Ionic Bonding Learning Objectives 1 Describe how ions

Ionic Bonding Learning Objectives: 1. Describe how ions are formed. 2. Draw dot and cross diagrams to represent ions and ionic compounds. 3. Describe the lattice structure of ionic compounds. 4. Explain how electrostatic forces hold ionic compounds together.
Review: What are the types of bonding? Different types of bonds are formed depending on the types of atoms involved: give/take el ionic bonding – occurs between metal and non-metal atoms. l covalent bonding – occurs between non-metals atoms only. share el metallic bonding – occurs between metal atoms only. share e- All bonds involve electrons and all bonding involve changes to the number of electrons in the outer shells of atoms.
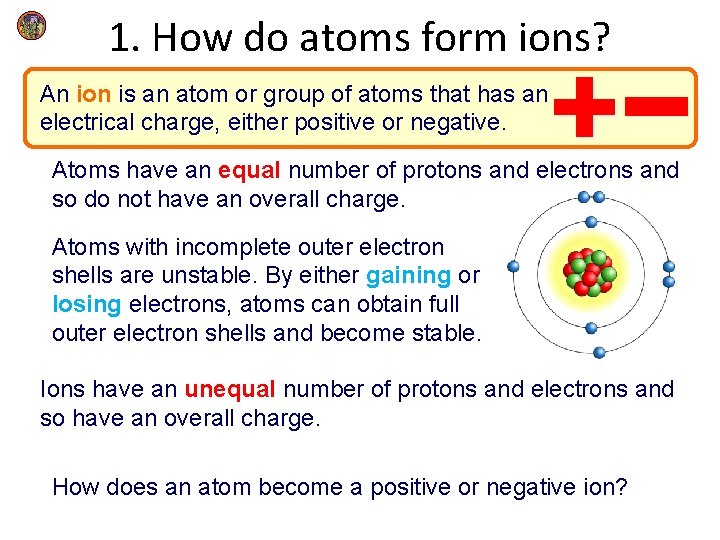
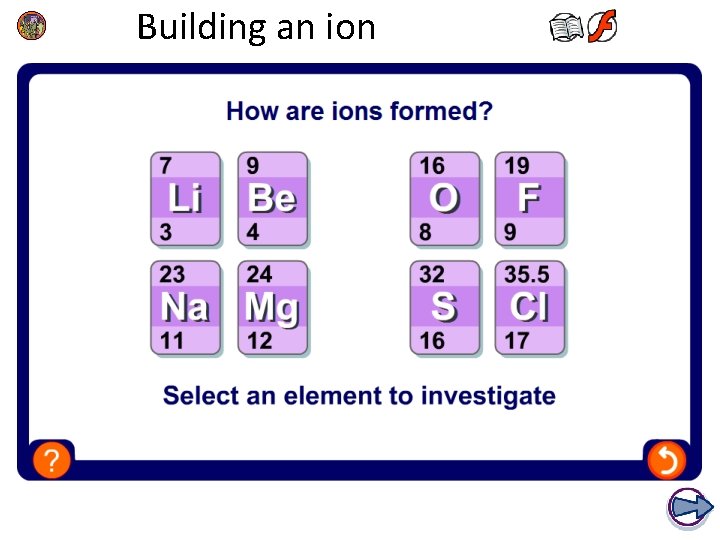
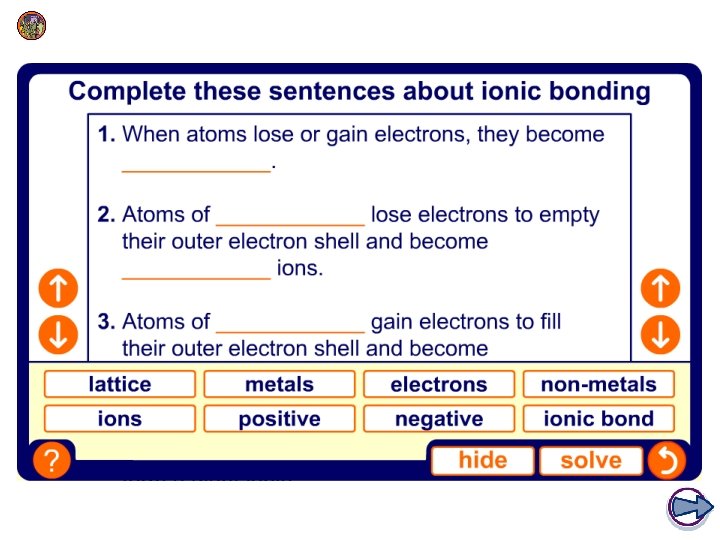
1. How do atoms form ions? An ion is an atom or group of atoms that has an electrical charge, either positive or negative. Atoms have an equal number of protons and electrons and so do not have an overall charge. Atoms with incomplete outer electron shells are unstable. By either gaining or losing electrons, atoms can obtain full outer electron shells and become stable. Ions have an unequal number of protons and electrons and so have an overall charge. How does an atom become a positive or negative ion?

2. Positive and negative ions? An atom that loses electrons has more protons than electrons and so has a positive overall charge. This is called a positive ion = cation. An atom that gains electrons has more electrons than protons and so has a negative overall charge. This is called a negative ion = anion. The electron configuration of an atom shows how many electrons it must lose or gain to have a filled outer shell. l Atoms with a nearly empty outer shell, will lose electrons to obtain a full outer shell. l Atoms with a nearly full outer shell, will gain electrons to obtain a full outer shell.
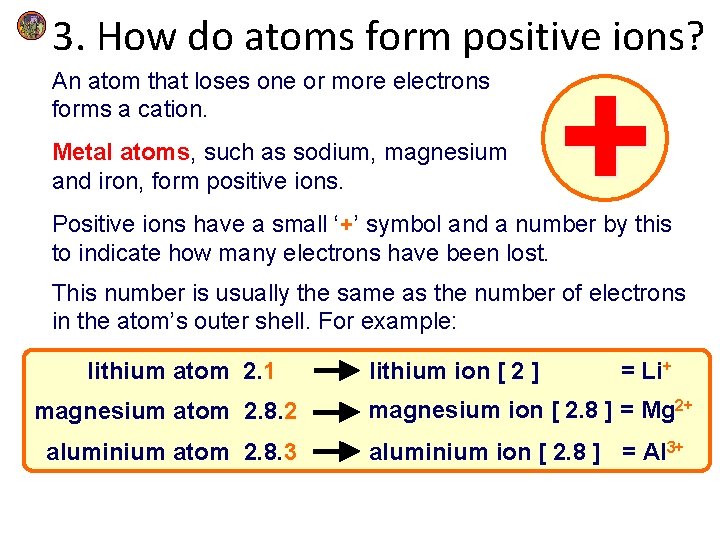
3. How do atoms form positive ions? An atom that loses one or more electrons forms a cation. Metal atoms, such as sodium, magnesium and iron, form positive ions. Positive ions have a small ‘+’ symbol and a number by this to indicate how many electrons have been lost. This number is usually the same as the number of electrons in the atom’s outer shell. For example: lithium atom 2. 1 lithium ion [ 2 ] = Li+ magnesium atom 2. 8. 2 magnesium ion [ 2. 8 ] = Mg 2+ aluminium atom 2. 8. 3 aluminium ion [ 2. 8 ] = Al 3+
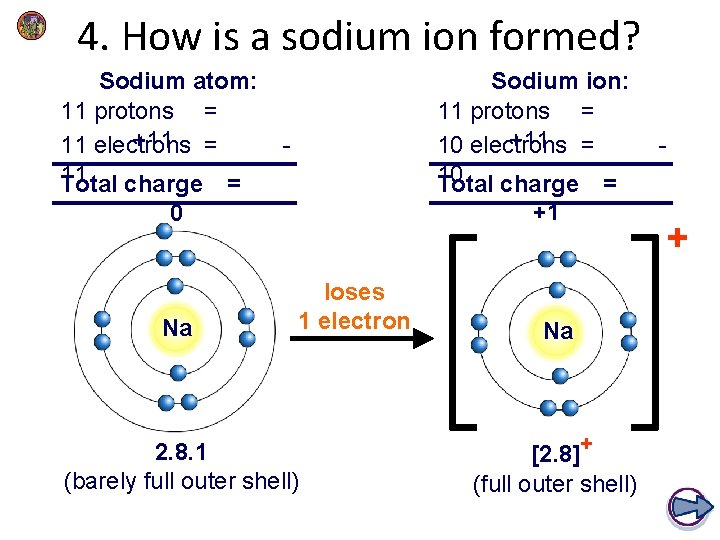
4. How is a sodium ion formed? Sodium atom: 11 protons = +11 = 11 electrons 11 Total charge = Sodium ion: 11 protons = +11 = 10 electrons 10 Total charge = - +1 0 Na loses 1 electron 2. 8. 1 (barely full outer shell) Na [2. 8]+ (full outer shell) - +
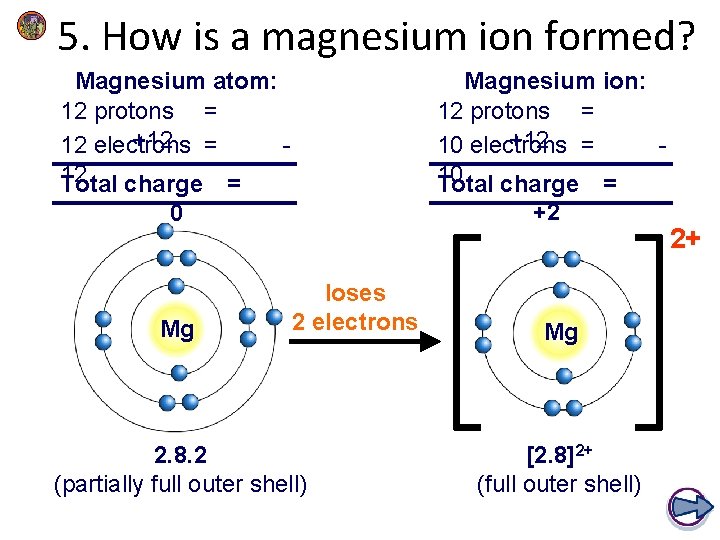
5. How is a magnesium ion formed? Magnesium atom: 12 protons = +12 = 12 electrons 12 Total charge = Magnesium ion: 12 protons = +12 = 10 electrons 10 Total charge = 0 +2 Mg loses 2 electrons 2. 8. 2 (partially full outer shell) Mg [2. 8]2+ (full outer shell) 2+
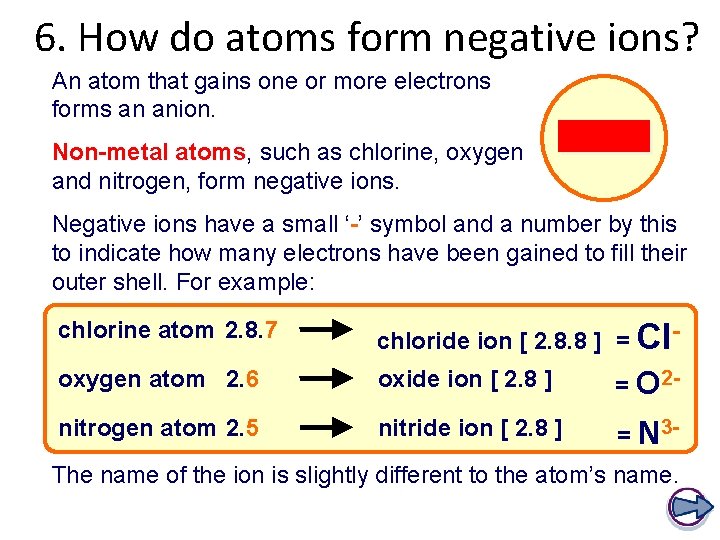
6. How do atoms form negative ions? An atom that gains one or more electrons forms an anion. Non-metal atoms, such as chlorine, oxygen and nitrogen, form negative ions. Negative ions have a small ‘-’ symbol and a number by this to indicate how many electrons have been gained to fill their outer shell. For example: chlorine atom 2. 8. 7 chloride ion [ 2. 8. 8 ] = Cl- oxygen atom 2. 6 oxide ion [ 2. 8 ] = O 2 - nitrogen atom 2. 5 nitride ion [ 2. 8 ] = N 3 - The name of the ion is slightly different to the atom’s name.
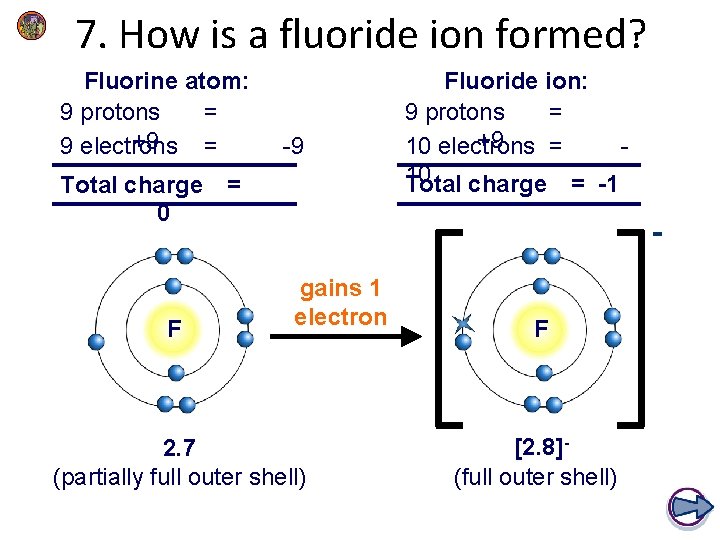
7. How is a fluoride ion formed? Fluorine atom: 9 protons = +9 9 electrons = -9 Total charge = 0 F Fluoride ion: 9 protons = +9 10 electrons = 10 Total charge = -1 gains 1 electron 2. 7 (partially full outer shell) F [2. 8](full outer shell)
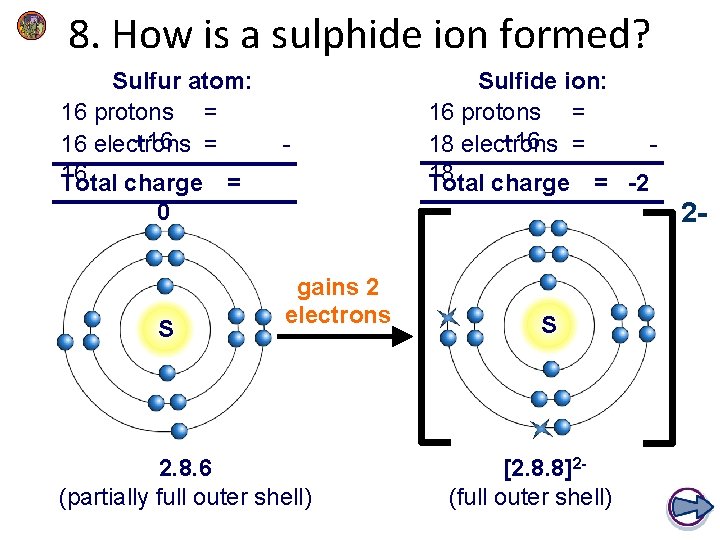
8. How is a sulphide ion formed? Sulfur atom: 16 protons = +16 = 16 electrons 16 Total charge = - Sulfide ion: 16 protons = +16 = 18 electrons 18 Total charge = -2 2 - 0 S gains 2 electrons 2. 8. 6 (partially full outer shell) S [2. 8. 8]2(full outer shell)
Building an ion
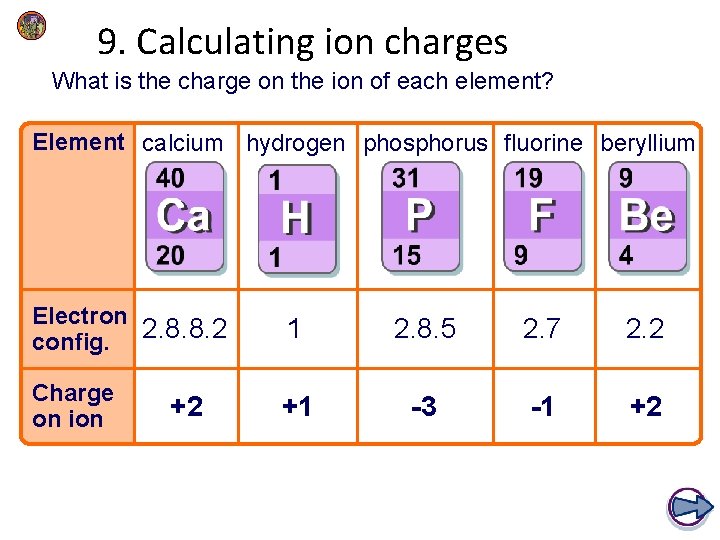
9. Calculating ion charges What is the charge on the ion of each element? Element calcium hydrogen phosphorus fluorine beryllium Electron config. 2. 8. 8. 2 1 2. 8. 5 2. 7 2. 2 Charge on ion +1 -3 -1 +2 +2
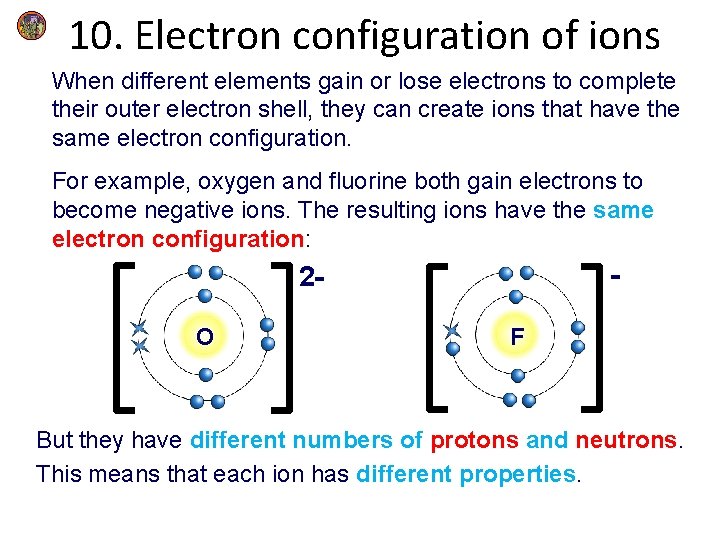
10. Electron configuration of ions When different elements gain or lose electrons to complete their outer electron shell, they can create ions that have the same electron configuration. For example, oxygen and fluorine both gain electrons to become negative ions. The resulting ions have the same electron configuration: - 2 O F But they have different numbers of protons and neutrons. This means that each ion has different properties.
Comparing electron configurations
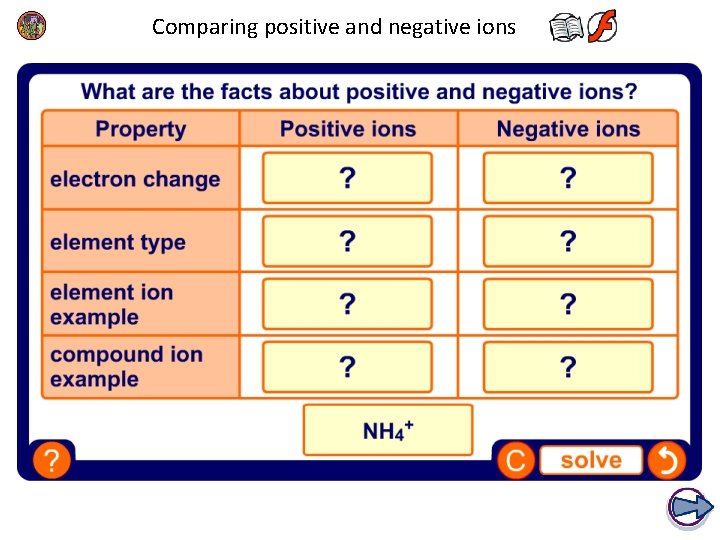
Comparing positive and negative ions
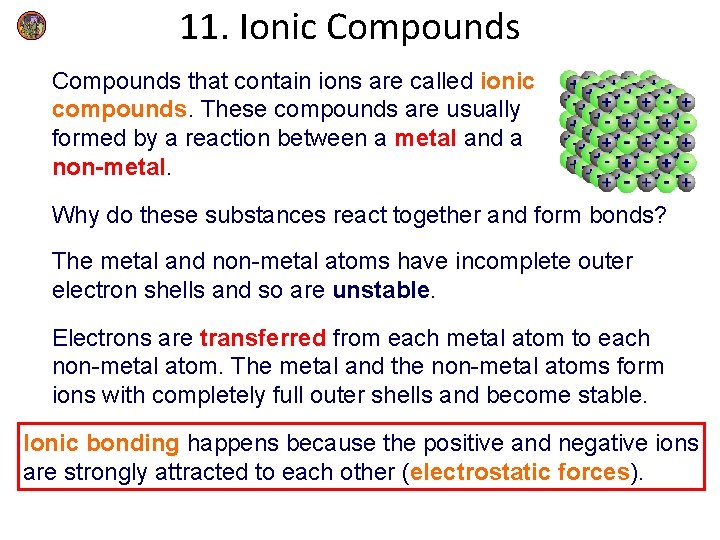
11. Ionic Compounds that contain ions are called ionic compounds. These compounds are usually formed by a reaction between a metal and a non-metal. Why do these substances react together and form bonds? The metal and non-metal atoms have incomplete outer electron shells and so are unstable. Electrons are transferred from each metal atom to each non-metal atom. The metal and the non-metal atoms form ions with completely full outer shells and become stable. Ionic bonding happens because the positive and negative ions are strongly attracted to each other (electrostatic forces).
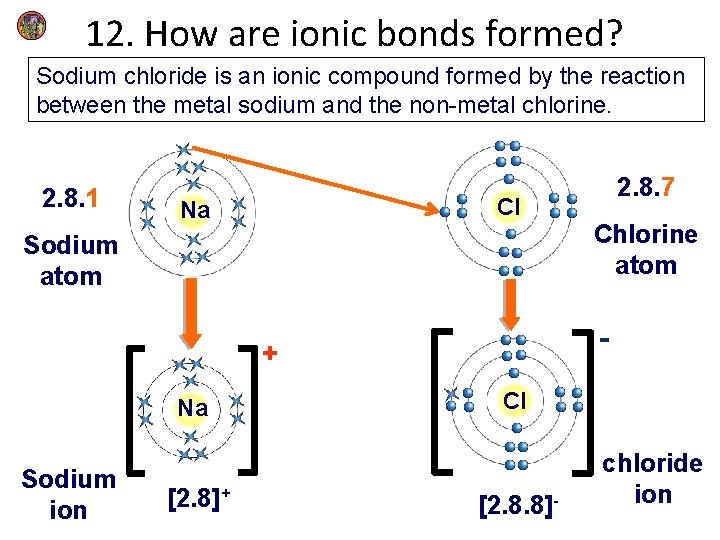
12. How are ionic bonds formed? Sodium chloride is an ionic compound formed by the reaction between the metal sodium and the non-metal chlorine. 2. 8. 1 Cl Na Sodium atom Sodium ion [2. 8]+ Chlorine atom - + Na 2. 8. 7 Cl [2. 8. 8]- chloride ion
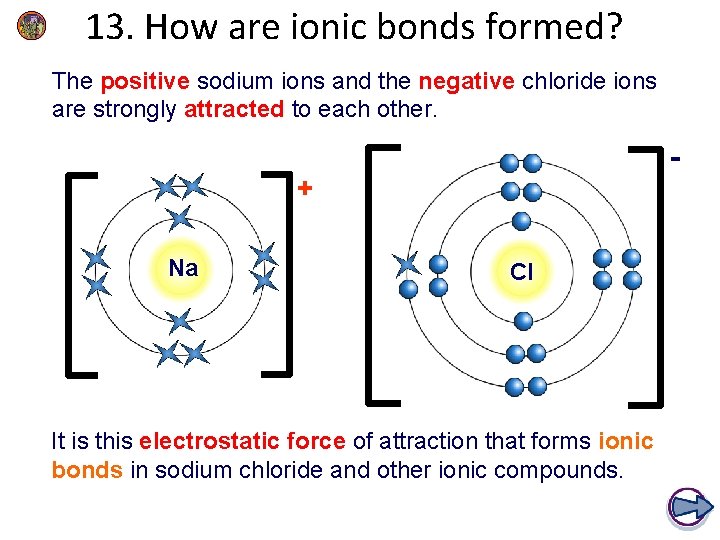
13. How are ionic bonds formed? The positive sodium ions and the negative chloride ions are strongly attracted to each other. + Na Cl It is this electrostatic force of attraction that forms ionic bonds in sodium chloride and other ionic compounds.
![14. example Lithium bonding to Fluorine 2. 1 - + Li [2]+ 2. 7 14. example Lithium bonding to Fluorine 2. 1 - + Li [2]+ 2. 7](http://slidetodoc.com/presentation_image_h/142129947dc558b30c58fa9b96691816/image-19.jpg)
14. example Lithium bonding to Fluorine 2. 1 - + Li [2]+ 2. 7 F Li F [2. 8]-

So what do we call ionic compounds? C e d i r o l h C m u i c al

15. Naming Simple Ionic Compounds Metal + Non-Metal 1. First word: Write out the full name of the first element (metal) of the chemical formula. 2. Second word: Write out the root of the second element (non-metal) and add –ide to the end. Numbers do NOT matter! Example: Mg. O = magnesium oxide Ca. F 2 = calcium fluoride

16. Name that Compound! Chemical Formula A. Na. Cl B. Cu. O C. KBr D. Ca. Cl 2 E. Zn. S Name A. Sodium Chloride B. Copper Oxide C. Potassium Bromide D. Calcium Chloride E. Zinc Sulphide
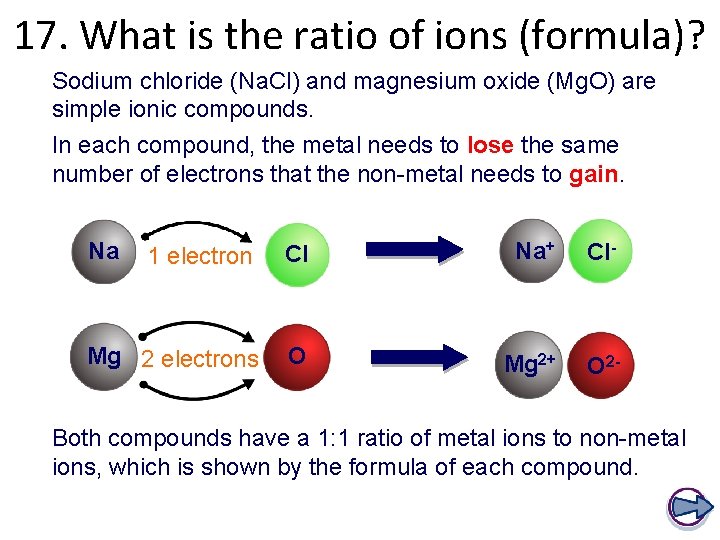
17. What is the ratio of ions (formula)? Sodium chloride (Na. Cl) and magnesium oxide (Mg. O) are simple ionic compounds. In each compound, the metal needs to lose the same number of electrons that the non-metal needs to gain. Na 1 electron Cl Na+ Cl- Mg 2 electrons O Mg 2+ O 2 - Both compounds have a 1: 1 ratio of metal ions to non-metal ions, which is shown by the formula of each compound.
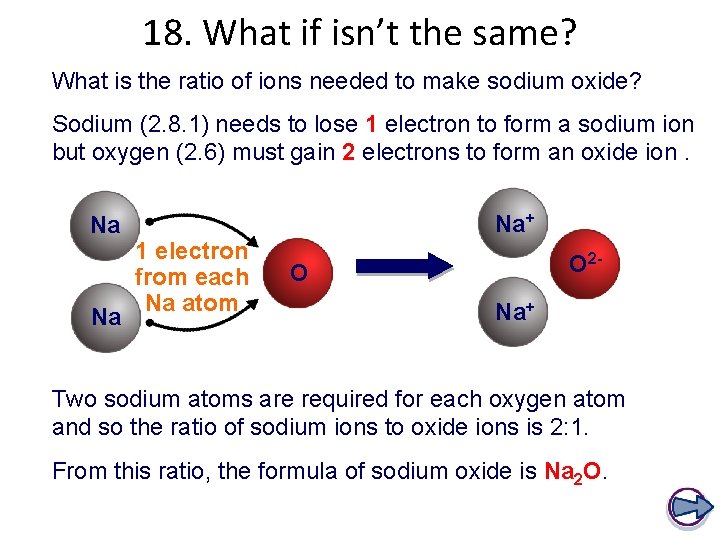
18. What if isn’t the same? What is the ratio of ions needed to make sodium oxide? Sodium (2. 8. 1) needs to lose 1 electron to form a sodium ion but oxygen (2. 6) must gain 2 electrons to form an oxide ion. Na Na Na+ 1 electron from each Na atom O 2 - O Na+ Two sodium atoms are required for each oxygen atom and so the ratio of sodium ions to oxide ions is 2: 1. From this ratio, the formula of sodium oxide is Na 2 O.
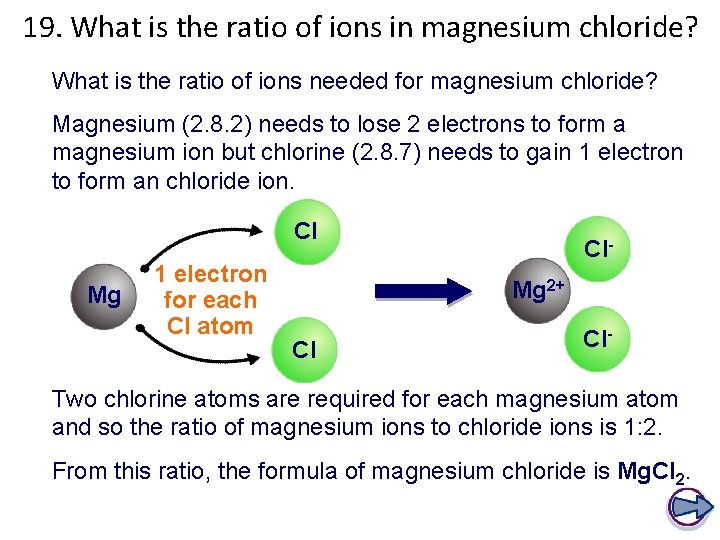
19. What is the ratio of ions in magnesium chloride? What is the ratio of ions needed for magnesium chloride? Magnesium (2. 8. 2) needs to lose 2 electrons to form a magnesium ion but chlorine (2. 8. 7) needs to gain 1 electron to form an chloride ion. Cl Mg 1 electron for each Cl atom Cl. Mg 2+ Cl Cl- Two chlorine atoms are required for each magnesium atom and so the ratio of magnesium ions to chloride ions is 1: 2. From this ratio, the formula of magnesium chloride is Mg. Cl 2.
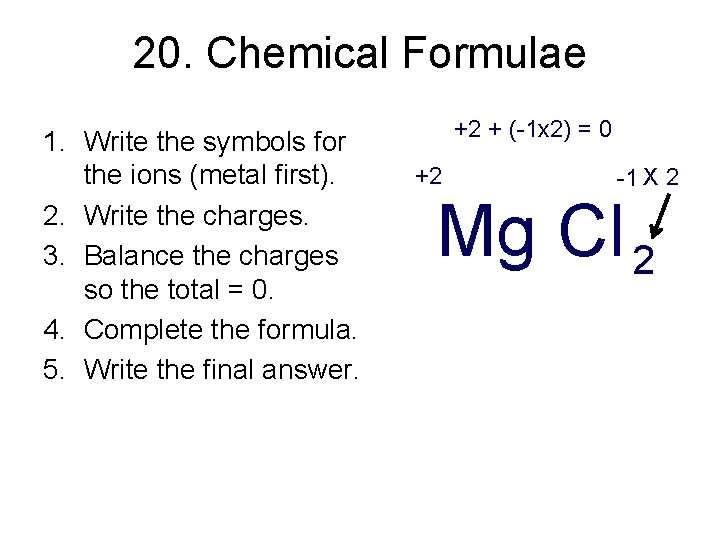
20. Chemical Formulae 1. Write the symbols for the ions (metal first). 2. Write the charges. 3. Balance the charges so the total = 0. 4. Complete the formula. 5. Write the final answer. +2 + (-1 x 2) = 0 +2 -1 X 2 Mg Cl 2
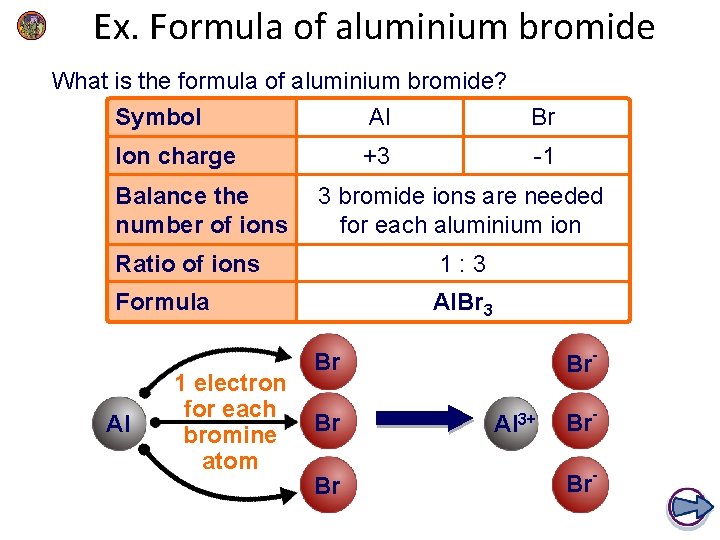
Ex. Formula of aluminium bromide What is the formula of aluminium bromide? Symbol Al Br Ion charge +3 -1 Balance the number of ions 3 bromide ions are needed for each aluminium ion Ratio of ions 1: 3 Formula Al. Br 3 Al 1 electron for each bromine atom Br- Br Br Br Al 3+ Br. Br-
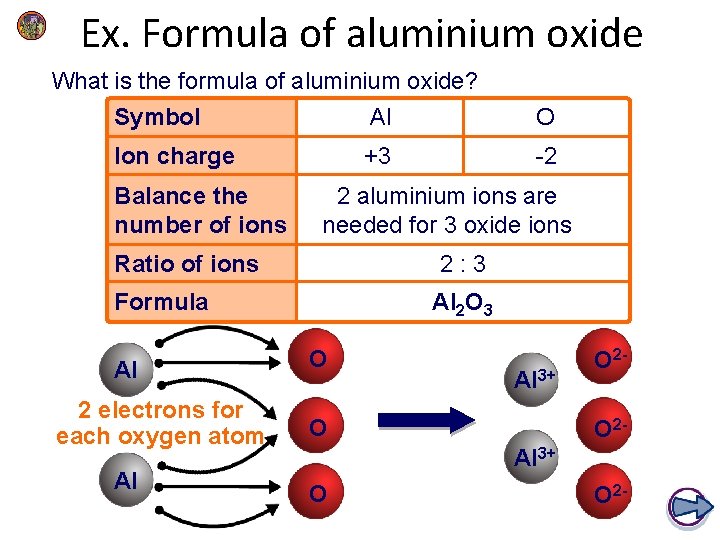
Ex. Formula of aluminium oxide What is the formula of aluminium oxide? Symbol Al O Ion charge +3 -2 Balance the number of ions 2 aluminium ions are needed for 3 oxide ions Ratio of ions 2: 3 Formula Al 2 electrons for each oxygen atom Al Al 2 O 3 O Al 3+ O O 2 O 2 - Al 3+ O O 2 -
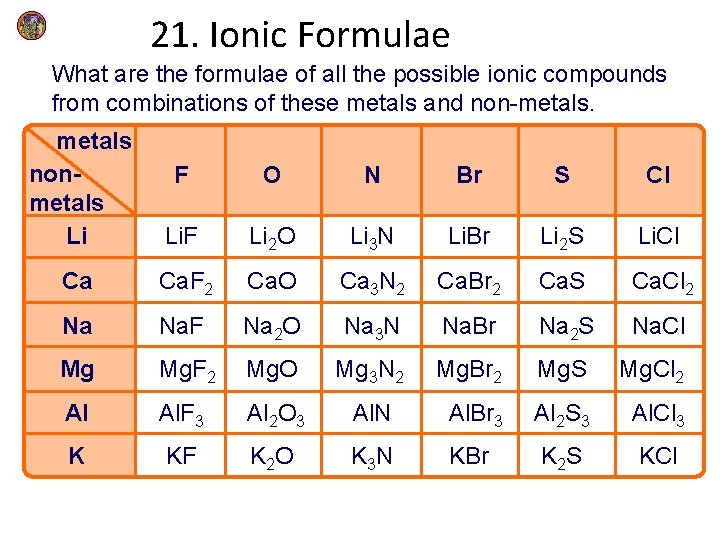
21. Ionic Formulae What are the formulae of all the possible ionic compounds from combinations of these metals and non-metals nonmetals Li F O N Br S Cl Li. F Li 2 O Li 3 N Li. Br Li 2 S Li. Cl Ca Ca. F 2 Ca. O Ca 3 N 2 Ca. Br 2 Ca. S Ca. Cl 2 Na Na. F Na 2 O Na 3 N Na. Br Na 2 S Na. Cl Mg Mg. F 2 Mg. O Mg 3 N 2 Mg. Br 2 Mg. S Mg. Cl 2 Al Al. F 3 Al 2 O 3 Al. N Al. Br 3 Al 2 S 3 Al. Cl 3 K KF K 2 O K 3 N KBr K 2 S KCl
- Slides: 31