COLLISION THEORY OF REACTION RATES Class xii COLLISION









- Slides: 16

COLLISION THEORY OF REACTION RATES Class xii

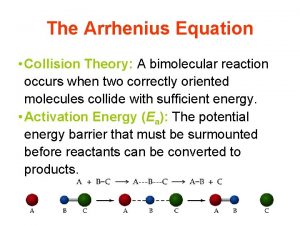
COLLISION THEORY OF REACTION RATES �Reaction occur when � contact with each other or collide each other. �The theory / concepts regarding this is called collision theory.

According to collision theory : �the basic condition for a chemical reaction is the collision among the reacting species. �Collision frequency ( Z AB ) : the number of collisions per second per unit volume of the reaction mixture. �

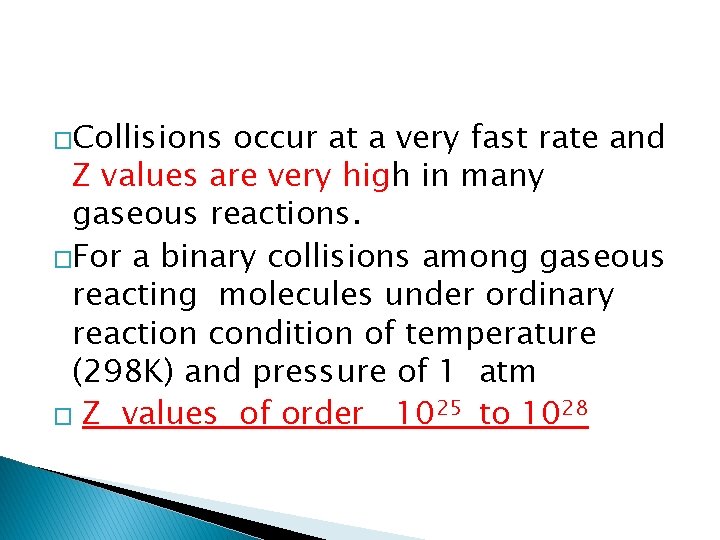
�Collisions occur at a very fast rate and Z values are very high in many gaseous reactions. �For a binary collisions among gaseous reacting molecules under ordinary reaction condition of temperature (298 K) and pressure of 1 atm � Z values of order 1025 to 1028

�Z values 1025 to 1028 ……. Means reactions should be very fast or rather instantaneous. �But it is not so… �Many reactions under such conditions are very slow or do not occur at all.

�This means it is not necessary that all collisions lead to the products. �Effective collisions : “The collisions among reacting species which results in the formation of products “

EFFECTIVE COLLISIONS AND THE BARRIERS � Energy barrier � Orientation Barrier

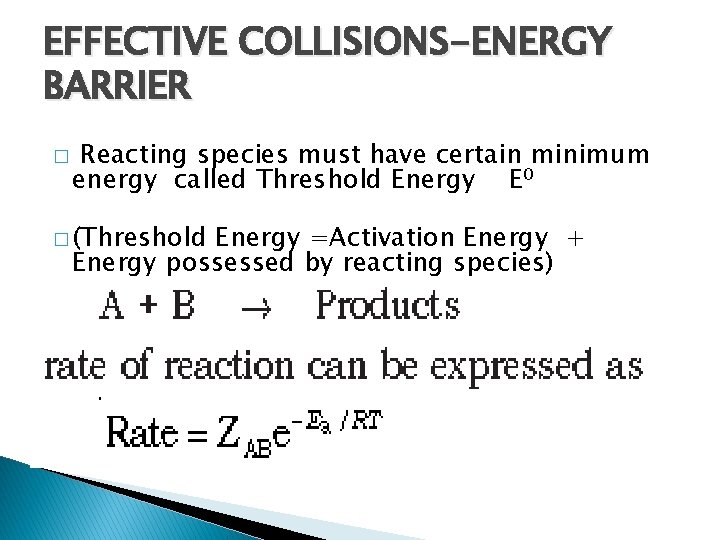
EFFECTIVE COLLISIONS-ENERGY BARRIER � Reacting species must have certain minimum energy called Threshold Energy E 0 � (Threshold Energy =Activation Energy + Energy possessed by reacting species) � � =

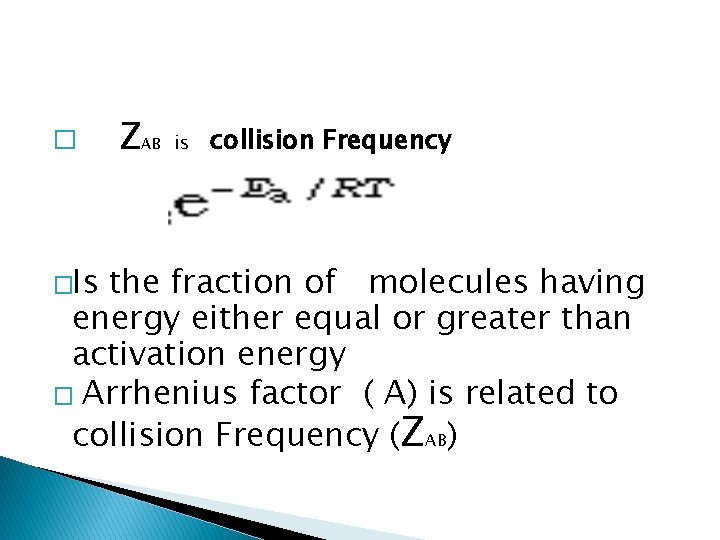
� �Is ZAB is collision Frequency the fraction of molecules having energy either equal or greater than activation energy � Arrhenius factor ( A) is related to collision Frequency (ZAB)

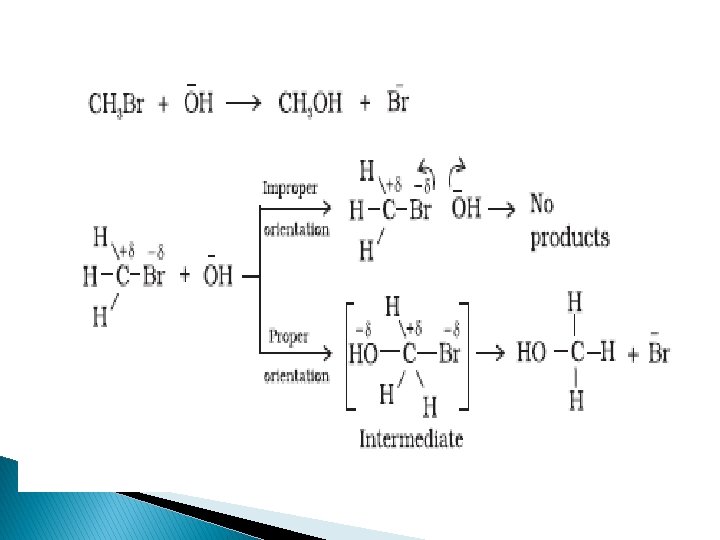
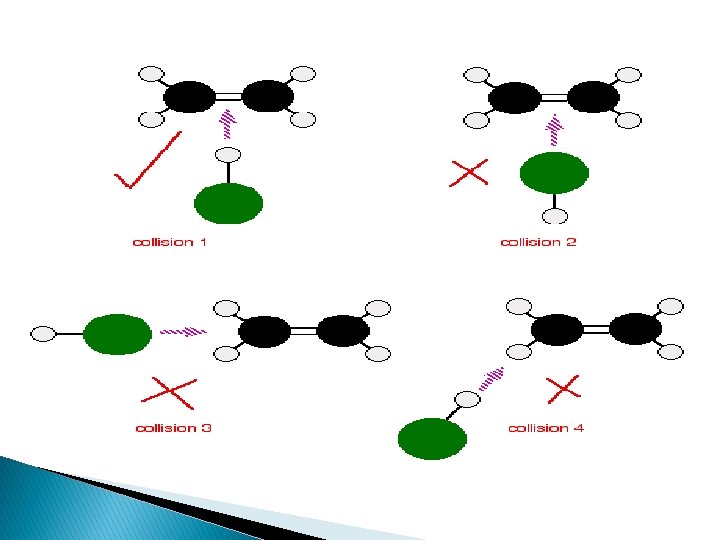
EFFECTIVE COLLISIONSORIENTATION BARRIER



�To account for effective collisions another factor P, The probability factor or steric factor is introduced.

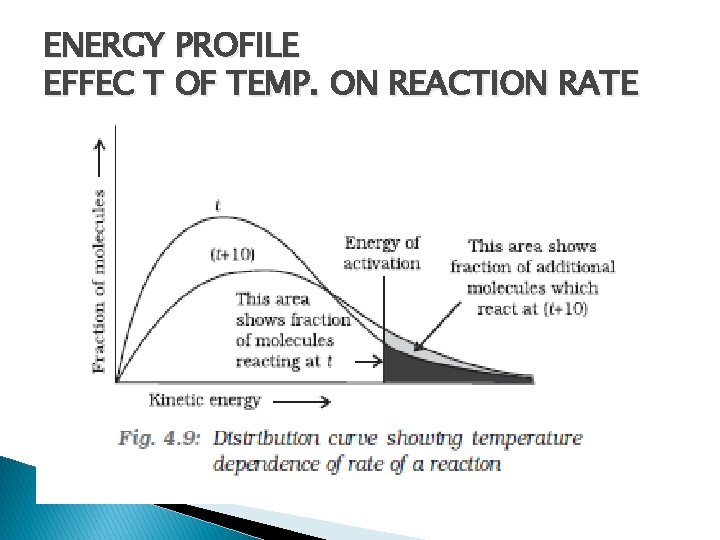
ENERGY PROFILE EFFEC T OF TEMP. ON REACTION RATE

Conclusion : � �In collision theory Activation energy and Proper Orientation of the molecules together determine criteria for effective collisions and hence the rate of chemical reactions.

Assignments : Define Collision frequency ? 2. What do you mean by Effective collisions ? 3. Explain the factors which influence the chemical reactions ? 4. Explain how Energy barrier and Orientation barrier affect the rate of a chemical reaction ? 1.
 Arrhenius equation collision theory
Arrhenius equation collision theory Is a ratio a rate
Is a ratio a rate Equivalent ratios
Equivalent ratios Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Collision theory class 12
Collision theory class 12 Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Reaction rate
Reaction rate Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Collision theory of kinetics
Collision theory of kinetics