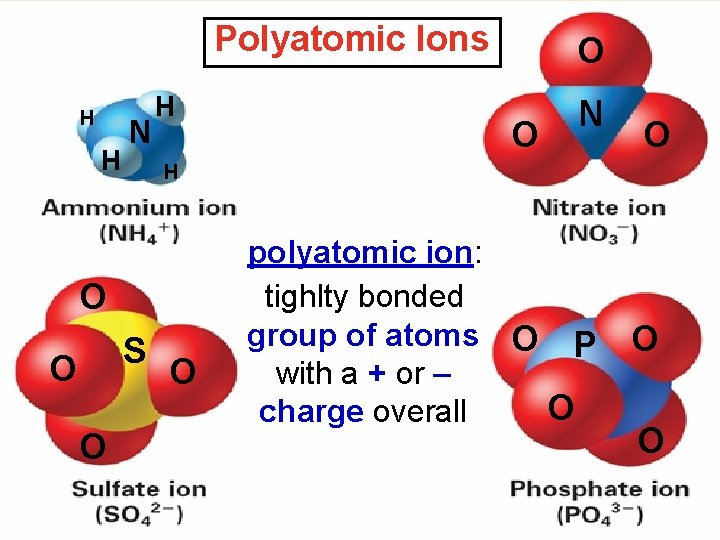
Polyatomic Ions H H N H S O



- Slides: 9

Polyatomic Ions H H N H S O O N O H O O polyatomic ion: tighlty bonded group of atoms O P with a + or – O charge overall O O

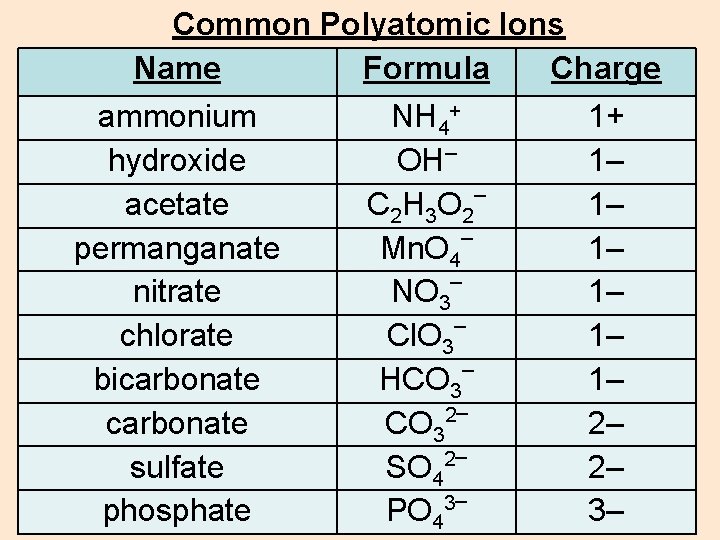
Common Polyatomic Ions Name Formula Charge ammonium hydroxide acetate permanganate nitrate chlorate bicarbonate sulfate phosphate NH 4+ OH– C 2 H 3 O 2– Mn. O 4– NO 3– Cl. O 3– HCO 3– CO 32– SO 42– 3– PO 4 1+ 1– 1– 1– 2– 2– 3–

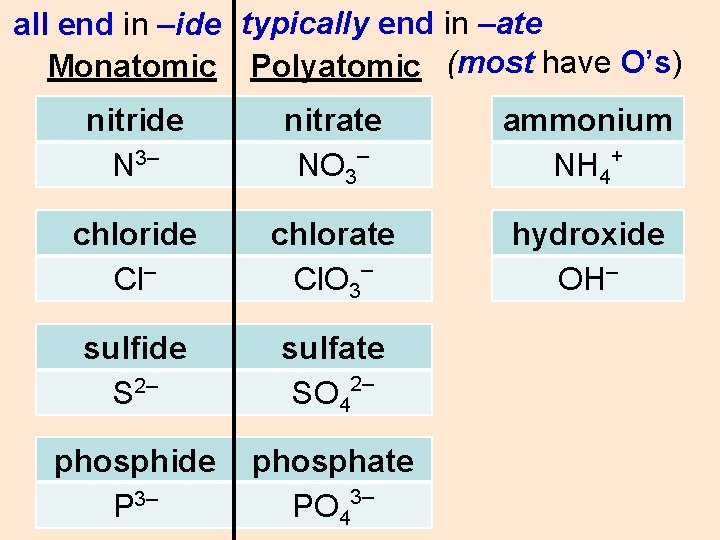
all end in –ide typically end in –ate Monatomic Polyatomic (most have O’s) nitride N 3– nitrate – NO 3 ammonium + NH 4 chloride Cl– chlorate Cl. O 3– hydroxide OH– sulfide S 2– sulfate 2– SO 4 phosphide P 3– phosphate 3– PO 4

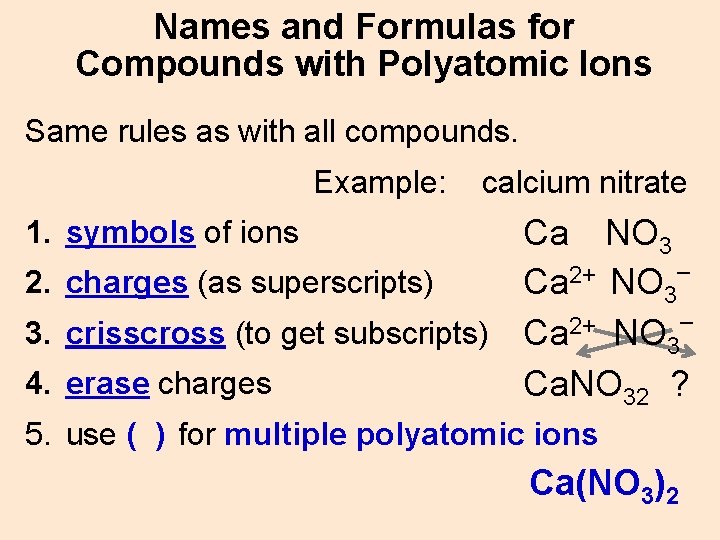
Names and Formulas for Compounds with Polyatomic Ions Same rules as with all compounds. Example: calcium nitrate Ca NO 3 – 2+ 2. charges (as superscripts) Ca NO 3 3. crisscross (to get subscripts) Ca 2+ NO 3– 4. erase charges Ca. NO 32 ? 1. symbols of ions 5. use ( ) for multiple polyatomic ions Ca(NO 3)2

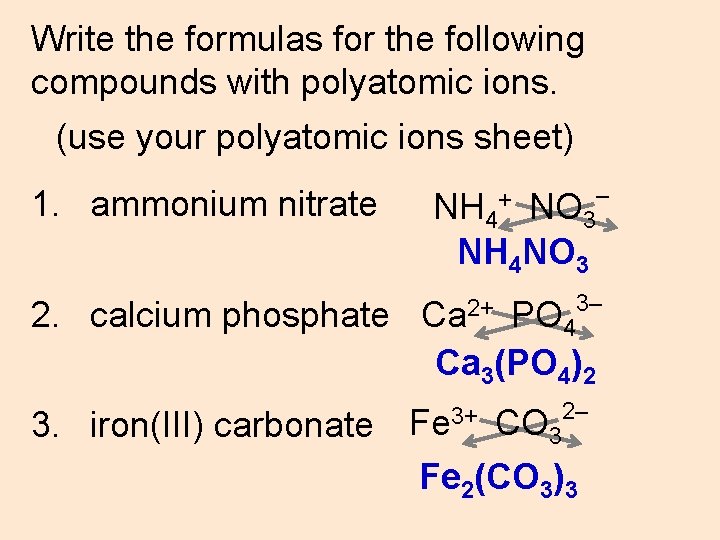
Write the formulas for the following compounds with polyatomic ions. (use your polyatomic ions sheet) 1. ammonium nitrate 2. calcium phosphate 3. iron(III) carbonate NH 4 NO 3 NH 4 NO 3 + Ca 2+ – 3– PO 4 Ca 3(PO 4)2 Fe 3+ 2– CO 3 Fe 2(CO 3)3

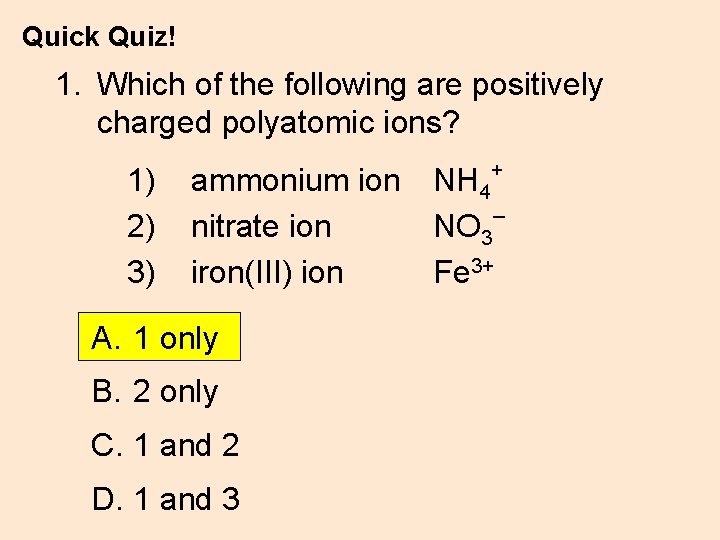
Quick Quiz! 1. Which of the following are positively charged polyatomic ions? 1) 2) 3) ammonium ion nitrate ion iron(III) ion A. 1 only B. 2 only C. 1 and 2 D. 1 and 3 NH 4+ – NO 3 Fe 3+

Quick Quiz. 2. What is the correct name for Mg(OH)2 ? A. magnesium oxide B. magnesium hydroxide C. magnesium(II) hydroxide D. magnesium(II) hydroxate

Quick Quiz. 3. What is the correct formula for copper(I) sulfate? A. Cu. S B. Cu. SO 4 C. Cu 2 SO 4 D. Cu(SO 4)2 Cu+ SO 4 2–

Quick Quiz. 4. Which one of the following formulas is named correctly? A. sodium bicarbonate, Na 2 CO 3 B. potassium nitrate, KNO 2 C. sodium acetate, Na. C 2 H 3 O 2 D. lithium sulfide, Li 2 SO 4