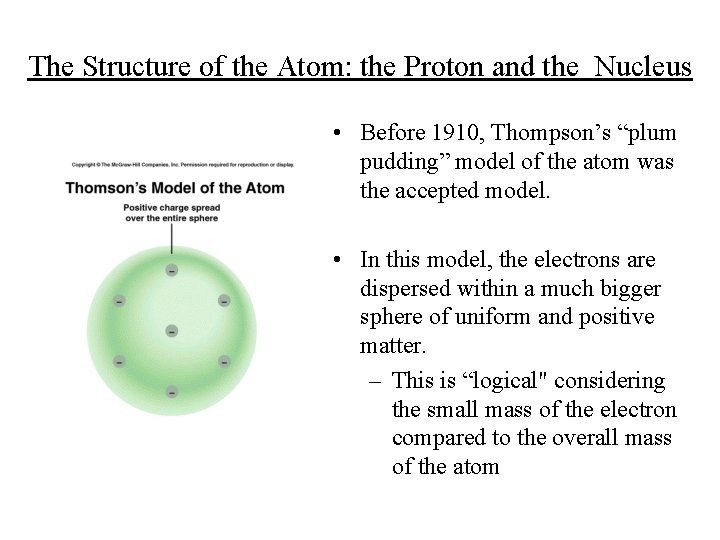
The Structure of the Atom: the Proton and the Nucleus • Before 1910, Thompson’s “plum pudding” model of the atom was the accepted model. • In this model, the electrons are dispersed within a much bigger sphere of uniform and positive matter. – This is “logical" considering the small mass of the electron compared to the overall mass of the atom

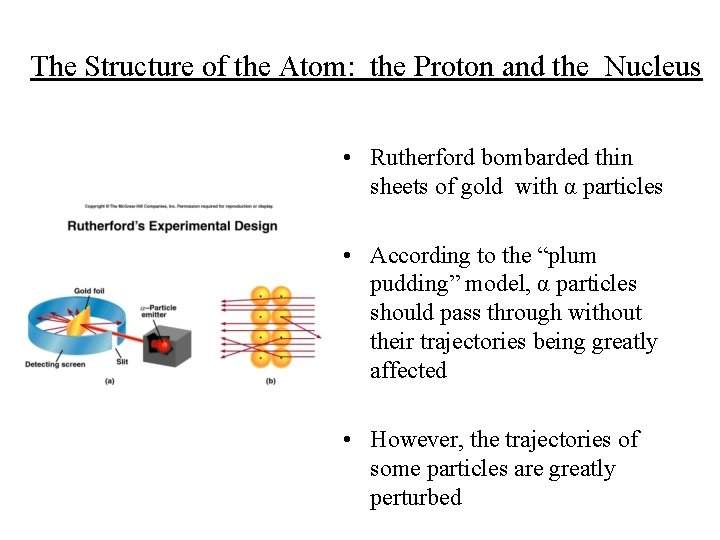
The Structure of the Atom: the Proton and the Nucleus • Rutherford bombarded thin sheets of gold with α particles • According to the “plum pudding” model, α particles should pass through without their trajectories being greatly affected • However, the trajectories of some particles are greatly perturbed

The Structure of the Atom: the Proton and the Nucleus • In Rutherford’s experiment, the deviations are the result of large repulsions • Because α particles are positively charged, the positive charge in the atom must be concentrated in a very small and solid nucleus in the center of the atom (about 10 -13 of the volume of the atom, but almost 100% of the mass) • The positive charges that are located in the nucleus are the protons • The charge of a proton is the same as the electron, but with the opposite sign (i. e. , positive) • The mass of the proton is 1. 67252 x 10 -24 g (about 1840 times that of the electron)

The Structure of the Atom: the Neutron • Before 1932, a great mystery was the fact that H had one proton and He had two, but He was four times more massive than H! • Many people predicted the existence of the neutron, but it was Chadwick that discovered it in 1932 • The neutron has no charge and is slightly larger than the proton (1. 67493 x 10 -24 g for the neutron vs 1. 67252 x 10 -24 g for the proton)

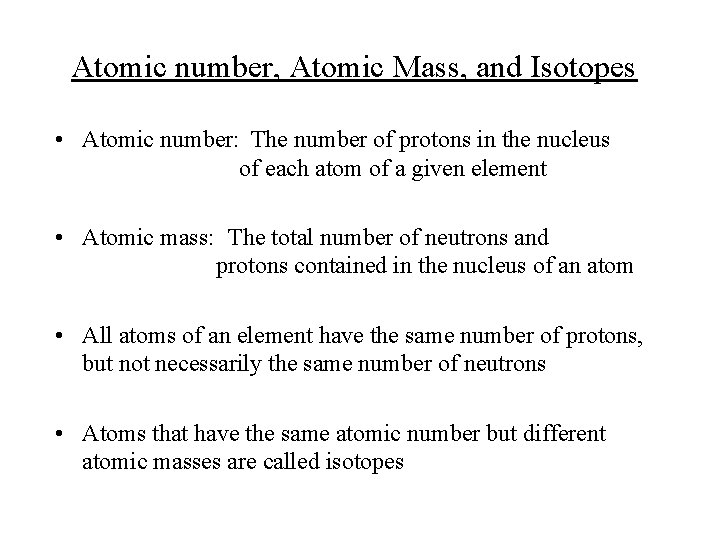
Atomic number, Atomic Mass, and Isotopes • Atomic number: The number of protons in the nucleus of each atom of a given element • Atomic mass: The total number of neutrons and protons contained in the nucleus of an atom • All atoms of an element have the same number of protons, but not necessarily the same number of neutrons • Atoms that have the same atomic number but different atomic masses are called isotopes

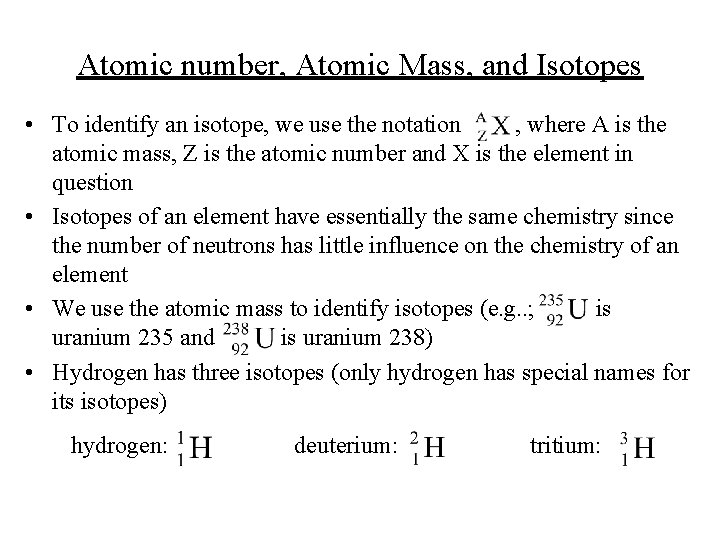
Atomic number, Atomic Mass, and Isotopes • To identify an isotope, we use the notation , where A is the atomic mass, Z is the atomic number and X is the element in question • Isotopes of an element have essentially the same chemistry since the number of neutrons has little influence on the chemistry of an element • We use the atomic mass to identify isotopes (e. g. . ; is uranium 235 and is uranium 238) • Hydrogen has three isotopes (only hydrogen has special names for its isotopes) hydrogen: deuterium: tritium:

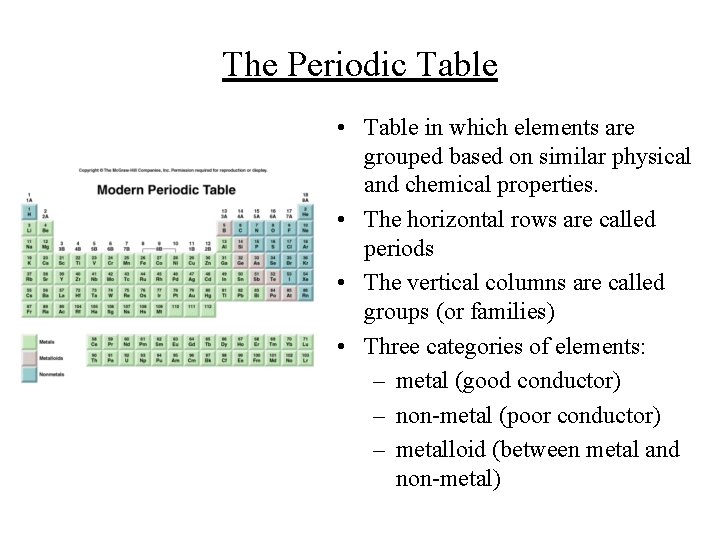
The Periodic Table • Table in which elements are grouped based on similar physical and chemical properties. • The horizontal rows are called periods • The vertical columns are called groups (or families) • Three categories of elements: – metal (good conductor) – non-metal (poor conductor) – metalloid (between metal and non-metal)

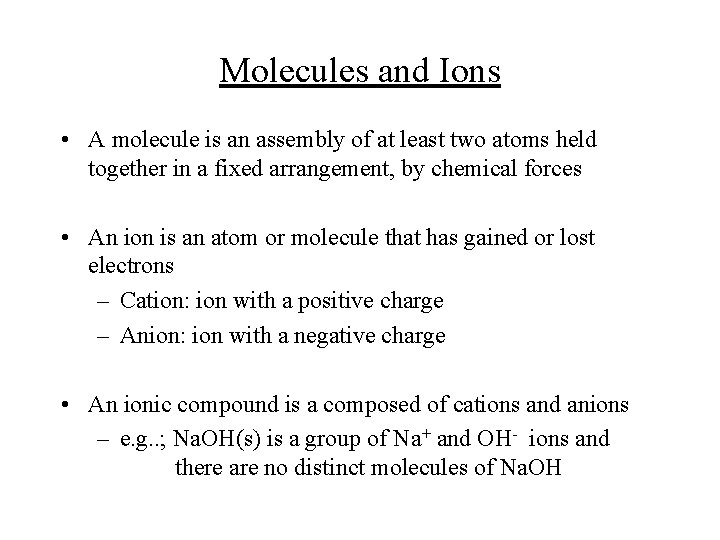
Molecules and Ions • A molecule is an assembly of at least two atoms held together in a fixed arrangement, by chemical forces • An ion is an atom or molecule that has gained or lost electrons – Cation: ion with a positive charge – Anion: ion with a negative charge • An ionic compound is a composed of cations and anions – e. g. . ; Na. OH(s) is a group of Na+ and OH- ions and there are no distinct molecules of Na. OH

Chemical Formulas • Molecular formula: indicates the exact number of atoms of each element contained in the smallest unit of a substance • Empirical Formula: indicates the lowest whole number ratio of atoms present in a substance • e. g. ; hydrogen peroxide is a molecule that contains two atoms of oxygen and two atoms of hydrogen. • The molecular formula is H 2 O 2 • The empirical formula is HO • For many molecules, the molecular and empirical formulas are identical (e. g. ; H 2 O)

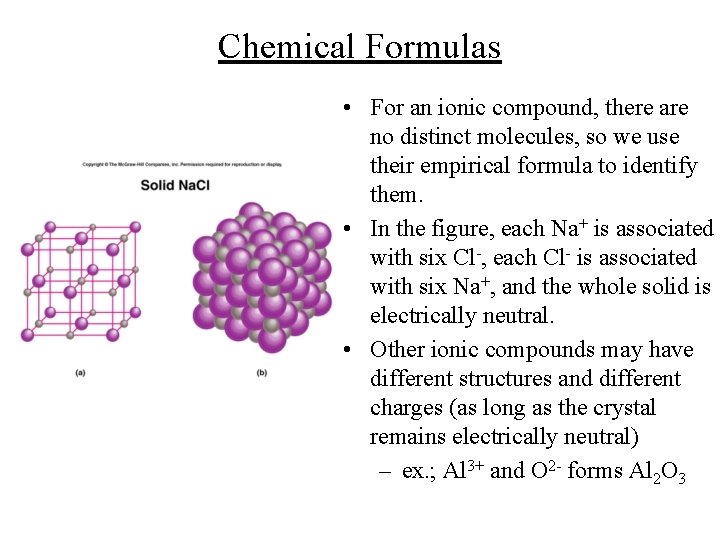
Chemical Formulas • For an ionic compound, there are no distinct molecules, so we use their empirical formula to identify them. • In the figure, each Na+ is associated with six Cl-, each Cl- is associated with six Na+, and the whole solid is electrically neutral. • Other ionic compounds may have different structures and different charges (as long as the crystal remains electrically neutral) – ex. ; Al 3+ and O 2 - forms Al 2 O 3

Nomenclature of Inorganic Compounds: Ionic Compounds • For ionic compounds, we name the cation followed by the anion • The anion or the cation is sometimes a polyatomic ion – ex. ; NH 4+ : ammonium CO 32 - : carbonate OH- : hydroxide PO 43 - : phosphate NO 3 - : nitrate SO 42 - : sulfate NO 2 - : nitrite SO 32 -: sulfite • ex. ; KBr : potassium bromide Zn. I 2 : zinc iodide Al 2 O 3 : aluminium oxide Na. OH : sodium hydroxide KCN : potassium cyanide NH 4 Cl : ammonium chloride

Nomenclature of Inorganic Compounds: Covalent Compounds • Covalent compounds consist of distinct molecules • For a binary covalent compound, we name the first element in the formula first – ex. ; HCl : hydrogen chloride NO : nitric oxide Si. C : silicon carbide

Nomenclature of Inorganic Compounds: Covalent Compounds • When two elements form several compounds, Greek prefixes are used to specify the number of atoms of each element – e. g. ; CO : carbon monoxide CO 2 : carbon dioxide NO 2 : nitrogen dioxide N 2 O 4 : dinitrogen tetroxide • Many covalent compounds containing hydrogen are referred to by their common name – e. g. ; B 2 H 6 : diborane CH 4 : methane NH 3 : ammonia H 2 O : water Si. H 4 : silane PH 3 : phosphine

Nomenclature of Inorganic Compounds: Acids and Bases • A definition of an acid is: a substance that releases an H+ when dissolved in water. • If the acid is not an oxyacid, i. e. , does not contain oxygen, we use the prefix “hydro” and the suffix “ic” – ex. ; HF : hydrofluoric acid HI : hydroiodic acid H 2 S : hydrosulfuric acid • N. B. HCl(g) is hydrogen chloride, but once it is dissolved in water, it become hydrochloric acid and releases H+

Nomenclature of Inorganic Compounds: Acids and Bases • For an oxyacid (general formula Hm. XOn) it often happens that there are multiple possible values of n for each element X, and as such, within this series of compounds, – There is always an acid in the series that ends with “ic” • Adding another oxygen to the “ic” acid produces the “per…. ic” acid • Removing an oxygen atom to the “ic” acid produces the “ous” acid • Removing two oxygen atoms to the “ic” acid produces the “hypo…ous” acid

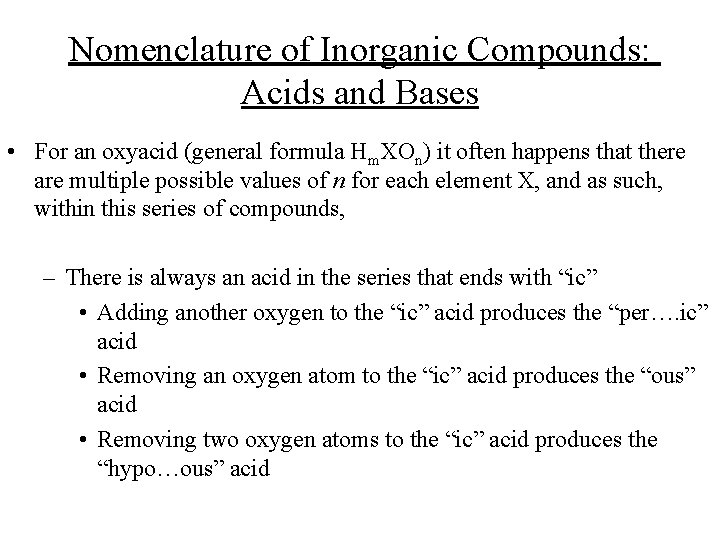
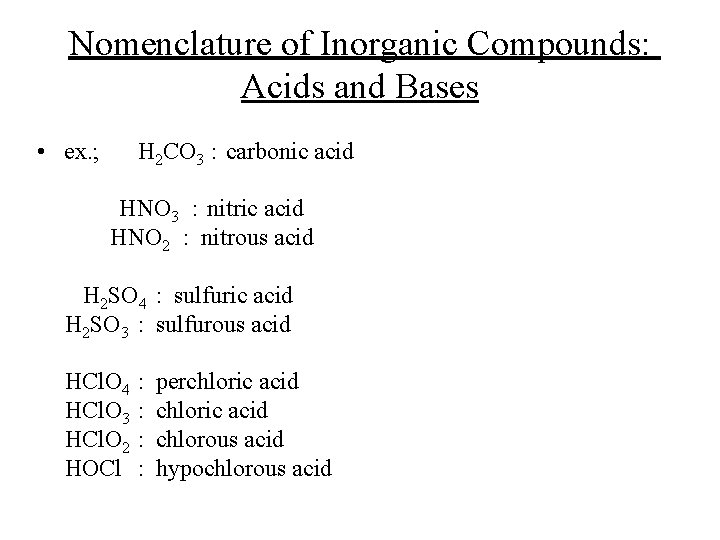
Nomenclature of Inorganic Compounds: Acids and Bases • ex. ; H 2 CO 3 : carbonic acid HNO 3 : nitric acid HNO 2 : nitrous acid H 2 SO 4 : sulfuric acid H 2 SO 3 : sulfurous acid HCl. O 4 HCl. O 3 HCl. O 2 HOCl : : perchloric acid chlorous acid hypochlorous acid

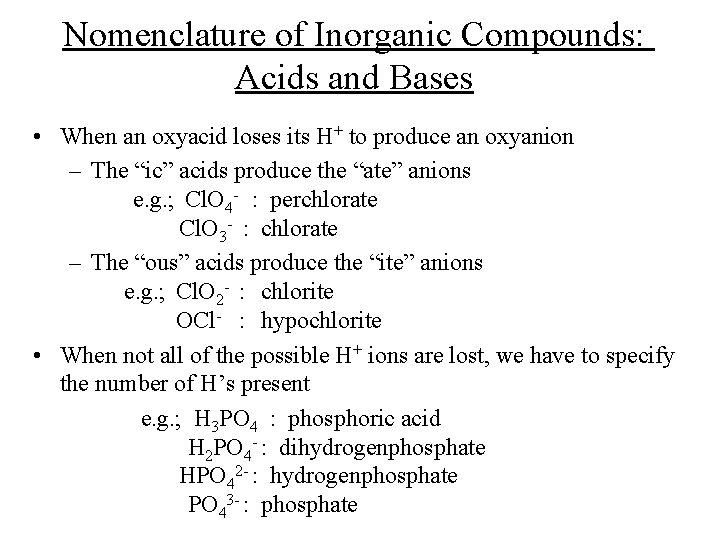
Nomenclature of Inorganic Compounds: Acids and Bases • When an oxyacid loses its H+ to produce an oxyanion – The “ic” acids produce the “ate” anions e. g. ; Cl. O 4 - : perchlorate Cl. O 3 - : chlorate – The “ous” acids produce the “ite” anions e. g. ; Cl. O 2 - : chlorite OCl- : hypochlorite • When not all of the possible H+ ions are lost, we have to specify the number of H’s present e. g. ; H 3 PO 4 : phosphoric acid H 2 PO 4 - : dihydrogenphosphate HPO 42 - : hydrogenphosphate PO 43 - : phosphate
 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Atomic model (1808)
Atomic model (1808) 1808 atomic model
1808 atomic model Thomson atom modeli
Thomson atom modeli Positive ions and negative ions table
Positive ions and negative ions table Daltons gas law
Daltons gas law Daltons gas law
Daltons gas law Frumeindamassi
Frumeindamassi Dalton atomic theory
Dalton atomic theory Daltons experiment
Daltons experiment Relationship between atoms and molecules
Relationship between atoms and molecules Study jams mixtures
Study jams mixtures 3bacl2 counting atoms
3bacl2 counting atoms Interacting molecules or ions
Interacting molecules or ions Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Magnesium and fluorine ionic compound
Magnesium and fluorine ionic compound Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Why do atoms combine to form molecules
Why do atoms combine to form molecules