Collision Theory Reaction Rates Collision Theory 1 Atoms













- Slides: 22

Collision Theory Reaction Rates

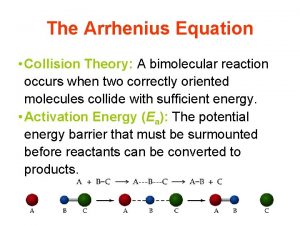
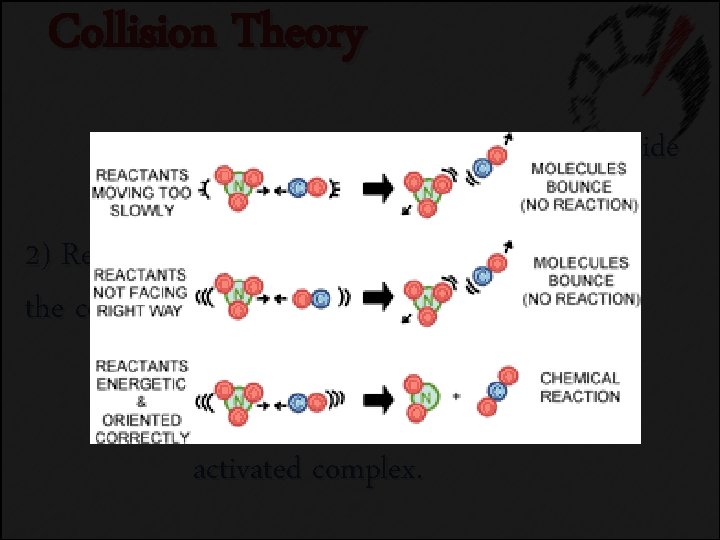
Collision Theory 1) Atoms, ions, and molecules must collide in order to react. No Reaction

Collision Theory 2) Reacting substances must collide with the correct orientation. No Reaction

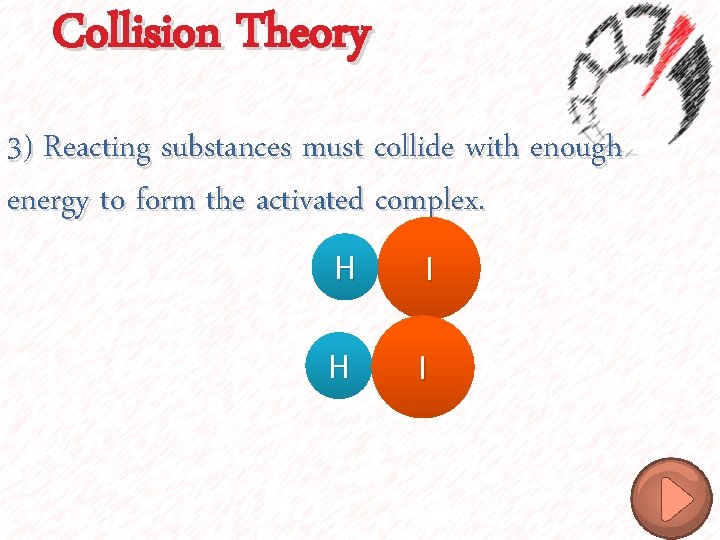
Collision Theory 3) Reacting substances must collide with enough energy to form the activated complex. No Reaction

Collision Theory 3) Reacting substances must collide with enough energy to form the activated complex. H I

Collision Theory 1) Atoms, ions, and molecules must collide in order to react. 2) Reacting substances must collide with the correct orientation. 3) Reacting substances must collide with enough energy to form the activated complex.

Activated Complex A temporary, unstable arrangement of atoms that may form products or reactants.

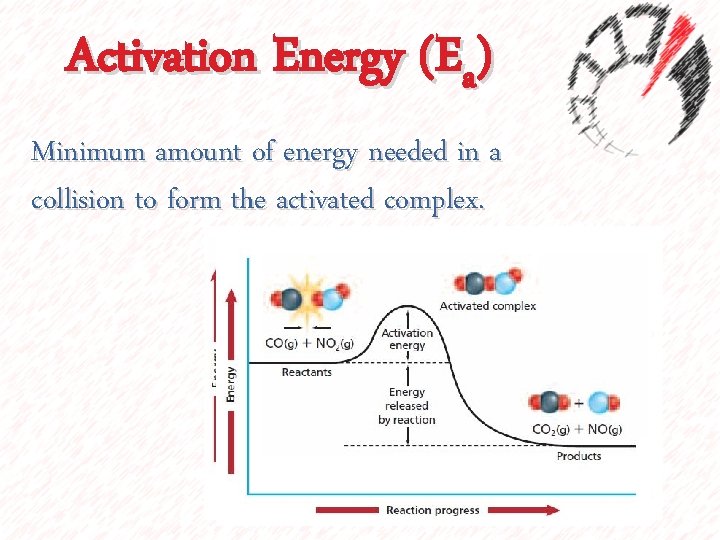
Activation Energy (Ea) Minimum amount of energy needed in a collision to form the activated complex.

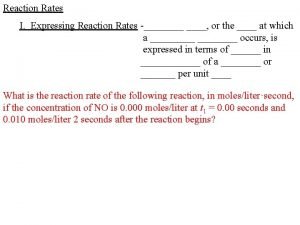
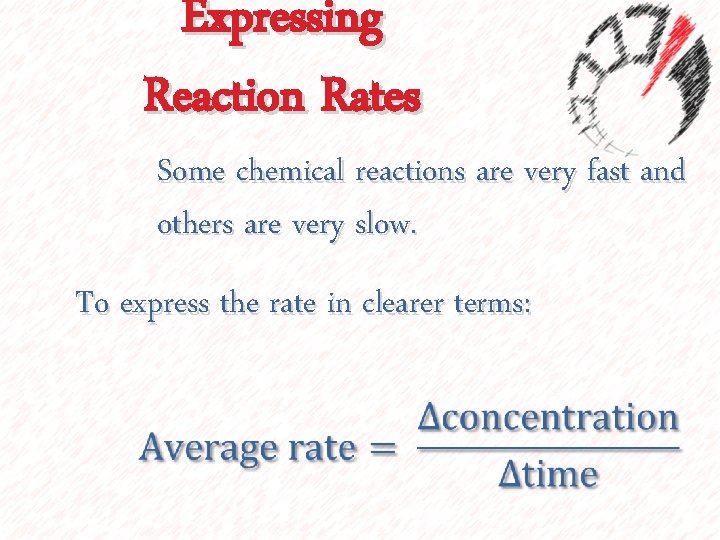
Expressing Reaction Rates Some chemical reactions are very fast and others are very slow. To express the rate in clearer terms:

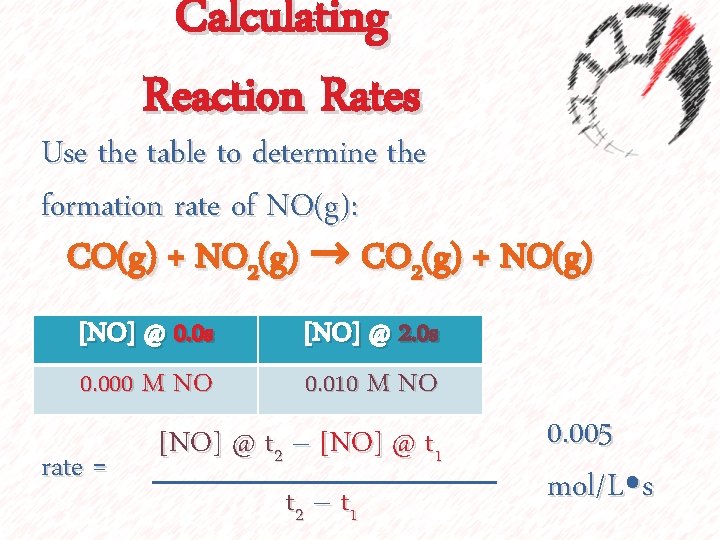
Calculating Reaction Rates Use the table to determine the formation rate of NO(g): CO(g) + NO 2(g) → CO 2(g) + NO(g) [NO] @ 0. 0 s 0. 000 M NO rate = [NO] @ 2. 0 s 0. 010 M NO [NO] @ t 2 – [NO] @ t 1 t 2 – t 1 0. 005 mol/L⦁s

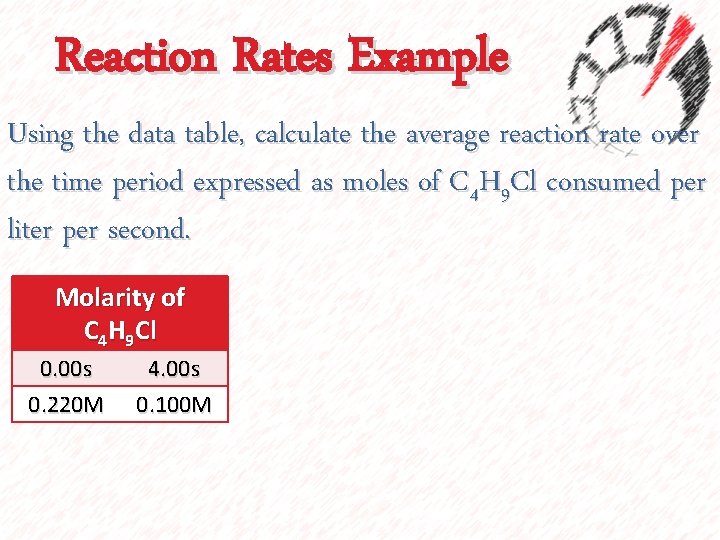
Reaction Rates Example Using the data table, calculate the average reaction rate over the time period expressed as moles of C 4 H 9 Cl consumed per liter per second. Molarity of C 4 H 9 Cl 0. 00 s 0. 220 M 4. 00 s 0. 100 M

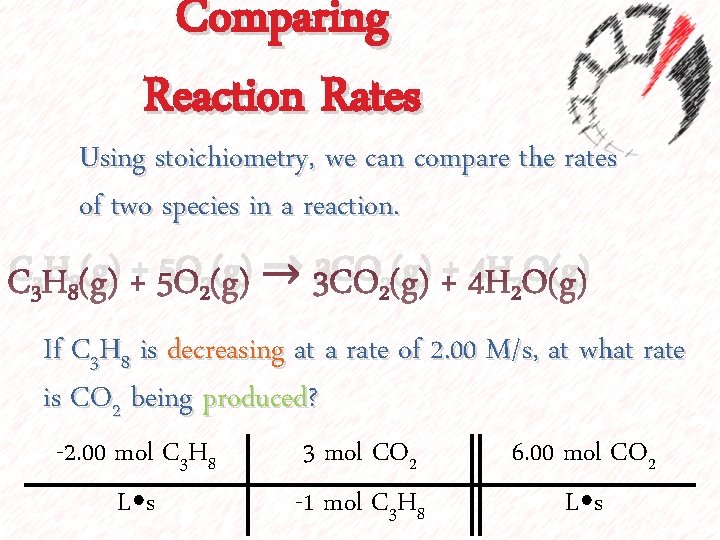
Comparing Reaction Rates Using stoichiometry, we can compare the rates of two species in a reaction. C 3 H 8(g) + 5 O 2(g) → 3 CO 2(g) + 4 H 2 O(g) If C 3 H 8 is decreasing at a rate of 2. 00 M/s, at what rate is CO 2 being produced? -2. 00 mol C 3 H 8 L⦁s 3 mol CO 2 -1 mol C 3 H 8 6. 00 mol CO 2 L⦁s

Discussion Questions 1) What criteria must be met for reactant collisions to result in a successful product? A) B) The reactants must collide with each other The reactants must collide with enough energy and be in the right positions C) The reactants must have enough energy to form the activated complex

Discussion Questions 2) The most common way of expressing a reaction rate is in terms of: A) B) C) D) mol/L g/L⦁s mol/s

Discussion Questions 3) What is the average rate of formation of salt in the first 10 seconds of this reaction? HCl + Na. OH → H 2 O + Na. Cl 0. 00 M Na. Cl at 0. 00 s 2. 87 M Na. Cl at 10. 00 s

Discussion Questions 4) What is the average rate of formation of CO 2 between 3. 00 and 7. 00 seconds of this reaction? H 2 CO 3→ H 2 O + CO 2 1. 08 M CO 2 at 3. 00 s 3. 42 M CO 2 at 7. 00 s

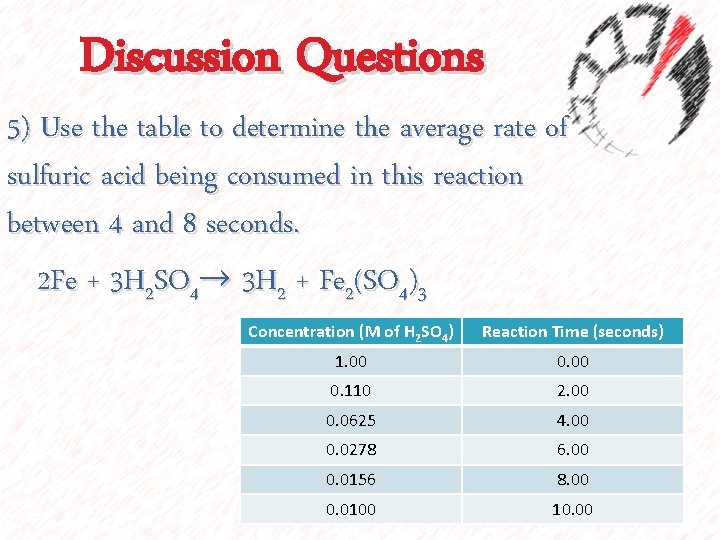
Discussion Questions 5) Use the table to determine the average rate of sulfuric acid being consumed in this reaction between 4 and 8 seconds. 2 Fe + 3 H 2 SO 4→ 3 H 2 + Fe 2(SO 4)3 Concentration (M of H 2 SO 4) Reaction Time (seconds) 1. 00 0. 110 2. 00 0. 0625 4. 00 0. 0278 6. 00 0. 0156 8. 00 0. 0100 10. 00

Discussion Questions 6) As a reaction proceeds, the concentration of reactants will _______ and the concentration of products will ____. A) increase, decrease B) increase, increase C) decrease, decrease D) decrease, increase

Discussion Questions 7) Calculate the rate of formation of iron(II) sulfide if sulfur is being consumed at a rate of 0. 440 mol/L⦁s. 8 Fe(s) + S 8(s) → 8 Fe. S(s)

Discussion Questions 8) Calculate the rate of formation of aluminum oxide if oxygen is being consumed at a rate of 0. 060 mol/L⦁s. 3 O 2(g) + 4 Al(s) → 2 Al 2 O 3(s)

Discussion Questions 9) At what rate is aluminum being consumed if aluminum oxide is being formed at 2. 50 mol/L⦁s. 3 O 2(g) + 4 Al(s) → 2 Al 2 O 3(s)

Discussion Questions 10) At what rate is nitrogen being consumed if hydrogen is being consumed at 0. 0093 mol/L⦁s. 3 H 2(g) + N 2(g) → 2 NH 3(g)
 Unit ratio
Unit ratio Equivalent ratios guided notes
Equivalent ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Arrhenius equation
Arrhenius equation Periodic table of elements regents
Periodic table of elements regents Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rates of reaction quiz
Rates of reaction quiz Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Rearranging atoms
Rearranging atoms Reaction order
Reaction order Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half time equation
Half time equation Lowest allowable energy state of an atom
Lowest allowable energy state of an atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom