ATOMS MOLECULES IONS Daltons Law Atomic Structure nucelus









- Slides: 43

ATOMS, MOLECULES, & IONS Dalton’s Law Atomic Structure - nucelus - proton (p+), neutron (n 0), electron (e-) What Determines - element, isotope, cation, anion Isotope - isotope notation, Atomic Mass (Z), Atomic Mass, Mass Number (A), amu Average Atomic Mass Chemical Symbol Molecules, Compounds - ionic, covalent

Ch. 2 More Concepts to Study & Focus On Chemical Nomenclature, Formulas - ionic/covalent/acids - regular/irregualar metals/polyatomic ions - diatomics Periodic Table - metals, nonmetal, metalloids, characteristics of - columns, families, groups, names - rows, periods, series, names - main group elements, transition, halogen (halides), inert gases (noble), alkaline, rare earth

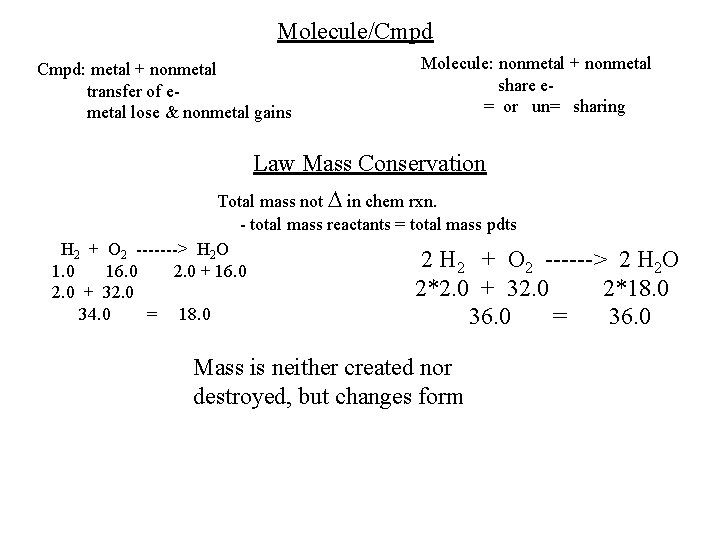
Molecule/Cmpd: metal + nonmetal transfer of emetal lose & nonmetal gains Molecule: nonmetal + nonmetal share e= or un= sharing Law Mass Conservation Total mass not in chem rxn. - total mass reactants = total mass pdts H 2 + O 2 -------> H 2 O 2 H 2 + O 2 ------> 2 H 2 O 1. 0 16. 0 2. 0 + 16. 0 2*2. 0 + 32. 0 2*18. 0 2. 0 + 32. 0 34. 0 = 18. 0 36. 0 = 36. 0 Mass is neither created nor destroyed, but changes form

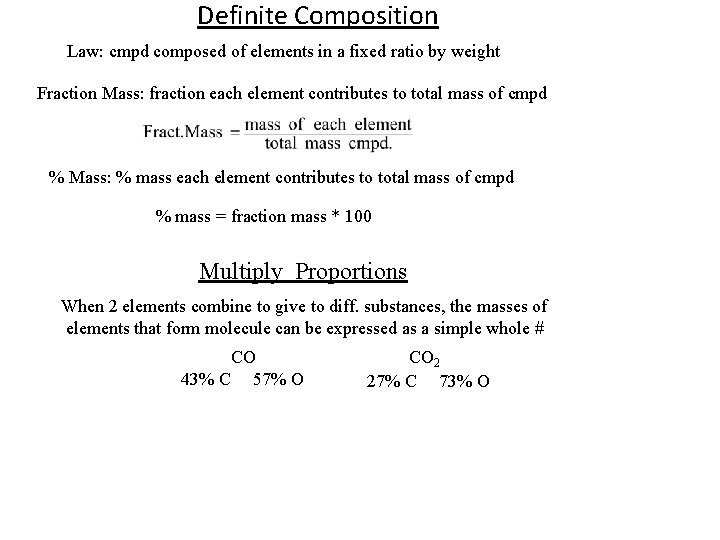
Definite Composition Law: cmpd composed of elements in a fixed ratio by weight Fraction Mass: fraction each element contributes to total mass of cmpd % Mass: % mass each element contributes to total mass of cmpd % mass = fraction mass * 100 Multiply Proportions When 2 elements combine to give to diff. substances, the masses of elements that form molecule can be expressed as a simple whole # CO 43% C 57% O CO 2 27% C 73% O

Elements: simplist form of matter; only 1 kind of atom; p. table; not broken down any further Compound: 2+ diff atoms bonded; fixed ratio by mass; properties diff than individual atoms; decomposed into simplier subst (chem) Mixture: 2+ diff subst; not chem react; mix in any diff amts; sep physical means

TERMS Atomic Number: identifies which element # of protons Atomic Mass: relative ave mass of element including % of all isotopes Mass Number: atomic mass rounded to nearest whole number # protons + # neutrons same!! What does that mean? Isotope: diff form of same element diff # of neutrons

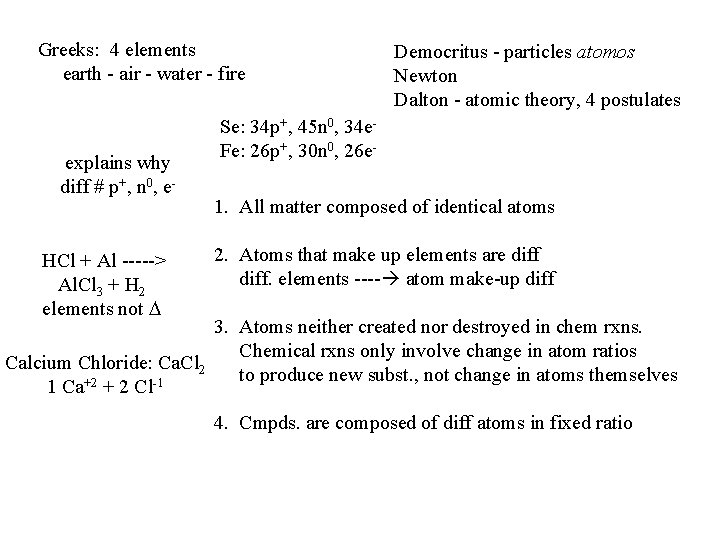
Greeks: 4 elements earth - air - water - fire explains why diff # p+, n 0, e- HCl + Al -----> Al. Cl 3 + H 2 elements not Democritus - particles atomos Newton Dalton - atomic theory, 4 postulates Se: 34 p+, 45 n 0, 34 e. Fe: 26 p+, 30 n 0, 26 e 1. All matter composed of identical atoms 2. Atoms that make up elements are diff. elements ---- atom make-up diff 3. Atoms neither created nor destroyed in chem rxns. Chemical rxns only involve change in atom ratios Calcium Chloride: Ca. Cl 2 to produce new subst. , not change in atoms themselves 1 Ca+2 + 2 Cl-1 4. Cmpds. are composed of diff atoms in fixed ratio

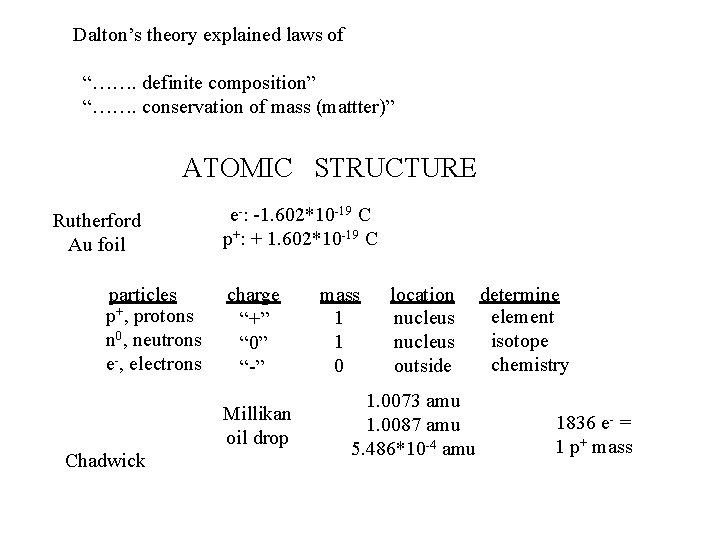
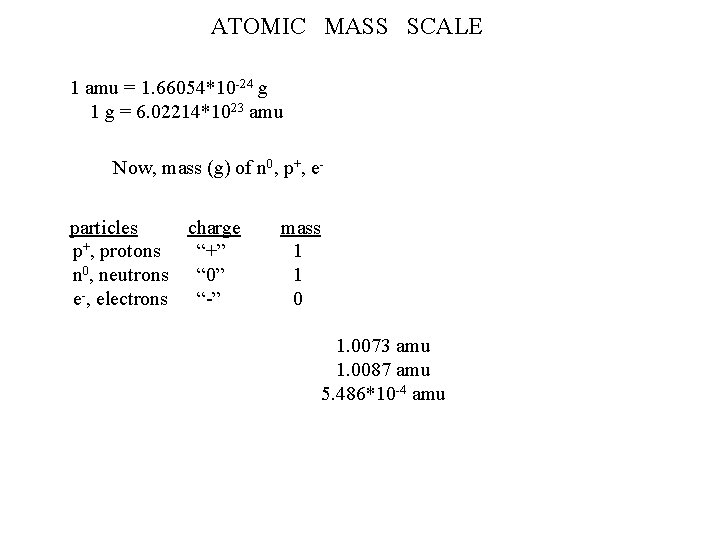
Dalton’s theory explained laws of “……. definite composition” “……. conservation of mass (mattter)” ATOMIC STRUCTURE Rutherford Au foil particles p+, protons n 0, neutrons e-, electrons e-: -1. 602*10 -19 C p+: + 1. 602*10 -19 C charge “+” “ 0” “-” Millikan oil drop Chadwick mass 1 1 0 location nucleus outside 1. 0073 amu 1. 0087 amu 5. 486*10 -4 amu determine element isotope chemistry 1836 e- = 1 p+ mass

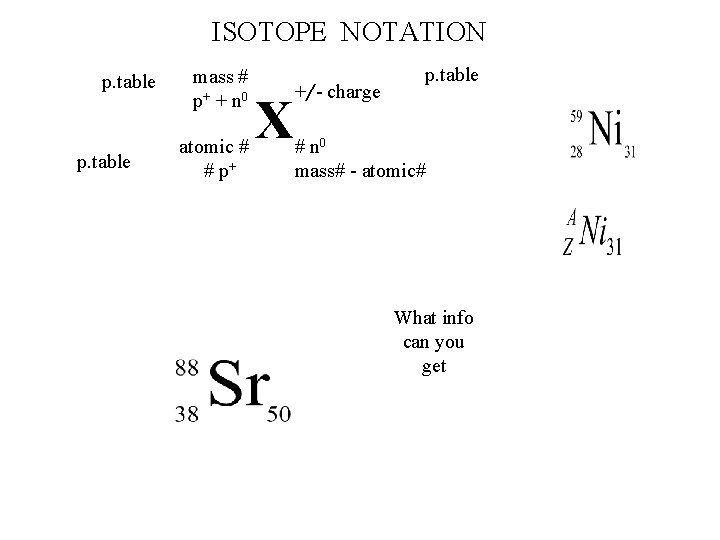
ISOTOPE NOTATION p. table mass # p+ + n 0 atomic # # p+ X +/- charge p. table # n 0 mass# - atomic# What info can you get

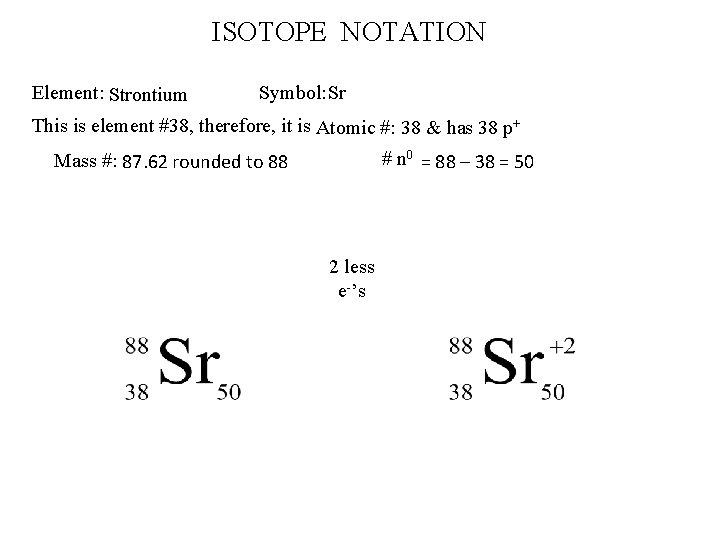
ISOTOPE NOTATION Element: Strontium Symbol: Sr This is element #38, therefore, it is Atomic #: 38 & has 38 p+ # n 0 = 88 – 38 = 50 Mass #: 87. 62 rounded to 88 2 less e-’s

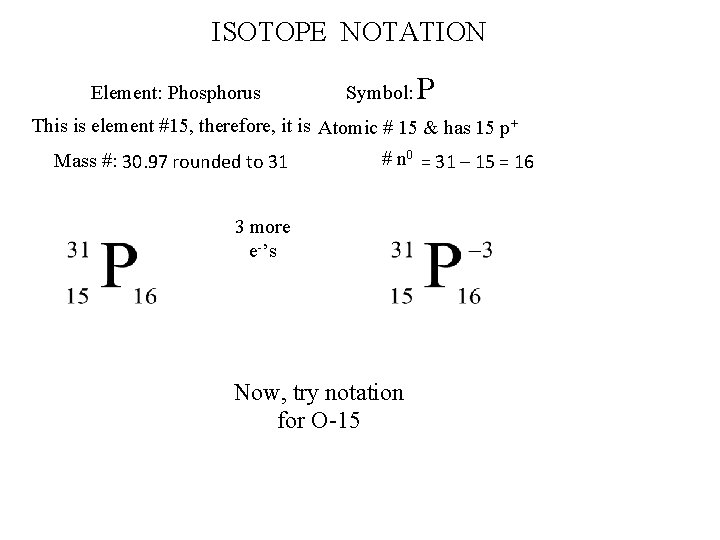
ISOTOPE NOTATION Element: Phosphorus Symbol: P This is element #15, therefore, it is Atomic # 15 & has 15 p+ Mass #: 30. 97 rounded to 31 # n 0 = 31 – 15 = 16 3 more e-’s Now, try notation for O-15

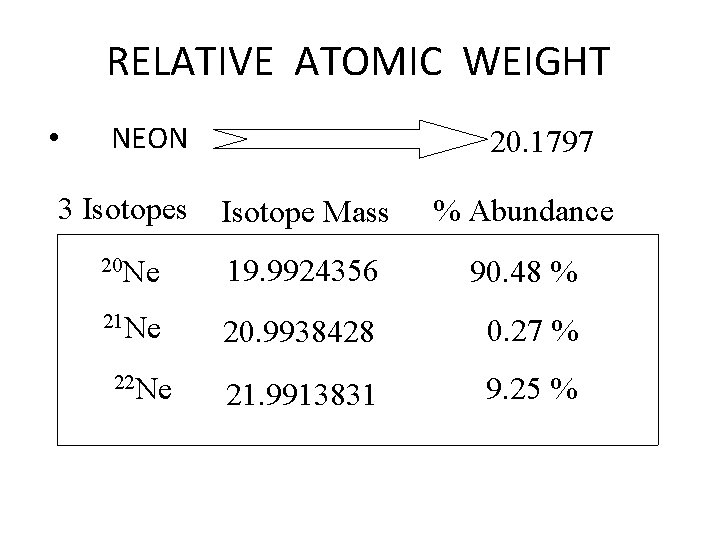
RELATIVE ATOMIC WEIGHT • NEON 3 Isotopes 20. 1797 Isotope Mass % Abundance 20 Ne 19. 9924356 90. 48 % 21 Ne 20. 9938428 0. 27 % 21. 9913831 9. 25 % 22 Ne

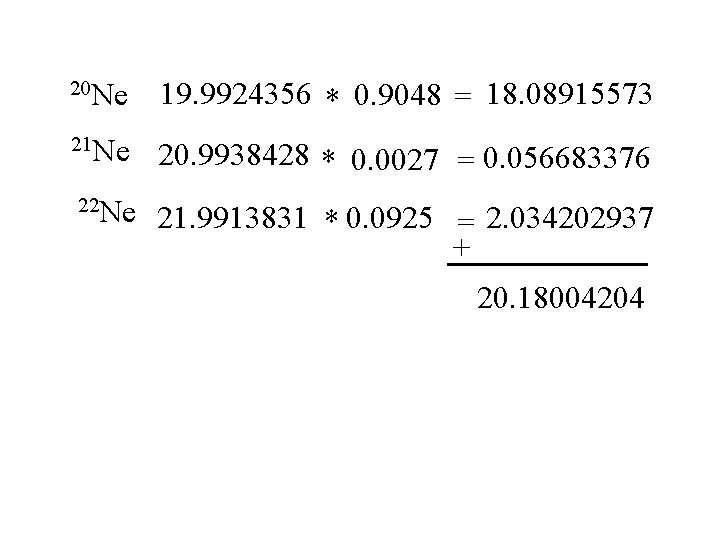
20 Ne 19. 9924356 * 0. 9048 = 18. 08915573 21 Ne 20. 9938428 * 0. 0027 = 0. 056683376 22 Ne 21. 9913831 * 0. 0925 = 2. 034202937 + 20. 18004204

ATOMIC MASS SCALE 1 amu = 1. 66054*10 -24 g 1 g = 6. 02214*1023 amu Now, mass (g) of n 0, p+, eparticles charge p+, protons “+” n 0, neutrons “ 0” e-, electrons “-” mass 1 1 0 1. 0073 amu 1. 0087 amu 5. 486*10 -4 amu

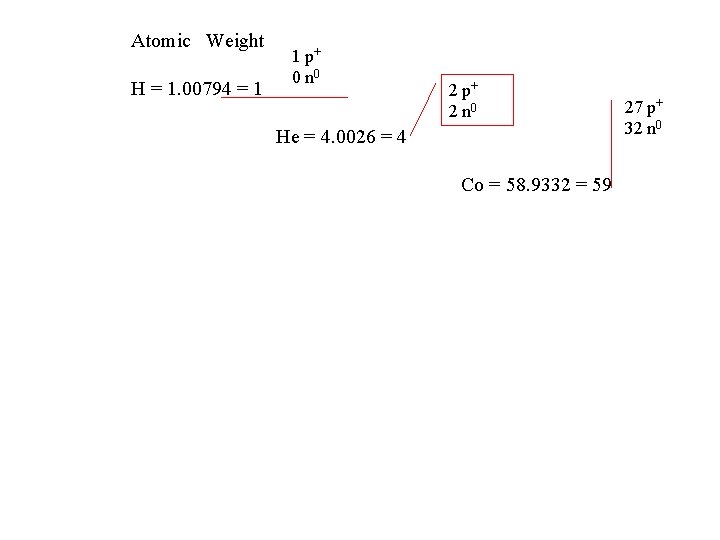
Atomic Weight H = 1. 00794 = 1 1 p+ 0 n 0 2 p+ 2 n 0 He = 4. 0026 = 4 Co = 58. 9332 = 59 27 p+ 32 n 0

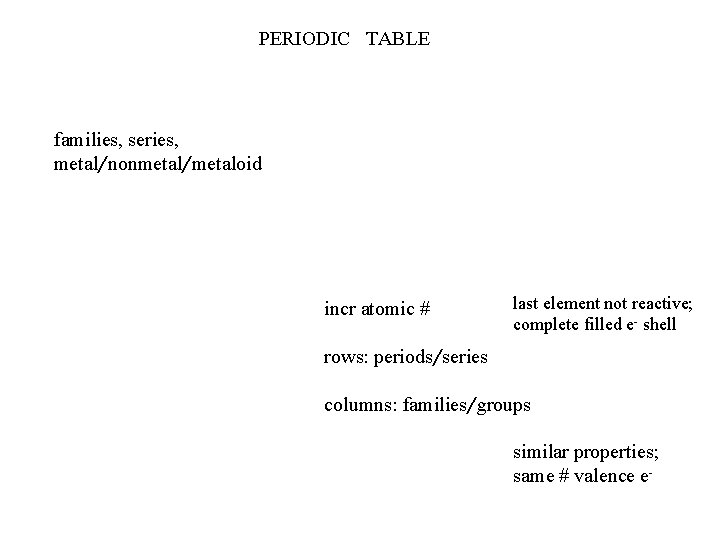
PERIODIC TABLE families, series, metal/nonmetal/metaloid incr atomic # last element not reactive; complete filled e- shell rows: periods/series columns: families/groups similar properties; same # valence e-

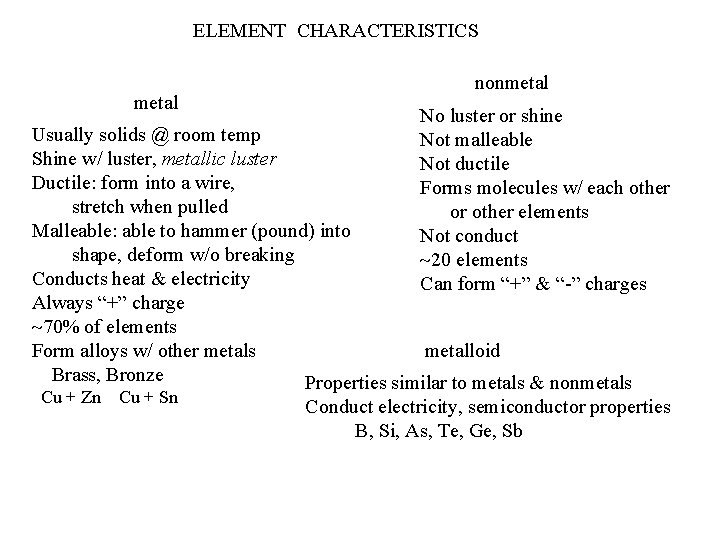
ELEMENT CHARACTERISTICS metal nonmetal No luster or shine Not malleable Not ductile Forms molecules w/ each other or other elements Not conduct 20 elements Can form “+” & “-” charges Usually solids @ room temp Shine w/ luster, metallic luster Ductile: form into a wire, stretch when pulled Malleable: able to hammer (pound) into shape, deform w/o breaking Conducts heat & electricity Always “+” charge 70% of elements metalloid Form alloys w/ other metals Brass, Bronze Properties similar to metals & nonmetals Cu + Zn Cu + Sn Conduct electricity, semiconductor properties B, Si, As, Te, Ge, Sb

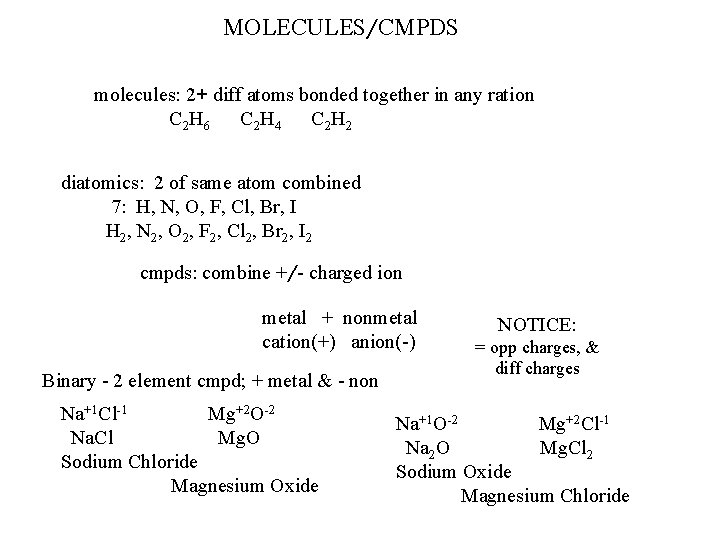
MOLECULES/CMPDS molecules: 2+ diff atoms bonded together in any ration C 2 H 6 C 2 H 4 C 2 H 2 diatomics: 2 of same atom combined 7: H, N, O, F, Cl, Br, I H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2 cmpds: combine +/- charged ion metal + nonmetal cation(+) anion(-) Binary - 2 element cmpd; + metal & - non Na+1 Cl-1 Mg+2 O-2 Na. Cl Mg. O Sodium Chloride Magnesium Oxide NOTICE: = opp charges, & diff charges Na+1 O-2 Mg+2 Cl-1 Na 2 O Mg. Cl 2 Sodium Oxide Magnesium Chloride

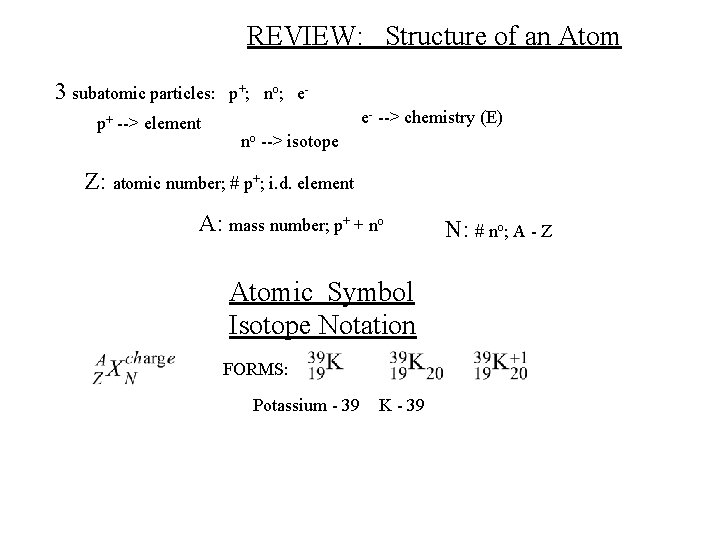
REVIEW: Structure of an Atom 3 subatomic particles: p+ --> element p + ; no ; e e- --> chemistry (E) no --> isotope Z: atomic number; # p+; i. d. element A: mass number; p+ + no Atomic Symbol Isotope Notation FORMS: Potassium - 39 K - 39 N: # no; A - Z

ISOTOPE same element, same # p+, only change # no Diff. make-up of same element 1) Not change element itself, same # p+ 2) Diff. # of n 0 3) Change atomic weight/mass result is change in Density

ATOM ---charged Termed: ION Cation: + charged # p+ > # e# p+ < # e- Anion: - charged “+” charge “-” charge same element, same # p+, only change # e# p+ = # eno charge to atom magnitude p+ charge = that of e- but “+” in sign

PERIODIC TABLE - arranged atomic # - 7 rows; periods/series - 18 columns; groups/families elements in each group – similar chemical properties Groups numbered in 2 ways Group A/B Group 1 - 18 Group A/B Main Group “A” : first 2 col. left, last 6 col. rgt. Transistion Metals “B”: 10 col. in middlde Group 1 – 18 Each col. numbered 1 thru 18, left to right Inner Transition Metals (Rare Earth) Lanthanide Series; elements 58 - 71 Actinide Series; elements 90 – 103 14 col. , not numbered

1 A : ALKALI METALS elements 1 st col. : Li, Na, K, Rb, Cs, Fr Shiny, soft, low melting pt. React violently w/ H 2 O produce alkaline (or basic) Not found in pure state, combined w/ other elements in cmpd. 2 A: ALKALINE METALS elements 2 nd col. : Be, Mg, Ca, Sr, Ba, Ra shiny, silvery less reactive than 1 A Not found in pure state

7 A: HALOGENS (HALIDES) elements next to last col. : F, Cl, Br, I, At colored, corrosive nonmentals found combined w/ elements Halogen (HALS) - salt 8 A: NOBLE GASES (INERT) elements last col. : He, Ne, Ar, Kr, Xe, Rn colorless gases, nonmetals low reactivity w/ other subst. INERT - nonreactive

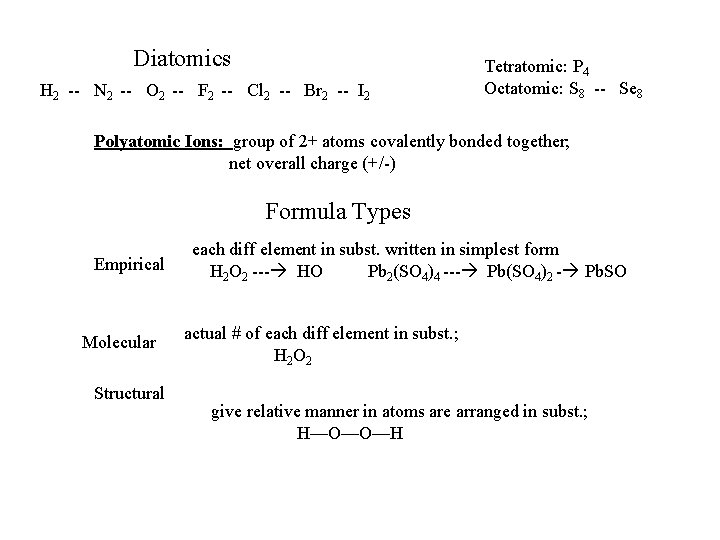
Diatomics H 2 -- N 2 -- O 2 -- F 2 -- Cl 2 -- Br 2 -- I 2 Tetratomic: P 4 Octatomic: S 8 -- Se 8 Polyatomic Ions: group of 2+ atoms covalently bonded together; net overall charge (+/-) Formula Types Empirical Molecular Structural each diff element in subst. written in simplest form H 2 O 2 --- HO Pb 2(SO 4)4 --- Pb(SO 4)2 - Pb. SO actual # of each diff element in subst. ; H 2 O 2 give relative manner in atoms are arranged in subst. ; H—O—O—H

Ionic or Covalent ? ? Ionic: metal + nonmetal joined together to form an ionic cmpd. by forming an ionic bond Covalent: nonmetal + nonmetal joined together to form a covalent molecule by forming a covalent bond COMPOUND Binary: compound comprised of only 2 different elements Name: first element is the name of the metal second element name ends in -ide Mg 3 N 2 Magnesium Nitride PCl 3 Phosphorus Trichloride

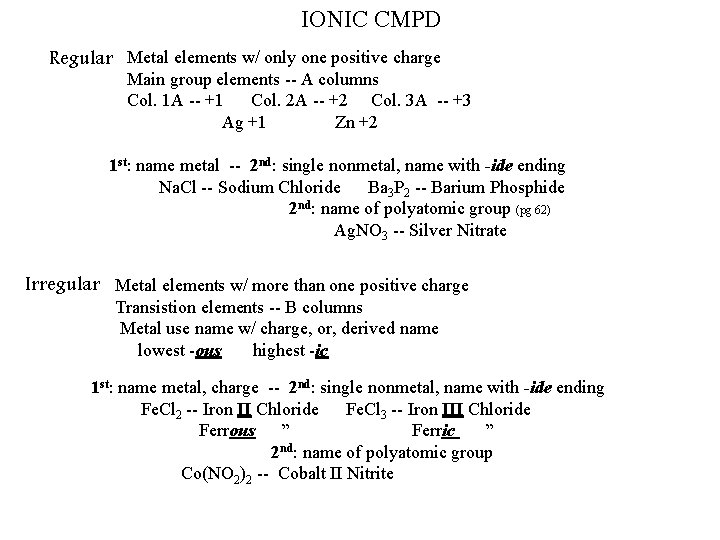
IONIC CMPD Regular Metal elements w/ only one positive charge Main group elements -- A columns Col. 1 A -- +1 Col. 2 A -- +2 Col. 3 A -- +3 Ag +1 Zn +2 1 st: name metal -- 2 nd: single nonmetal, name with -ide ending Na. Cl -- Sodium Chloride Ba 3 P 2 -- Barium Phosphide 2 nd: name of polyatomic group (pg 62) Ag. NO 3 -- Silver Nitrate Irregular Metal elements w/ more than one positive charge Transistion elements -- B columns Metal use name w/ charge, or, derived name lowest -ous highest -ic 1 st: name metal, charge -- 2 nd: single nonmetal, name with -ide ending Fe. Cl 2 -- Iron II Chloride Fe. Cl 3 -- Iron III Chloride Ferrous ” Ferric ” 2 nd: name of polyatomic group Co(NO 2)2 -- Cobalt II Nitrite

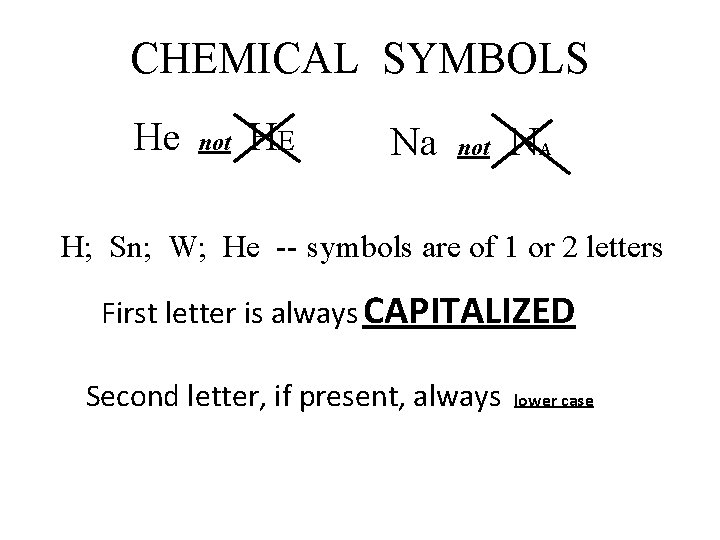
CHEMICAL SYMBOLS He not HE Na not NA H; Sn; W; He -- symbols are of 1 or 2 letters First letter is always CAPITALIZED Second letter, if present, always lower case

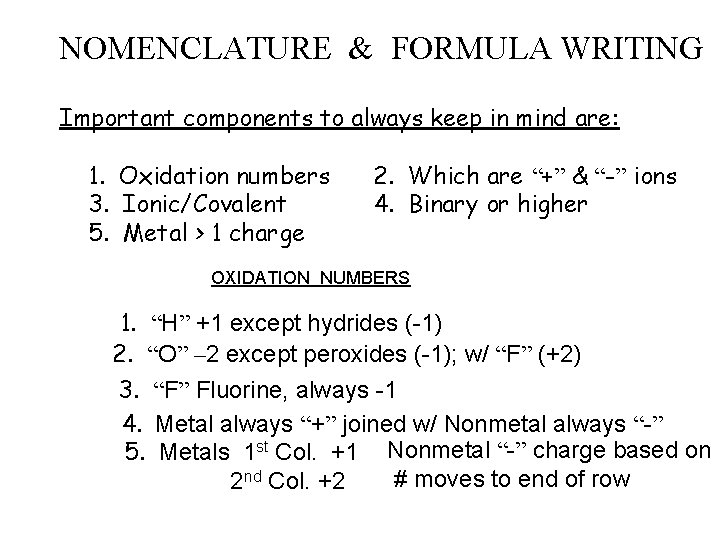
NOMENCLATURE & FORMULA WRITING Important components to always keep in mind are: 1. Oxidation numbers 3. Ionic/Covalent 5. Metal > 1 charge 2. Which are “+” & “-” ions 4. Binary or higher OXIDATION NUMBERS 1. “H” +1 except hydrides (-1) 2. “O” – 2 except peroxides (-1); w/ “F” (+2) 3. “F” Fluorine, always -1 4. Metal always “+” joined w/ Nonmetal always “-” 5. Metals 1 st Col. +1 Nonmetal “-” charge based on # moves to end of row 2 nd Col. +2

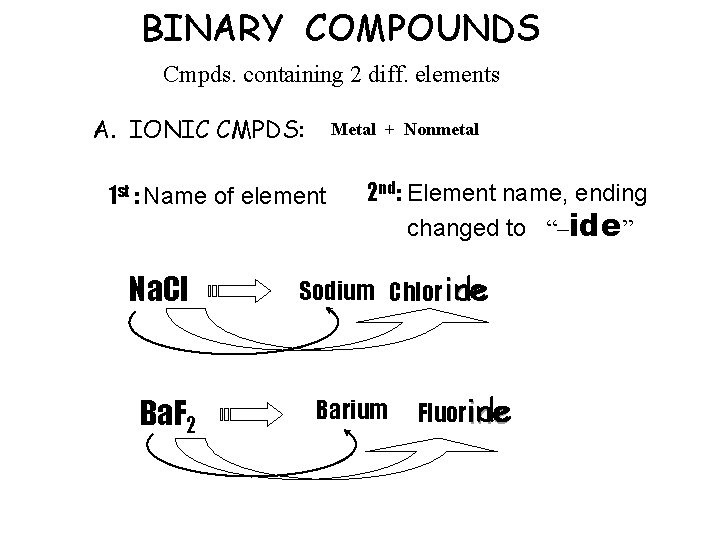
BINARY COMPOUNDS Cmpds. containing 2 diff. elements A. IONIC CMPDS: Metal + Nonmetal 1 st : Name of element Na. Cl Ba. F 2 2 nd: Element name, ending changed to “–ide” Sodium Chlor ine ide Barium ide Fluorine

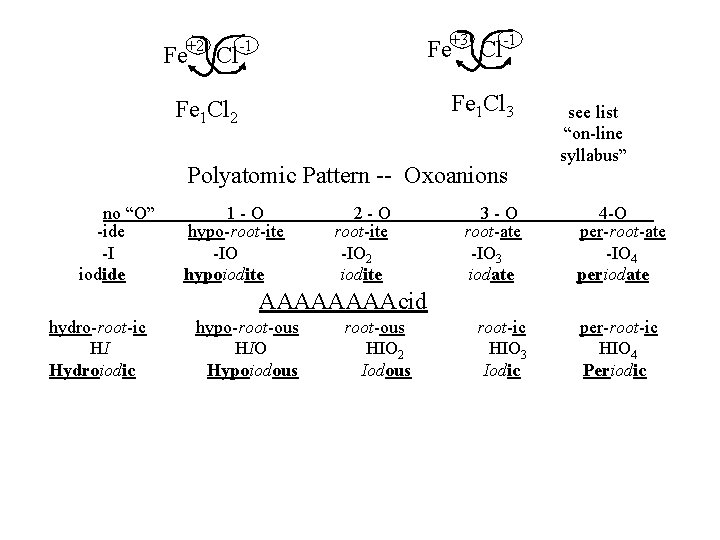
Fe +2 Cl Fe -1 +3 Cl -1 Fe 1 Cl 3 Fe 1 Cl 2 Polyatomic Pattern -- Oxoanions no “O” -ide -I iodide 1 -O hypo-root-ite -IO hypoiodite 2 -O root-ite -IO 2 iodite 3 -O root-ate -IO 3 iodate see list “on-line syllabus” 4 -O per-root-ate -IO 4 periodate AAAAcid hydro-root-ic HI Hydroiodic hypo-root-ous HIO Hypoiodous root-ous HIO 2 Iodous root-ic HIO 3 Iodic per-root-ic HIO 4 Periodic

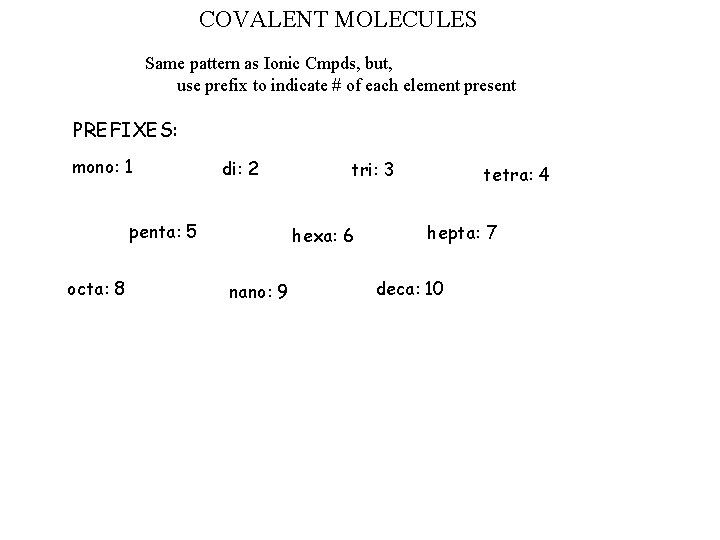
COVALENT MOLECULES Same pattern as Ionic Cmpds, but, use prefix to indicate # of each element present PREFIXES: mono: 1 di: 2 penta: 5 octa: 8 tri: 3 hexa: 6 nano: 9 tetra: 4 hepta: 7 deca: 10

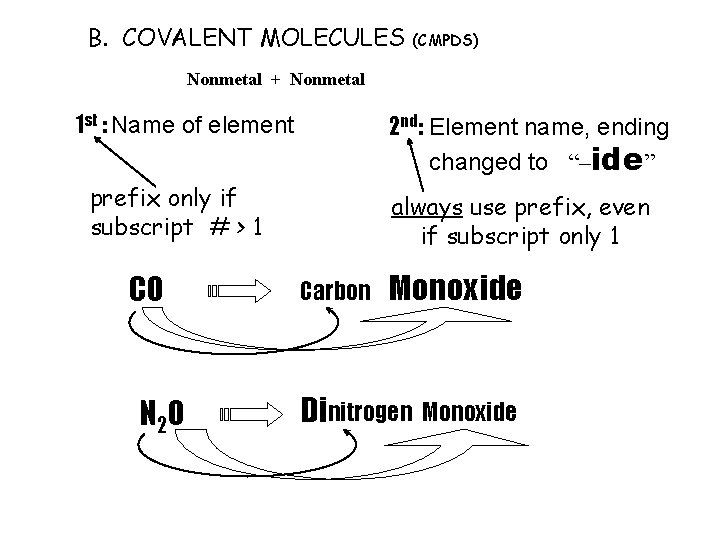
B. COVALENT MOLECULES (CMPDS) Nonmetal + Nonmetal 1 st : Name of element 2 nd: Element name, ending changed to “–ide” prefix only if subscript # > 1 CO N 2 O always use prefix, even if subscript only 1 Carbon Mon oxide Dinitrogen Monoxide

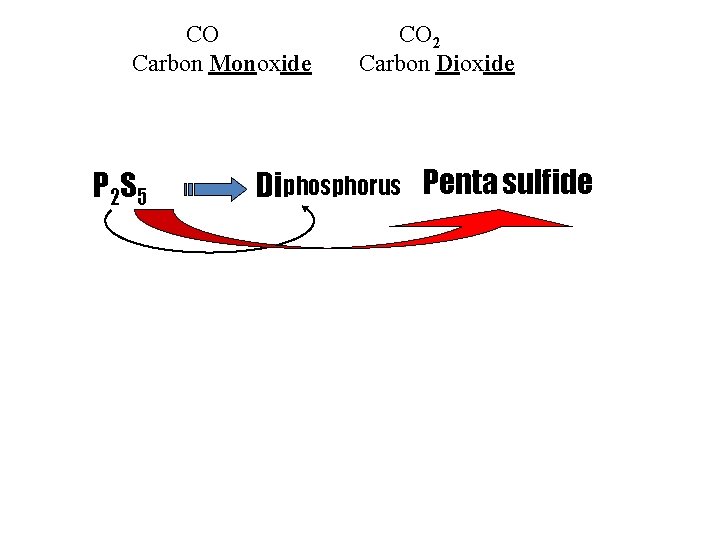
CO Carbon Monoxide P 2 S 5 CO 2 Carbon Dioxide Diphosphorus Penta sulfide

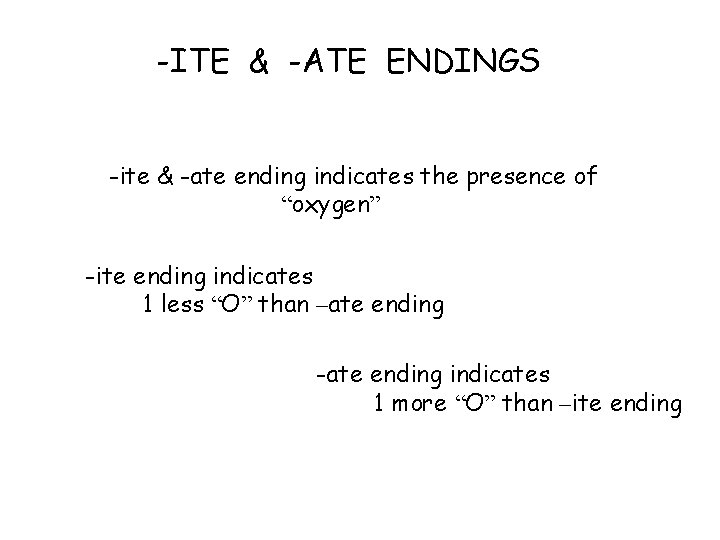
-ITE & -ATE ENDINGS -ite & -ate ending indicates the presence of “oxygen” -ite ending indicates 1 less “O” than –ate ending -ate ending indicates 1 more “O” than –ite ending

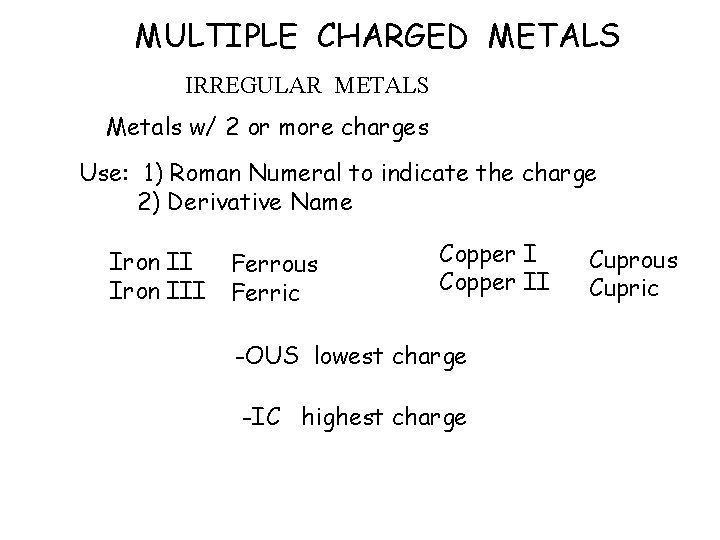
MULTIPLE CHARGED METALS IRREGULAR METALS Metals w/ 2 or more charges Use: 1) Roman Numeral to indicate the charge 2) Derivative Name Iron III Ferrous Ferric Copper II -OUS lowest charge -IC highest charge Cuprous Cupric

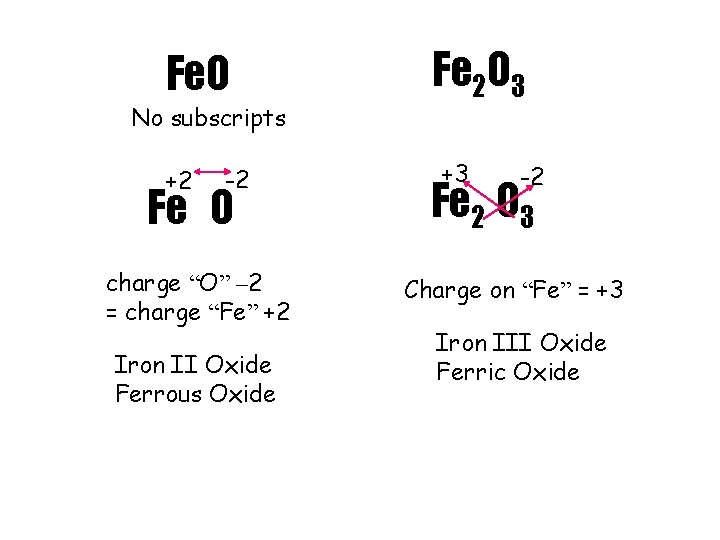
Fe. O No subscripts +2 -2 Fe O charge “O” – 2 = charge “Fe” +2 Iron II Oxide Ferrous Oxide Fe 2 O 3 +3 -2 Fe 2 O 3 Charge on “Fe” = +3 Iron III Oxide Ferric Oxide

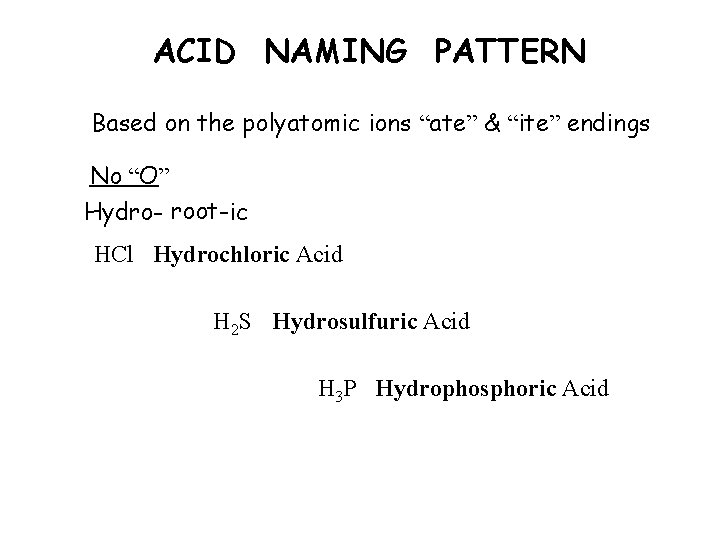
ACID NAMING PATTERN Based on the polyatomic ions “ate” & “ite” endings No “O” Hydro- root-ic HCl Hydrochloric Acid H 2 S Hydrosulfuric Acid H 3 P Hydrophosphoric Acid

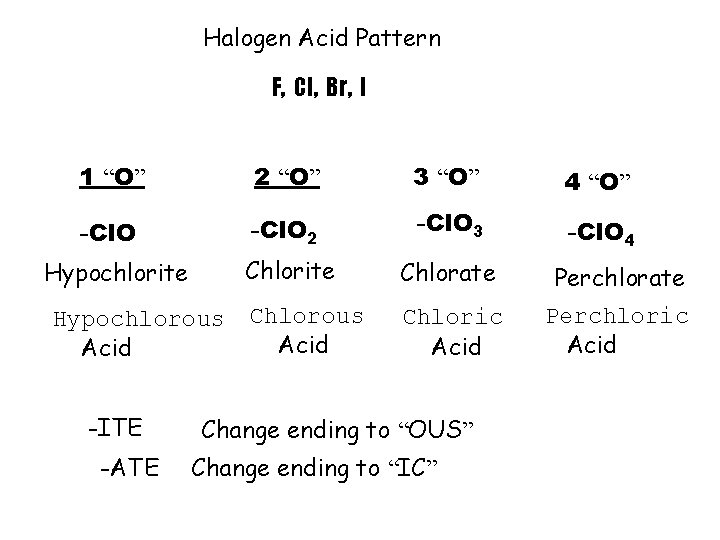
Halogen Acid Pattern F, Cl, Br, I 1 “O” 2 “O” 3 “O” 4 “O” -Cl. O 2 -Cl. O 3 -Cl. O 4 Chlorite Chlorate Perchlorate Chlorous Acid Chloric Acid Perchloric Acid Hypochlorite Hypochlorous Acid -ITE -ATE Change ending to “OUS” Change ending to “IC”

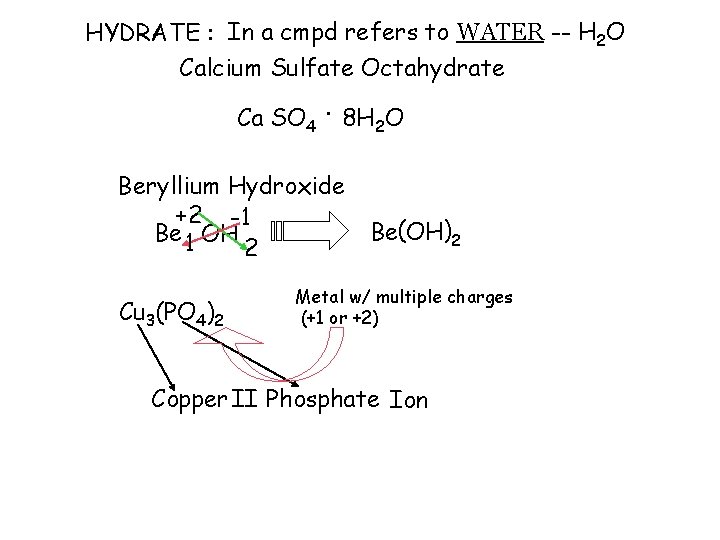
HYDRATE : In a cmpd refers to WATER -- H 2 O Calcium Sulfate Octahydrate Ca SO 4. 8 H 2 O Beryllium Hydroxide +2 -1 Be 1 OH 2 Cu 3(PO 4)2 Be(OH)2 Metal w/ multiple charges (+1 or +2) Copper II Phosphate Ion

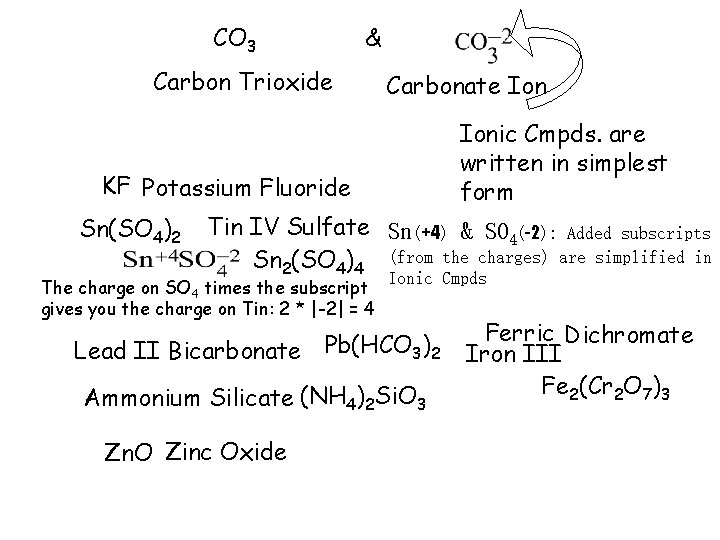
CO 3 & Carbon Trioxide Carbonate Ionic Cmpds. are written in simplest form KF Potassium Fluoride Sn(SO 4)2 Tin IV Sulfate Sn(+4) & SO 4(-2): Added subscripts Sn 2(SO 4)4 (from the charges) are simplified in The charge on SO 4 times the subscript gives you the charge on Tin: 2 * |-2| = 4 Ionic Cmpds Lead II Bicarbonate Pb(HCO 3)2 Ammonium Silicate (NH 4)2 Si. O 3 Zn. O Zinc Oxide Ferric Dichromate Iron III Fe 2(Cr 2 O 7)3

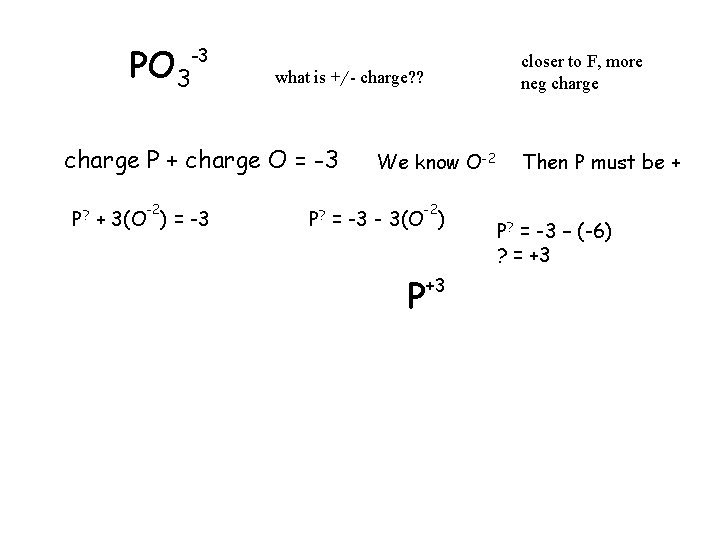
PO 3 -3 what is +/- charge? ? charge P + charge O = -3 -2 P? + 3(O ) = -3 closer to F, more neg charge We know O-2 -2 P? = -3 - 3(O ) P +3 Then P must be + P? = -3 – (-6) ? = +3

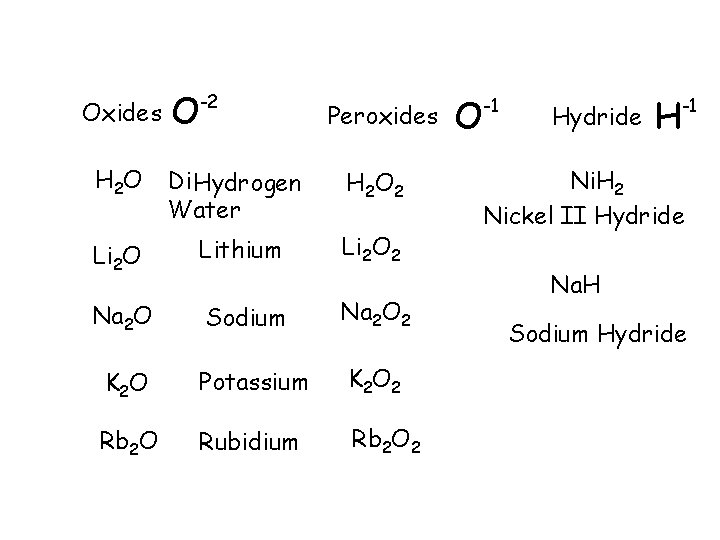
EXCEPTIONS Oxides O -2 Peroxides H 2 O Di Hydrogen Water H 2 O 2 Li 2 O Lithium Li 2 O 2 Na 2 O Sodium Na 2 O 2 K 2 O Potassium K 2 O 2 Rb 2 O Rubidium Rb 2 O 2 O -1 Hydride H -1 Ni. H 2 Nickel II Hydride Na. H Sodium Hydride