Unit 4 The Atom TEST Friday 15 th
























- Slides: 60

Unit 4: The Atom TEST Friday, 15 th Element Trading Cards Project DUE Tuesday th October 29

The Atom Poster • Draw what you think the atom looks like • Drawing must include the atom and a color coded key. Draw Today On Back Name, Date, Period The Atom – Day 1 The Atom – Day 2

SPS 1 a. Examine the structure of the atom in terms of proton, neutron, and electron. Activator: Draw what you think an atom looks like, including the protons, neutrons, and electrons (label).

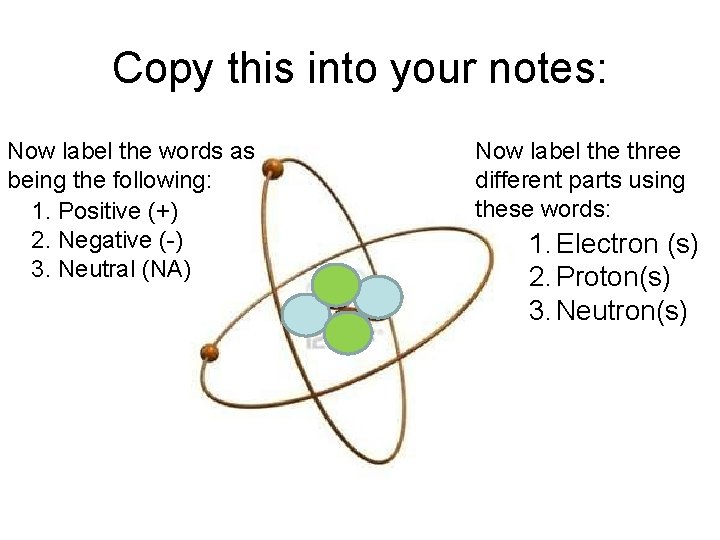
Copy this into your notes: Now label the words as being the following: 1. Positive (+) 2. Negative (-) 3. Neutral (NA) Now label the three different parts using these words: 1. Electron (s) 2. Proton(s) 3. Neutron(s)

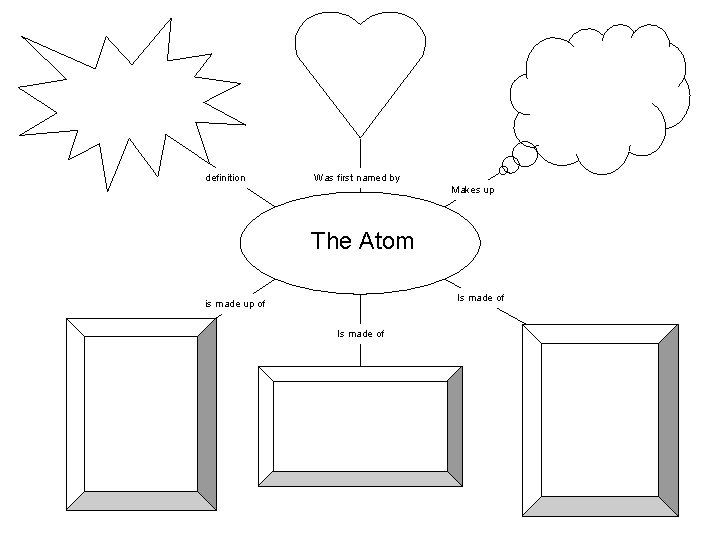
definition Was first named by Makes up The Atom Is made of is made up of Is made of

The smallest piece of matter that still has the chemical and physical properties of that matter.

Democritus in 400 B. C.

All the matter in the universe

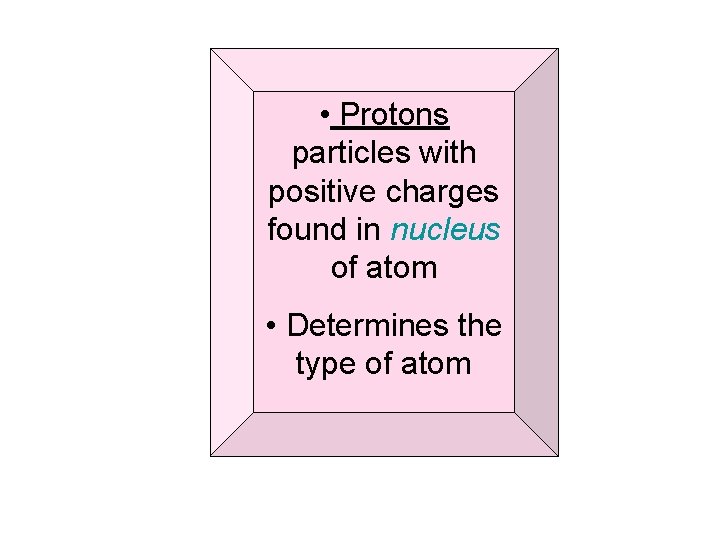
• Protons particles with positive charges found in nucleus of atom • Determines the type of atom

• Neutrons - particles with no charge found in the nucleus of the atom • # of neutrons can vary

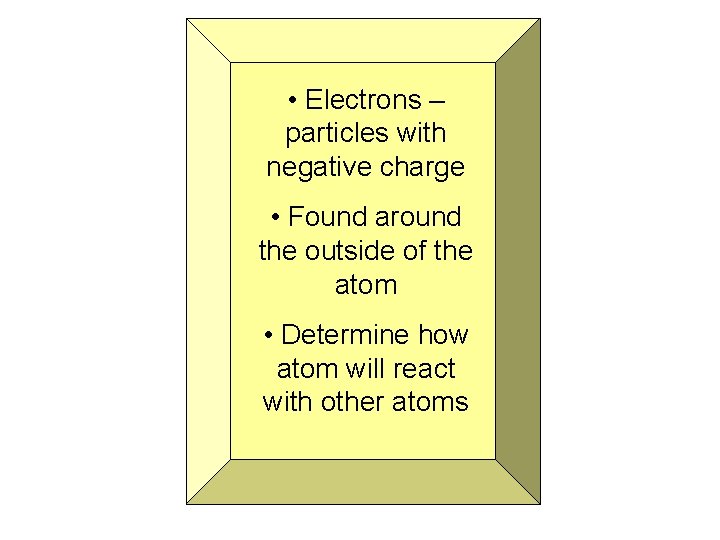
• Electrons – particles with negative charge • Found around the outside of the atom • Determine how atom will react with other atoms


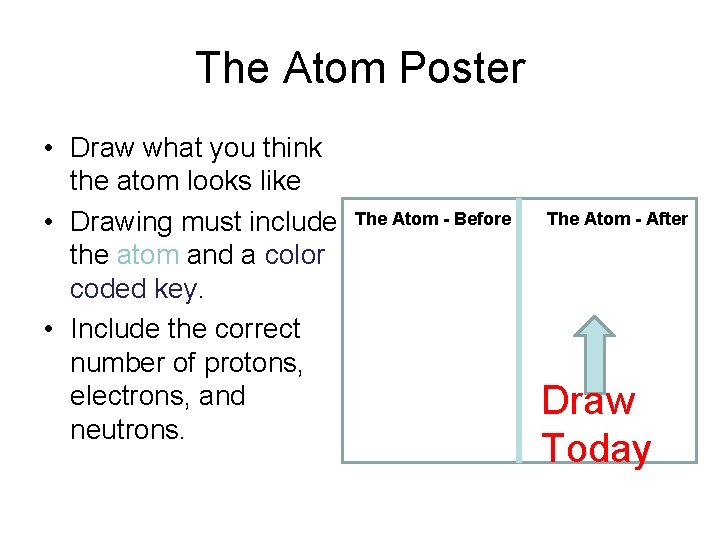
The Atom Poster • Draw what you think the atom looks like • Drawing must include the atom and a color coded key. • Include the correct number of protons, electrons, and neutrons. The Atom - Before The Atom - After Draw Today

TOD: • What was your drawing from yesterday missing?

Atoms

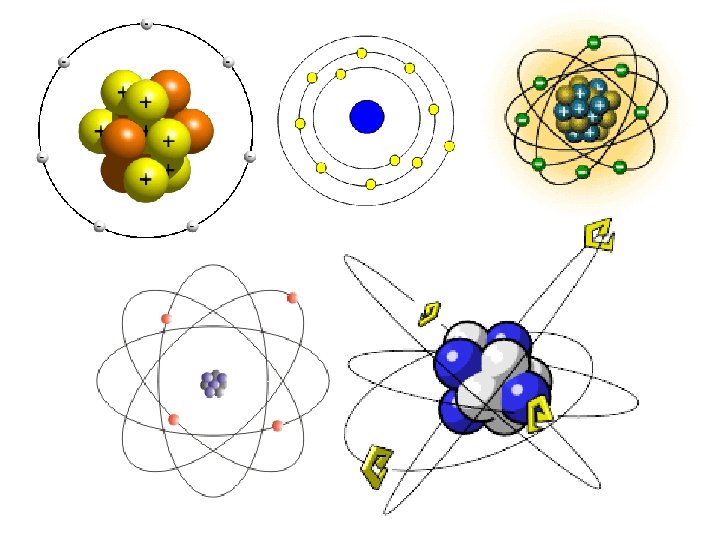
Look at the back of your notes from yesterday. • Are all the pictures of atoms? • How are they similar? • How are they different?

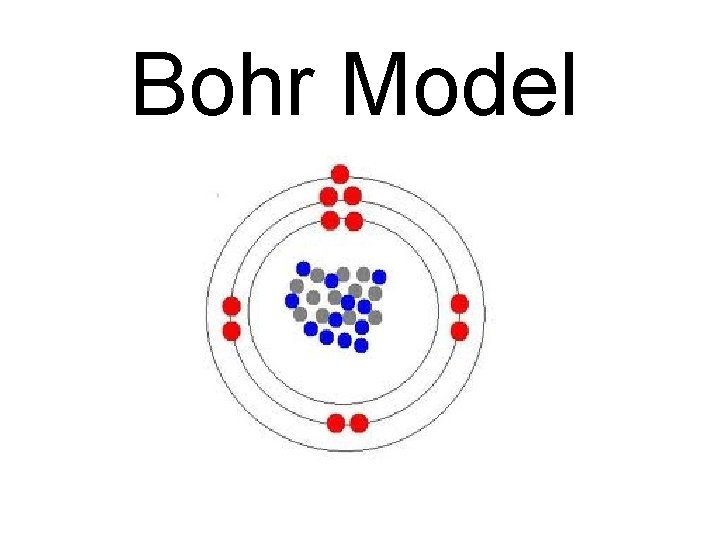
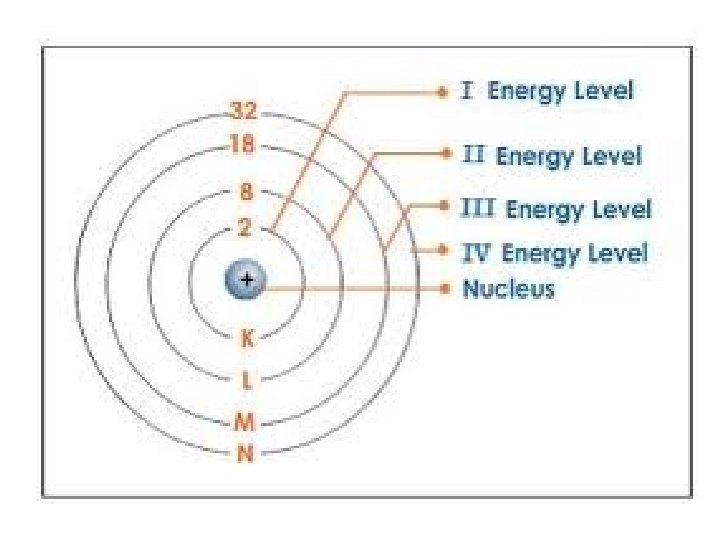
Bohr Model

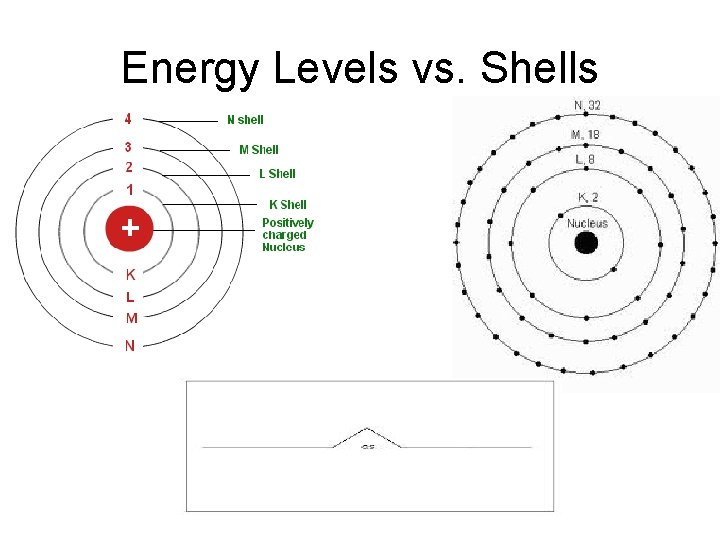
What is a Bohr Model? • Shows all the protons, neutrons, and electrons contained within an atom. • Protons and neutrons are drawn in the Nucleus. • Electrons are drawn on ENERGY LEVELS or SHELLS or ELECTRON CLOUDS (the rings found around the nucleus).

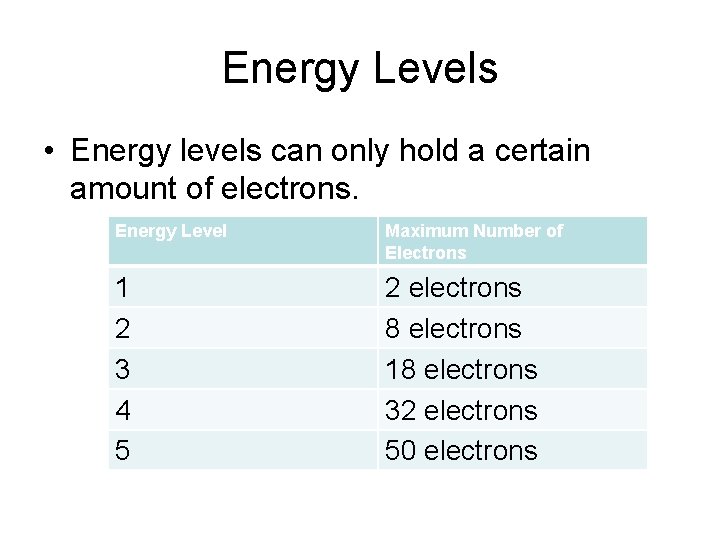
Energy Levels • Energy levels can only hold a certain amount of electrons. Energy Level Maximum Number of Electrons 1 2 3 4 5 2 electrons 8 electrons 18 electrons 32 electrons 50 electrons


Practice • Hydrogen

Practice • Helium

Practice • Magnesium

Finish the following alone: • • Sulfur Oxygen Copper Francium

How are electrons arranged in Atom? • Complete for HOMEWORK! • DUE TOMORROW • FRIDAY

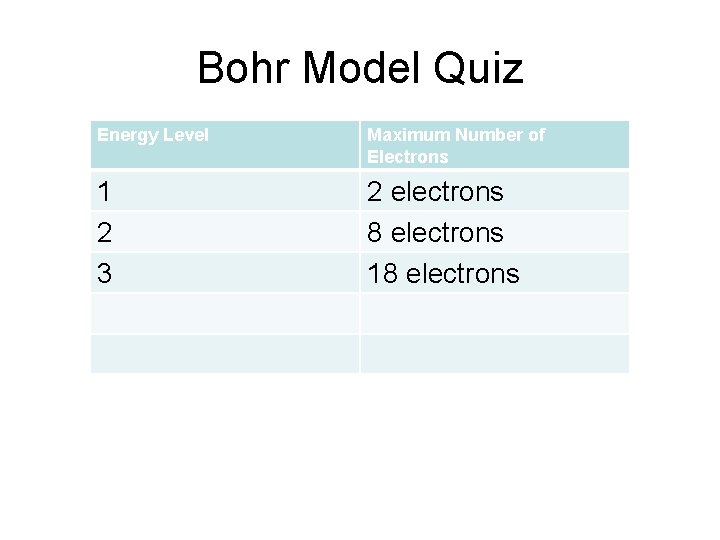
Bohr Model Quiz Energy Level Maximum Number of Electrons 1 2 3 2 electrons 8 electrons 18 electrons

Question 1: Flourine • Protons = 9 • Electrons = 9 • Neutrons = 5

Question 2: Magnesium • Protons = 12 • Electrons = 12 • Neutrons = 12

More Practice • Nitrogen

• Calcium

Do and Answer Draw a Bohr Model for Oxygen Hydrogen (twice) Using the drawing try to draw a Water Molecule. Define: atom, compound, molecule

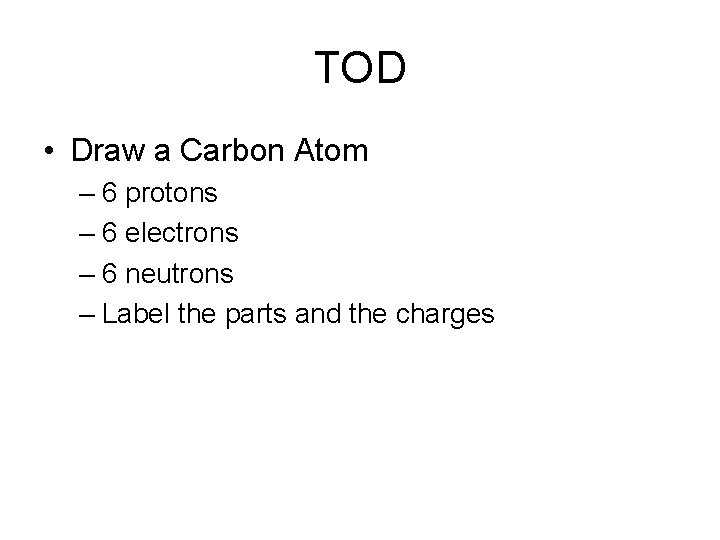
TOD • Draw a Carbon Atom – 6 protons – 6 electrons – 6 neutrons – Label the parts and the charges

In your notes: • Make 3 circle maps for: Electro n Proto n Neutro n

Energy Levels vs. Shells

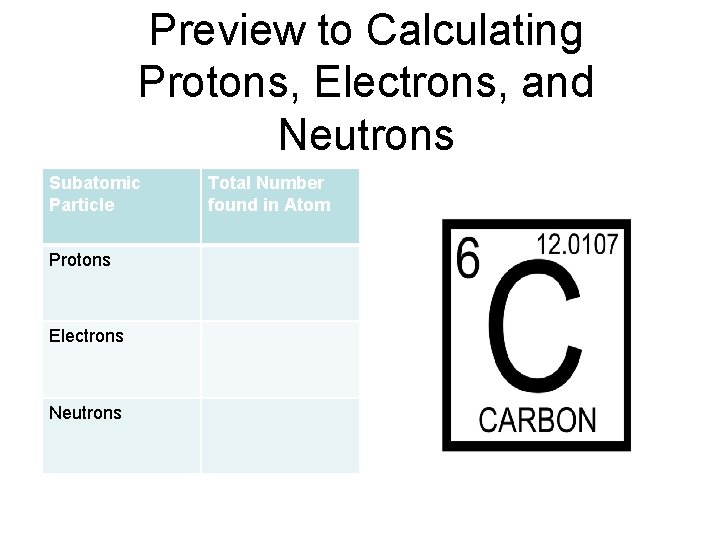
Preview to Calculating Protons, Electrons, and Neutrons Subatomic Particle Protons Electrons Neutrons Total Number found in Atom

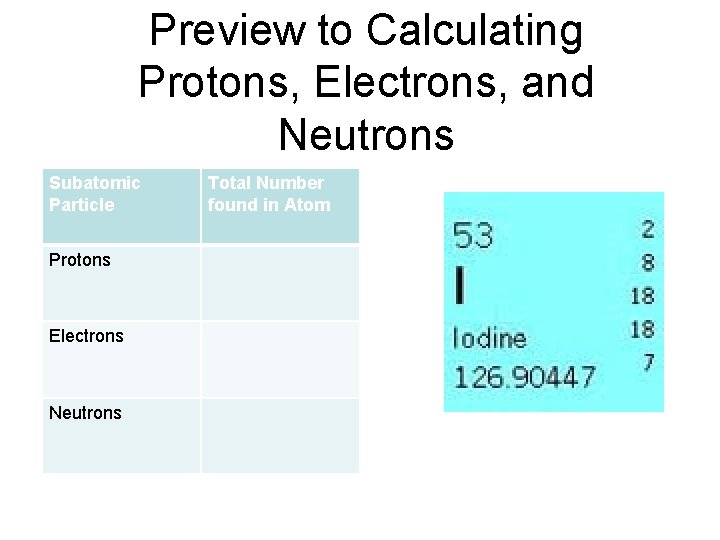
Preview to Calculating Protons, Electrons, and Neutrons Subatomic Particle Protons Electrons Neutrons Total Number found in Atom

The Atoms Family was created by Kathleen Crawford, 1994 Presentation developed by Tracy Trimpe, 2006, http: //sciencespot. net/

Unscramble these words: uceulns urntneo toonrp cneletro mota mocriusdet


How do the Structures of Atoms Differ? Atomic number Mass number Atomic mass

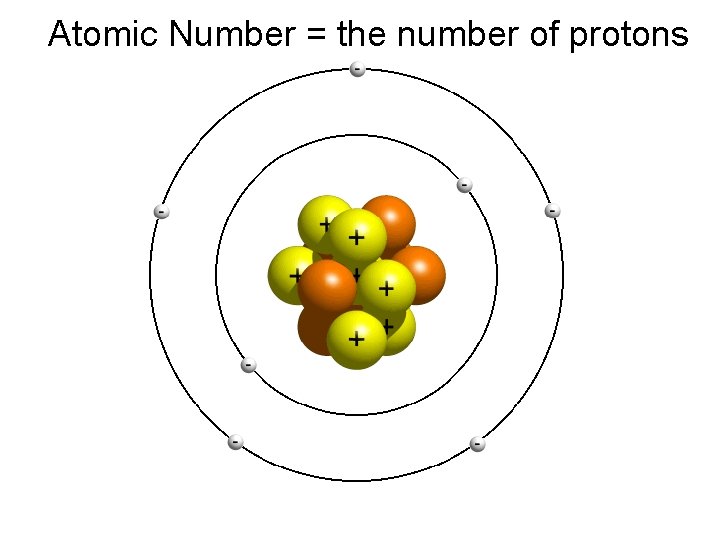
Atomic Number = the number of protons

Identify the type of atom, the number of protons and the number of electrons: Atomic number 16 43 4 6

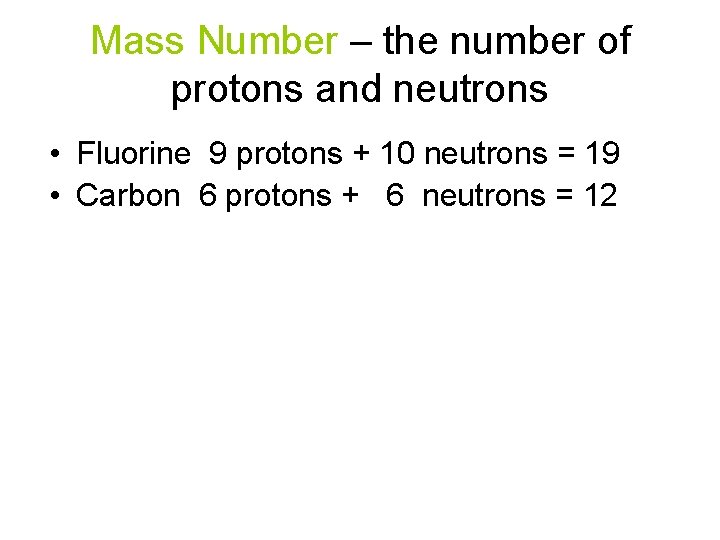
Mass Number – the number of protons and neutrons • Fluorine 9 protons + 10 neutrons = 19 • Carbon 6 protons + 6 neutrons = 12

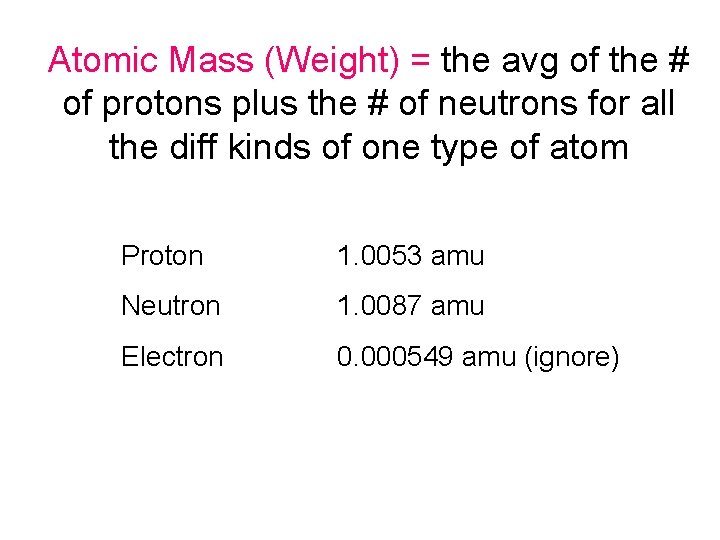
Atomic Mass (Weight) = the avg of the # of protons plus the # of neutrons for all the diff kinds of one type of atom Proton 1. 0053 amu Neutron 1. 0087 amu Electron 0. 000549 amu (ignore)

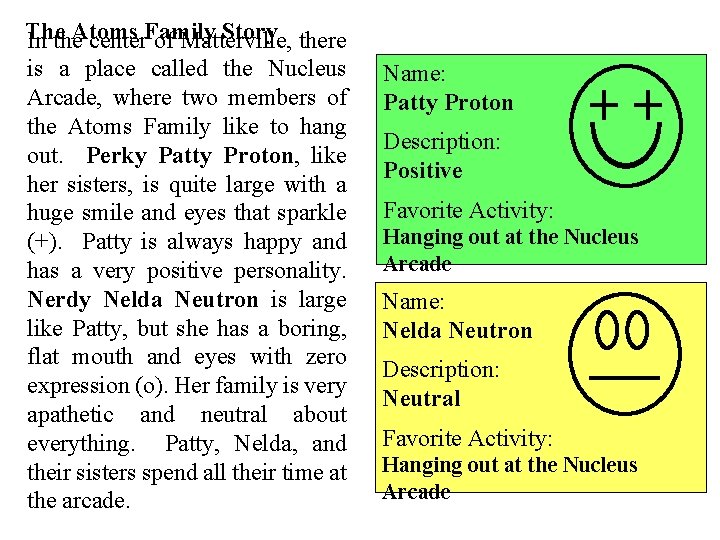
The Story there In the. Atoms center. Family of Matterville, is a place called the Nucleus Arcade, where two members of the Atoms Family like to hang out. Perky Patty Proton, like her sisters, is quite large with a huge smile and eyes that sparkle (+). Patty is always happy and has a very positive personality. Nerdy Nelda Neutron is large like Patty, but she has a boring, flat mouth and eyes with zero expression (o). Her family is very apathetic and neutral about everything. Patty, Nelda, and their sisters spend all their time at the arcade. Name: Patty Proton Description: Positive Favorite Activity: Hanging out at the Nucleus Arcade Name: Nelda Neutron Description: Neutral Favorite Activity: Hanging out at the Nucleus Arcade

Around the Nucleus Arcade, you will find a series of roadways that are used by another member of the Atoms Family, Enraged Elliott Electron. Elliott races madly around the Arcade on his bright red chromeplated Harley-Davidson. He rides so fast that no one can be sure where he is at any time. Elliott is much smaller than Patty and Nelda and he is always angry because these bigger relatives will not let him in the Arcade. He has a frown on his face, eyes that are squinted with anger, and a very negative (-) attitude. Name: Elliott Electron Description: Negative Favorite Activity: Racing around the arcade

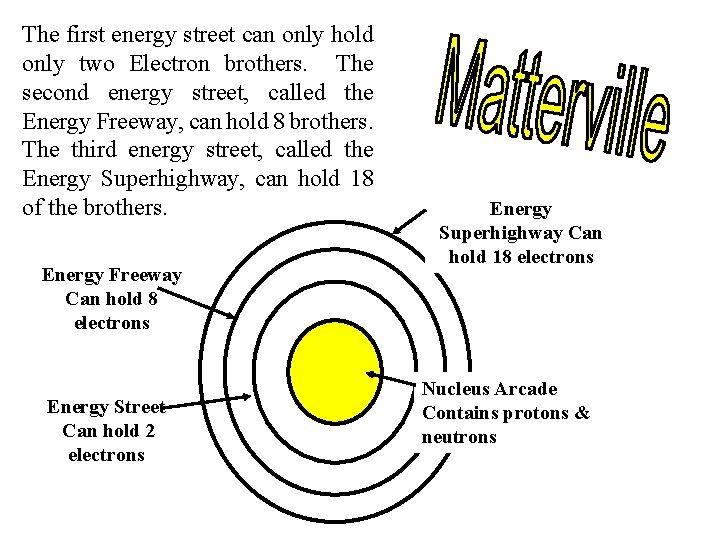
The first energy street can only hold only two Electron brothers. The second energy street, called the Energy Freeway, can hold 8 brothers. The third energy street, called the Energy Superhighway, can hold 18 of the brothers. Energy Freeway Can hold 8 electrons Energy Street Can hold 2 electrons Energy Superhighway Can hold 18 electrons Nucleus Arcade Contains protons & neutrons

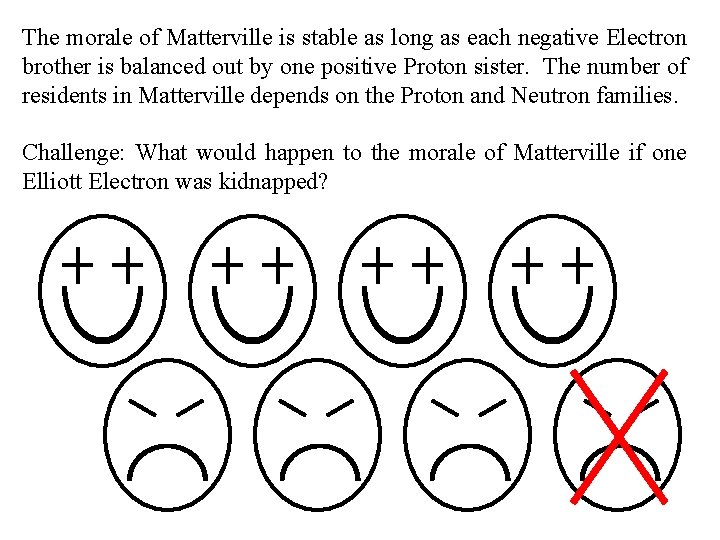
The morale of Matterville is stable as long as each negative Electron brother is balanced out by one positive Proton sister. The number of residents in Matterville depends on the Proton and Neutron families. Challenge: What would happen to the morale of Matterville if one Elliott Electron was kidnapped?

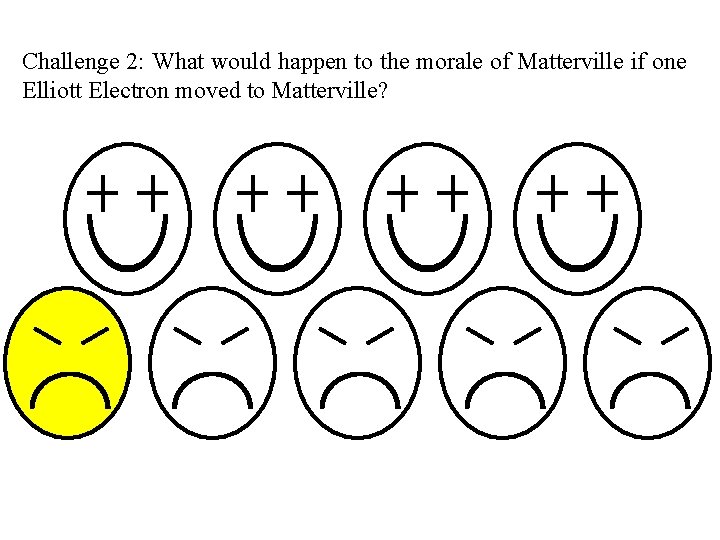
Challenge 2: What would happen to the morale of Matterville if one Elliott Electron moved to Matterville?

1 st Verse: They’re tiny and they’re teeny, Much smaller than a beany, They never can be seeny, The Atoms Family. Chorus 3 rd Verse: Neutrons can be found, Where protons hang around; Electrons they surround The Atoms Family. Chorus 2 nd Verse: Together they make gases, And liquids like molasses, And all the solid masses, The Atoms Family Chorus: They are so small. (Snap, snap) They’re round like a ball. (Snap, snap) They make up the air. They’re everywhere. Can’t see them at all. (Snap, snap) http: //www. youtube. com/ watch? v=porv. QQWJpv M&feature=related

They’re tiny and they’re teeny, Much smaller than a beany, They never can be seeny, The Atoms Family.
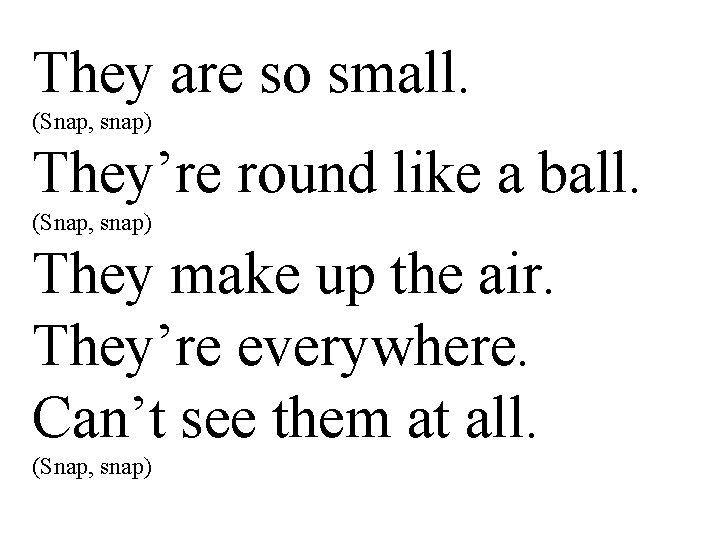
They are so small. (Snap, snap) They’re round like a ball. (Snap, snap) They make up the air. They’re everywhere. Can’t see them at all. (Snap, snap)

Together they make gases, And liquids like molasses, And all the solid masses, The Atoms Family
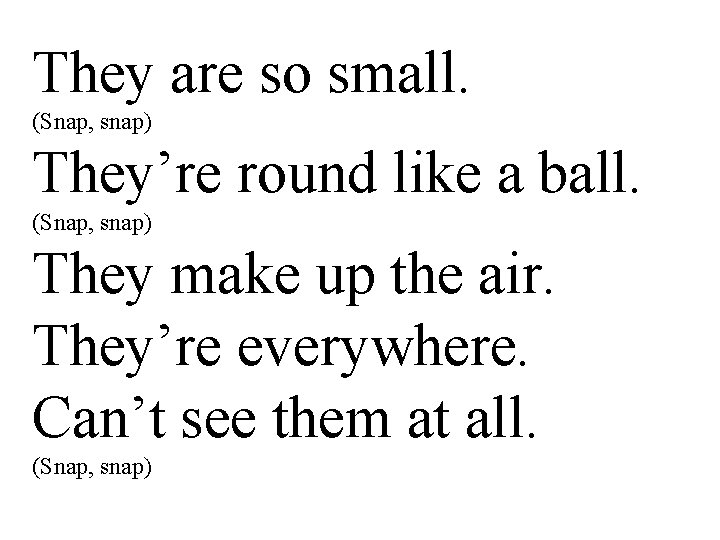
They are so small. (Snap, snap) They’re round like a ball. (Snap, snap) They make up the air. They’re everywhere. Can’t see them at all. (Snap, snap)

Neutrons can be found, Where protons hang around; Electrons they surround The Atoms Family.
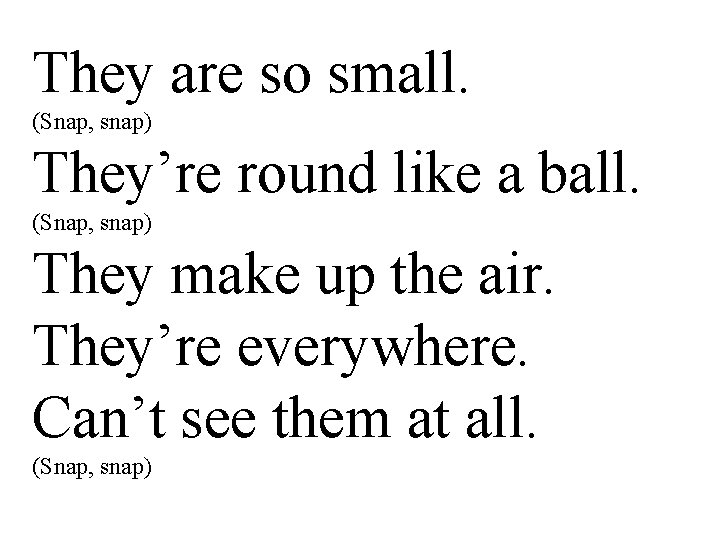
They are so small. (Snap, snap) They’re round like a ball. (Snap, snap) They make up the air. They’re everywhere. Can’t see them at all. (Snap, snap)

Ready to try it again? Click the music note to try the song again. If you do not want to sing it again, click the arrow to move to the next slide.

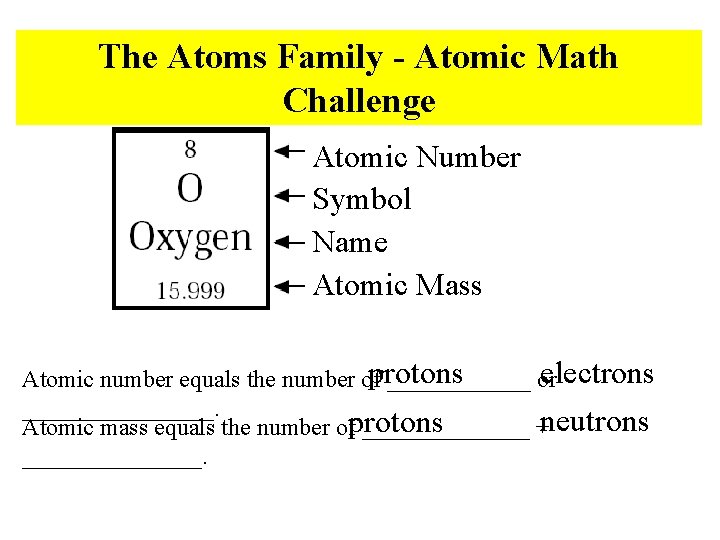
The Atoms Family - Atomic Math Challenge Atomic Number Symbol Name Atomic Mass electrons protons Atomic number equals the number of ______ or ________. Atomic mass equals the number ofprotons _______ +neutrons ________.


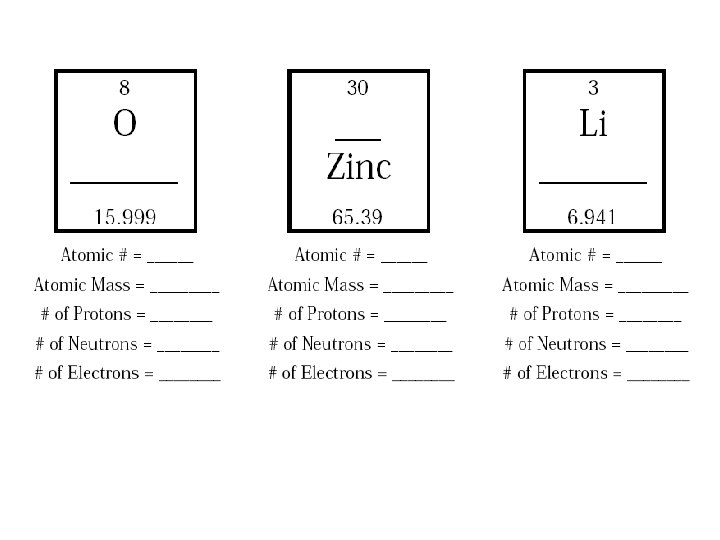
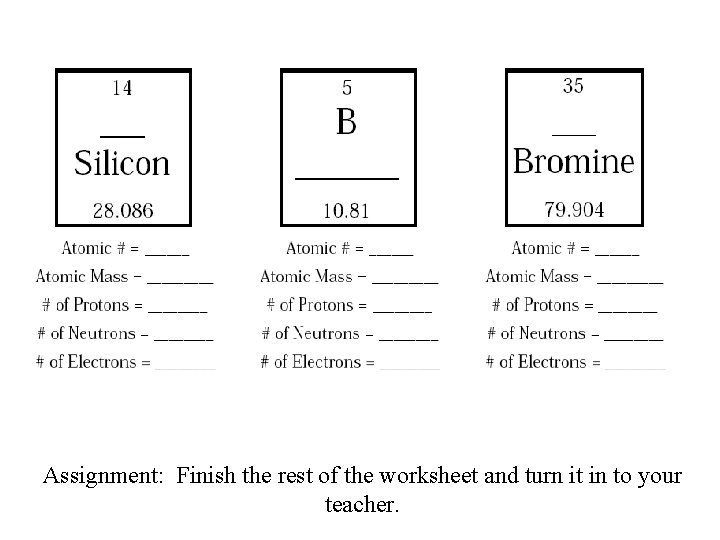
Assignment: Finish the rest of the worksheet and turn it in to your teacher.