COLLISION THEORY States that atoms ions and molecules




- Slides: 9

COLLISION THEORY: States that atoms, ions, and molecules must collide in order to react.

Collision Theory: • According to collision theory, particles will react to form products if they collide with enough kinetic energy. • Particles that do not have enough energy to react bounce apart unchanged when they collide (no reaction).

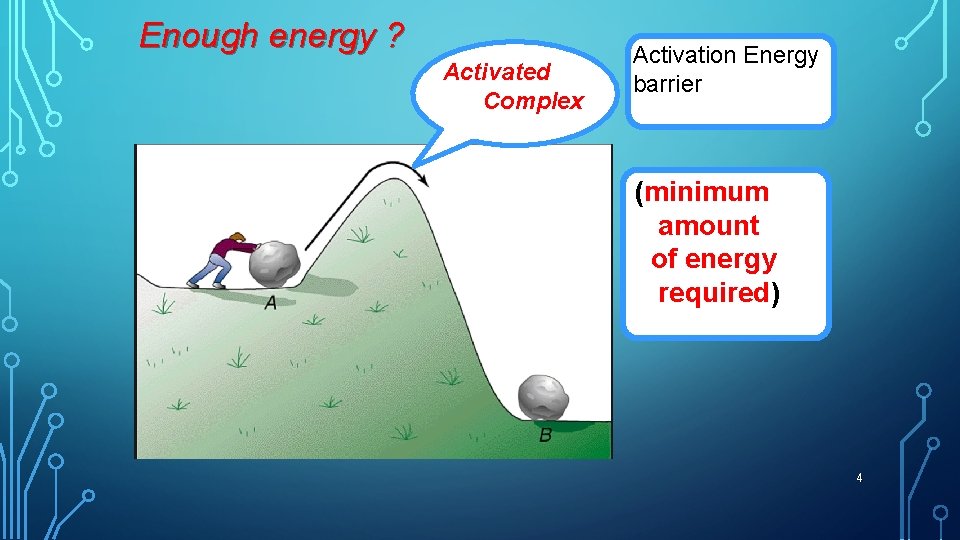
When two reactant particles collide, they may form an activated complex. • An activated complex (transition state) is an unstable arrangement of atoms that forms for a moment at the peak of the activation-energy barrier. • The activated complex forms only if the colliding particles have enough energy and correct orientation

Enough energy ? Activated Complex Activation Energy barrier (minimum amount of energy required) 4

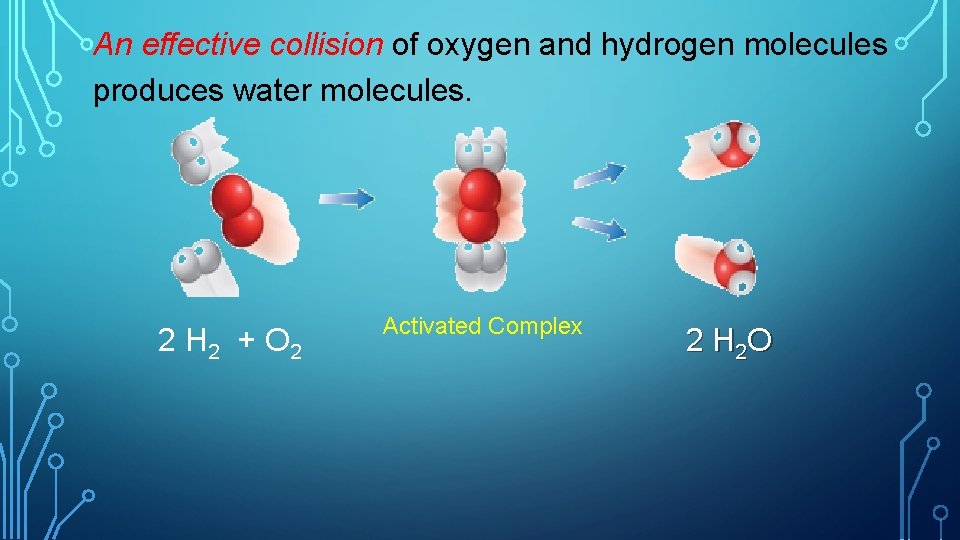
An effective collision of oxygen and hydrogen molecules produces water molecules. 2 H 2 + O 2 Activated Complex 2 H 2 O

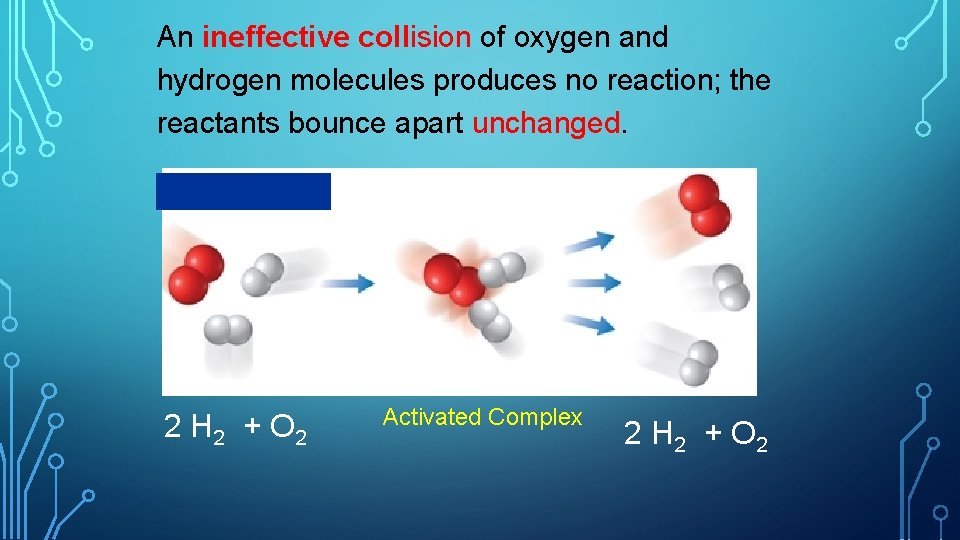
An ineffective collision of oxygen and hydrogen molecules produces no reaction; the reactants bounce apart unchanged. 2 H 2 + O 2 Activated Complex 2 H 2 + O 2

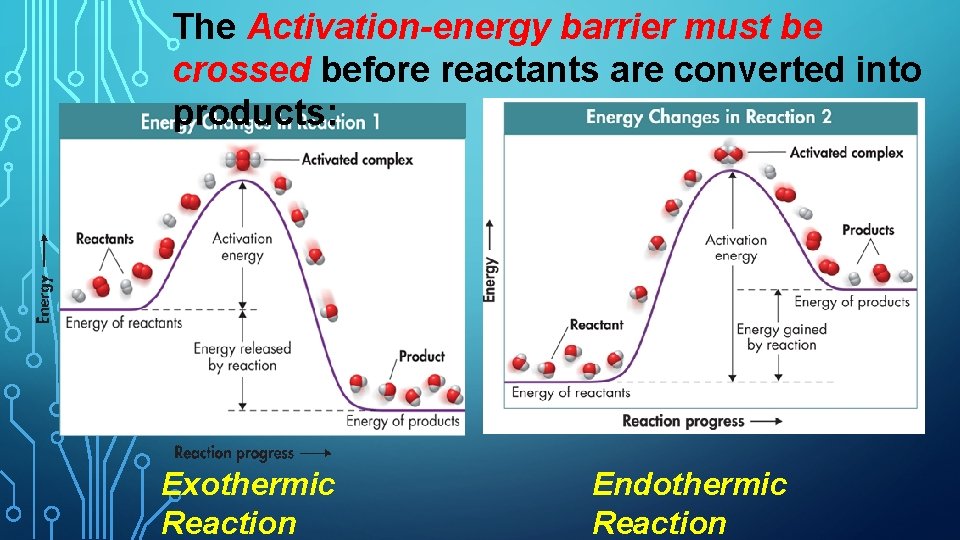
The Activation-energy barrier must be crossed before reactants are converted into products: Exothermic Reaction Endothermic Reaction

PARTICLES MUST COLLIDE WITH: (EFFECTIVE COLLISION) • Favorable (correct) orientation to form the activated complex (transition state). • Sufficient energy to form the activated complex, and overcome the Activation Energy, Ea (minimum amount of energy required for a reaction to occur)

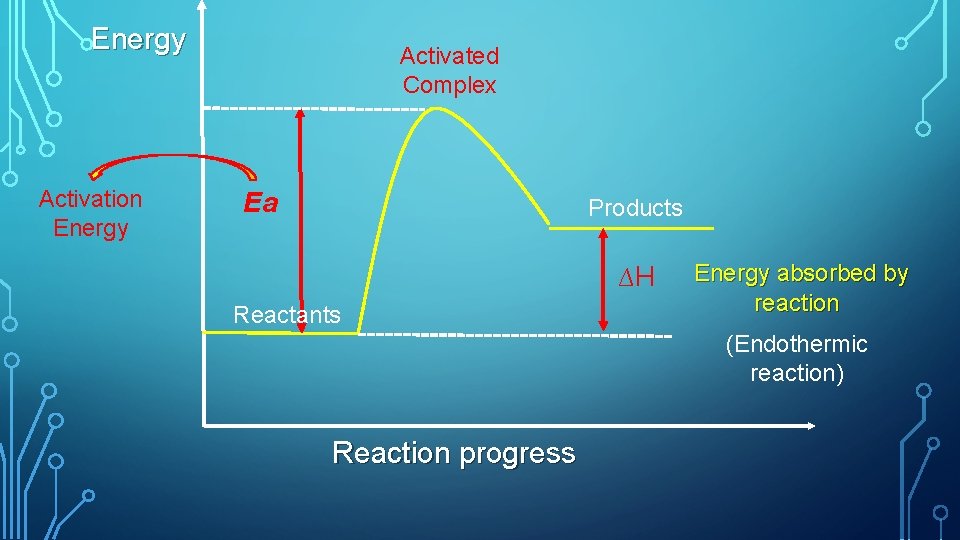
Energy Activation Energy Activated Complex Ea Products ∆H Reactants Energy absorbed by reaction (Endothermic reaction) Reaction progress