Atoms Molecules and Ions Chapter 2 Daltons Atomic












- Slides: 30

Atoms, Molecules, and Ions Chapter 2

Dalton’s Atomic Model l First model - early 1800’s l l l Developed through experiments All elements are composed of tiny indivisible particles called atoms All atoms of the same element are identical.

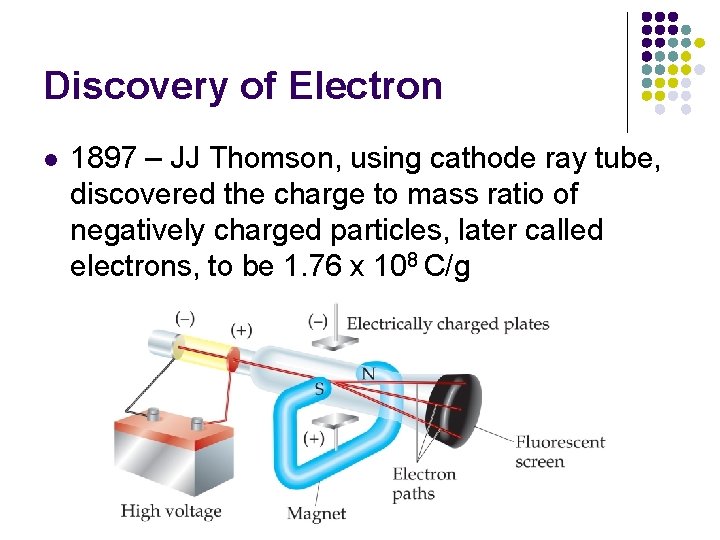
Discovery of Electron l 1897 – JJ Thomson, using cathode ray tube, discovered the charge to mass ratio of negatively charged particles, later called electrons, to be 1. 76 x 108 C/g

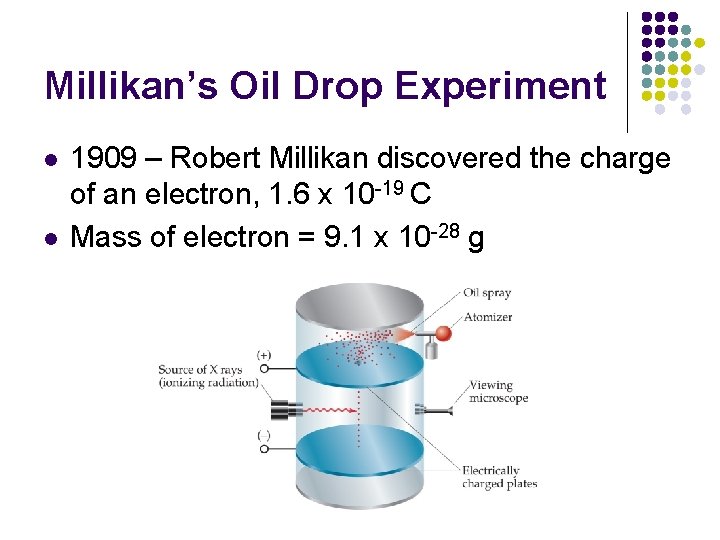
Millikan’s Oil Drop Experiment l l 1909 – Robert Millikan discovered the charge of an electron, 1. 6 x 10 -19 C Mass of electron = 9. 1 x 10 -28 g

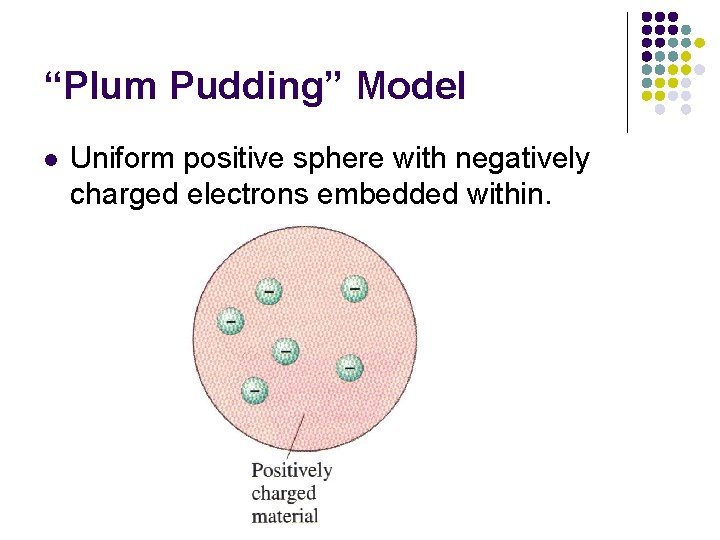
“Plum Pudding” Model l Uniform positive sphere with negatively charged electrons embedded within.

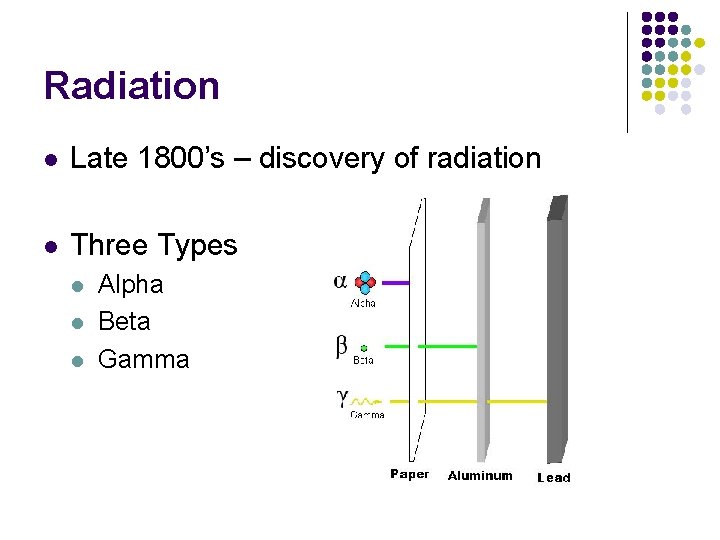
Radiation l Late 1800’s – discovery of radiation l Three Types l l l Alpha Beta Gamma

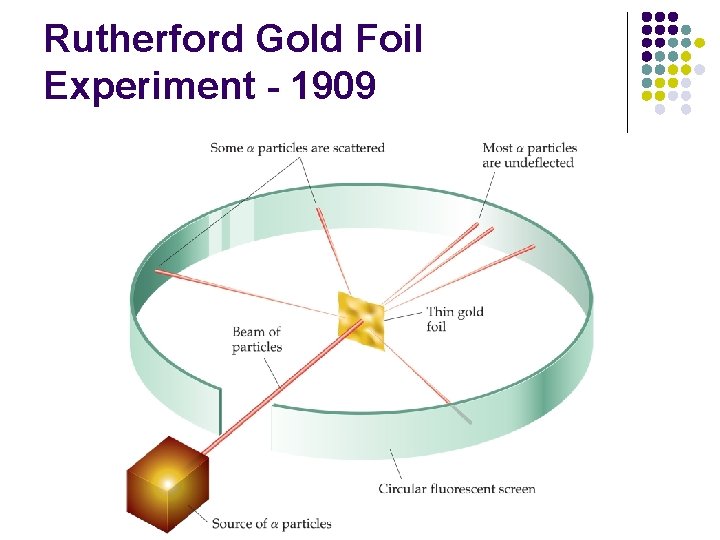
Rutherford Gold Foil Experiment - 1909 l l l Shot alpha particles at gold foil Most went through foil with little or no deflection. Some were deflected at large angle and some straight back.

Rutherford Gold Foil Experiment - 1909

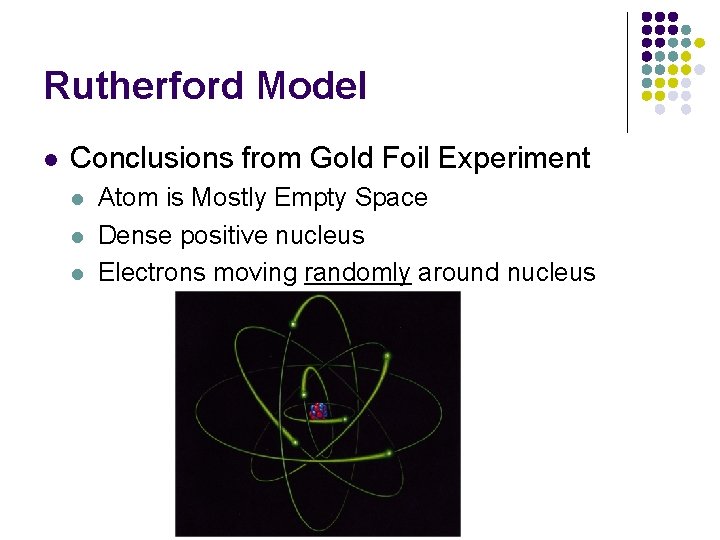
Rutherford Model l Conclusions from Gold Foil Experiment l l l Atom is Mostly Empty Space Dense positive nucleus Electrons moving randomly around nucleus

Bohr Model l Dense positive nucleus l Electrons in specified circular paths, called energy levels

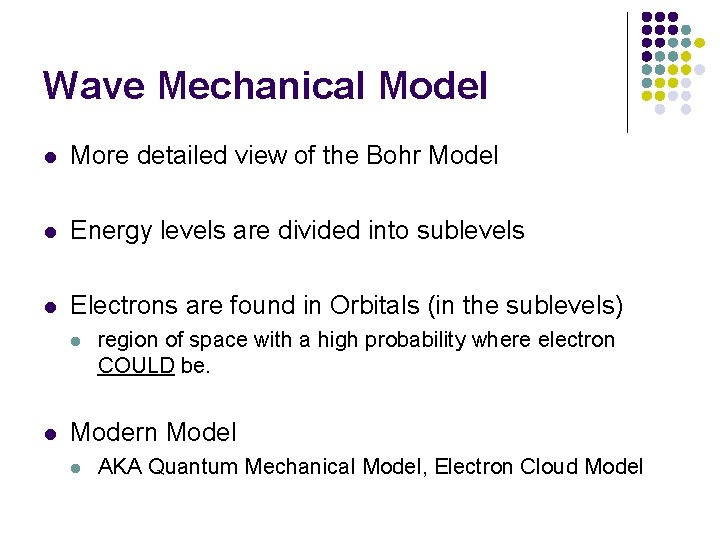
Wave Mechanical Model l More detailed view of the Bohr Model l Energy levels are divided into sublevels l Electrons are found in Orbitals (in the sublevels) l l region of space with a high probability where electron COULD be. Modern Model l AKA Quantum Mechanical Model, Electron Cloud Model

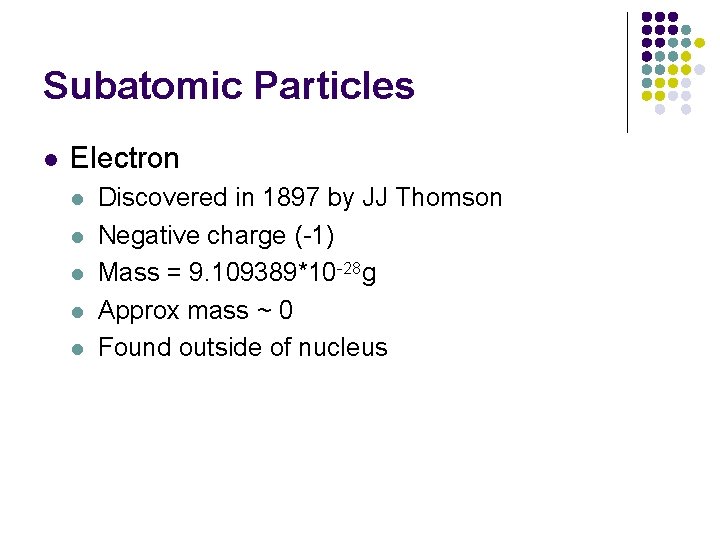
Subatomic Particles l Electron l l l Discovered in 1897 by JJ Thomson Negative charge (-1) Mass = 9. 109389*10 -28 g Approx mass ~ 0 Found outside of nucleus

Subatomic Particles l Proton l l l Discovered in 1919 by Rutherford Positive charge (+1) Mass = 1. 672623*10 -24 g Approx mass ~ 1 atomic mass unit (u) Found inside nucleus

Subatomic Particles l Neutron l l l Discovered in 1932 by James Chadwick No charge (0) Mass = 1. 6749286*10 -24 g Approx mass ~ 1 atomic mass unit (u) Just slightly larger than a proton Found inside nucleus

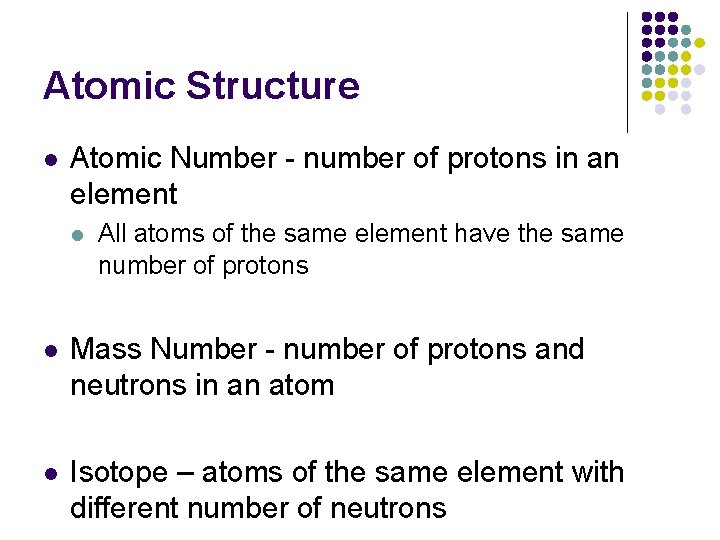
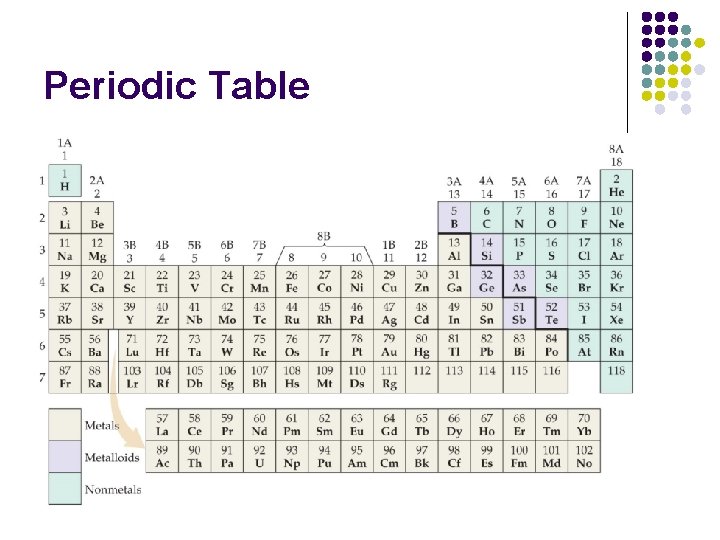
Atomic Structure l Atomic Number - number of protons in an element l All atoms of the same element have the same number of protons l Mass Number - number of protons and neutrons in an atom l Isotope – atoms of the same element with different number of neutrons

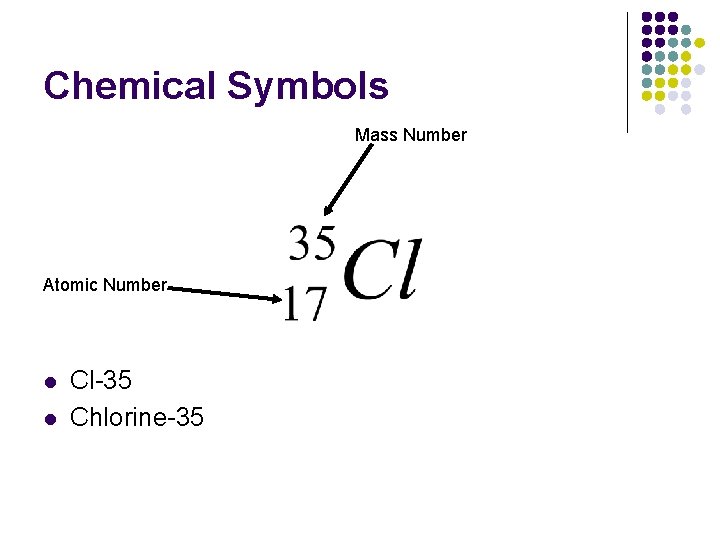
Chemical Symbols Mass Number Atomic Number l l Cl-35 Chlorine-35

Ion l Atom or group of atoms that have gained or lost one or more electrons l l Have a charge Example: l H+, Ca 2+, Cl-, OH-

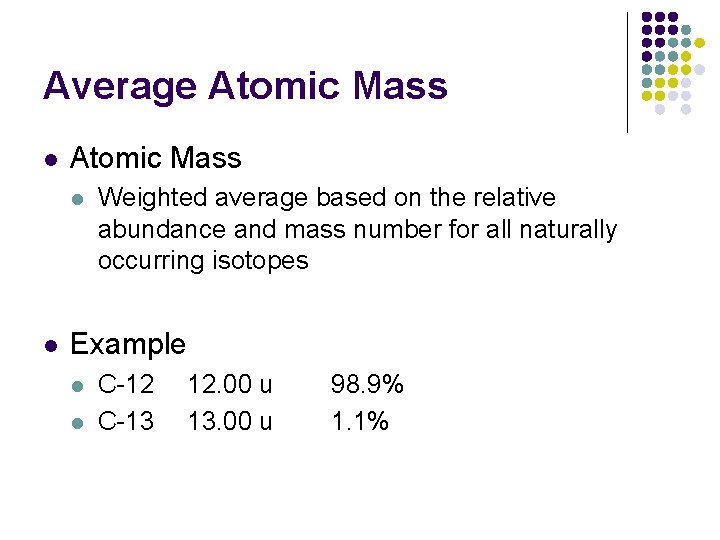
Average Atomic Mass l l Weighted average based on the relative abundance and mass number for all naturally occurring isotopes Example l l C-12 C-13 12. 00 u 13. 00 u 98. 9% 1. 1% 12. 011 u

Periodic Table

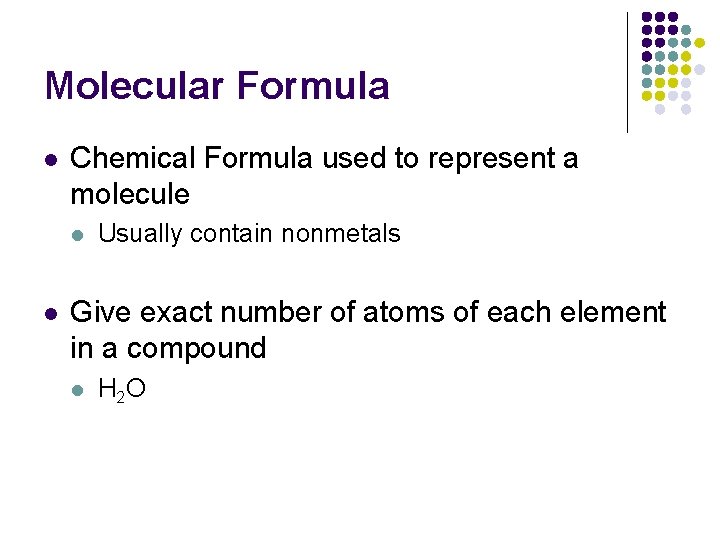
Molecular Formula l Chemical Formula used to represent a molecule l l Usually contain nonmetals Give exact number of atoms of each element in a compound l H 2 O

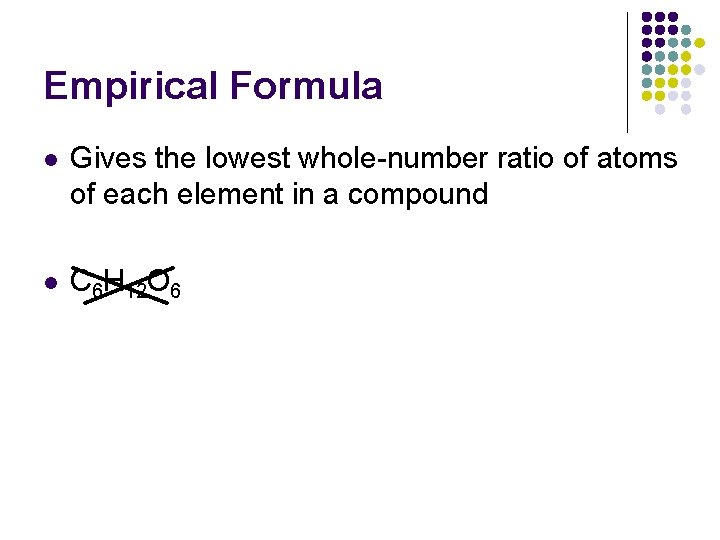
Empirical Formula l Gives the lowest whole-number ratio of atoms of each element in a compound l C 6 H 12 O 6 CH 2 O

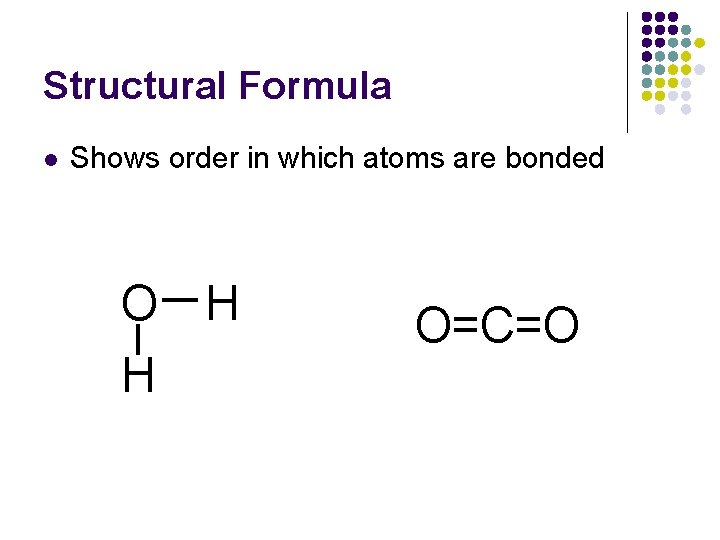
Structural Formula l Shows order in which atoms are bonded O H H O=C=O

Ionic Compounds l Usually contains a metal and a nonmetal or polyatomic ions l Ionic formula is an empirical formula l Na. Cl

Naming Ions and Ionic Compounds l Name positive ion first, then negative ion. l l Na. Cl, Sodium Chloride Ions of metals that have more than one positive oxidation number need a roman numeral

Oxyanions l l Polyatomic ions often contain oxygen The most common combination of oxygen and another ion will end with –ate l l Nitrate (NO 3 -), Sulfate (SO 42 -), Chlorate (Cl. O 3 -) The anion with one less oxygen will end with –ite l Nitrite (NO 2 -), Sulfite (SO 32 -), Chlorite (Cl. O 2 -)

Oxyanions l An anion with two less oxygens will start with Hypo- and end with –ite l l Hypochlorite (Cl. O-) An anion with one more oxygen that the most common ion will start with Per- and end with –ate l Perchlorate (Cl. O 4 -)

Naming Acids l Acid names are based on the anion in the acid l Anions that end with –ide become hydro_____ic acid l HCl Chloride Hydrochloric acid l

Naming Acids l Anions that end with –ate become _____ic acid l HNO 3 Nitrate Nitric acid l l l H 2 SO 4 Sulfate Sulfuric acid

Naming Acids l Anions that end with –ite become _____ous acid l HNO 2 Nitrite Nitrous acid l l l H 2 SO 3 Sulfite Sulfurous acid

Naming Molecular Compounds l Use a prefix to indicate the number of atoms for each element l l l Binary Covalent System Second element ends in –ide Exceptions l l When there is only one atom of the first element, do not use mono- prefix. When an element starts with a vowel, drop any o or a at the end of a prefix