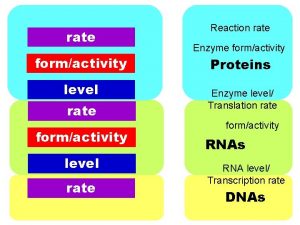
Rates of Reaction Rates of Reaction The rate



- Slides: 21

Rates of Reaction

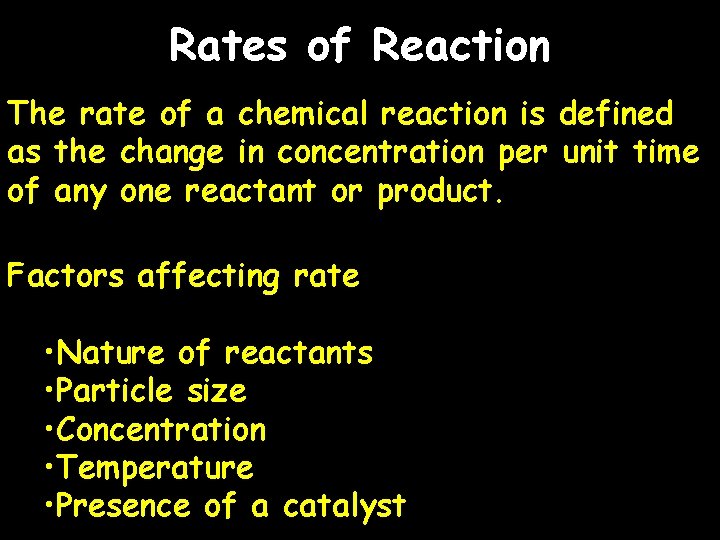
Rates of Reaction The rate of a chemical reaction is defined as the change in concentration per unit time of any one reactant or product. Factors affecting rate • Nature of reactants • Particle size • Concentration • Temperature • Presence of a catalyst

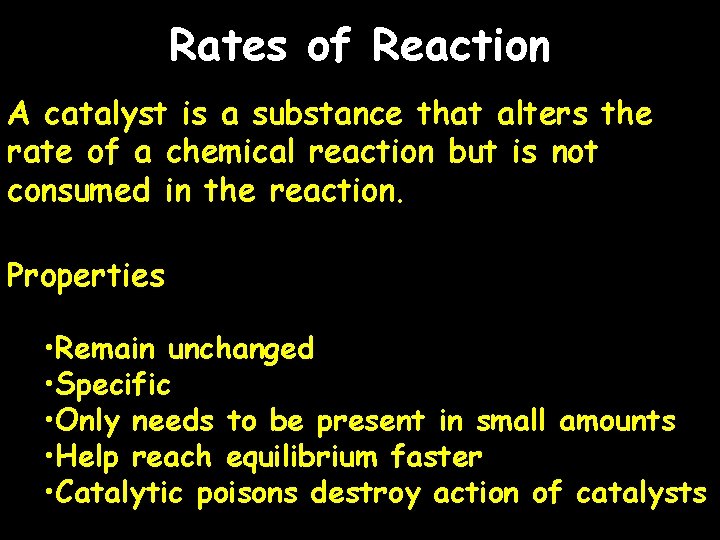
Rates of Reaction A catalyst is a substance that alters the rate of a chemical reaction but is not consumed in the reaction. Properties • Remain unchanged • Specific • Only needs to be present in small amounts • Help reach equilibrium faster • Catalytic poisons destroy action of catalysts

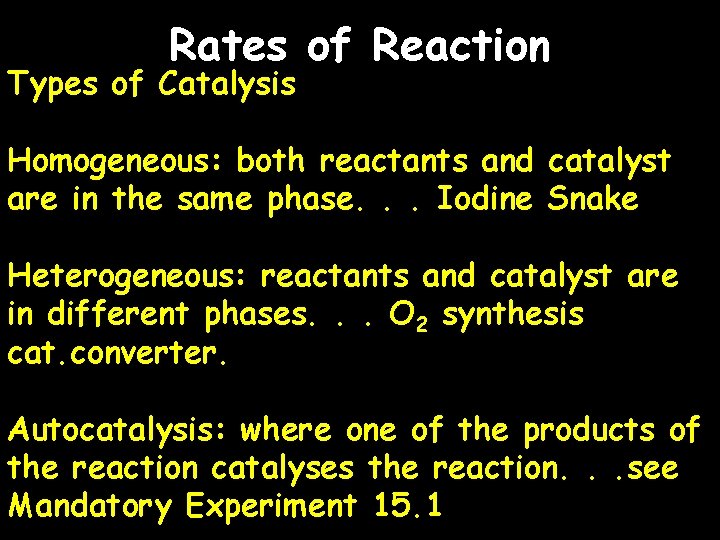
Rates of Reaction Types of Catalysis Homogeneous: both reactants and catalyst are in the same phase. . . Iodine Snake Heterogeneous: reactants and catalyst are in different phases. . . O 2 synthesis cat. converter. Autocatalysis: where one of the products of the reaction catalyses the reaction. . . see Mandatory Experiment 15. 1

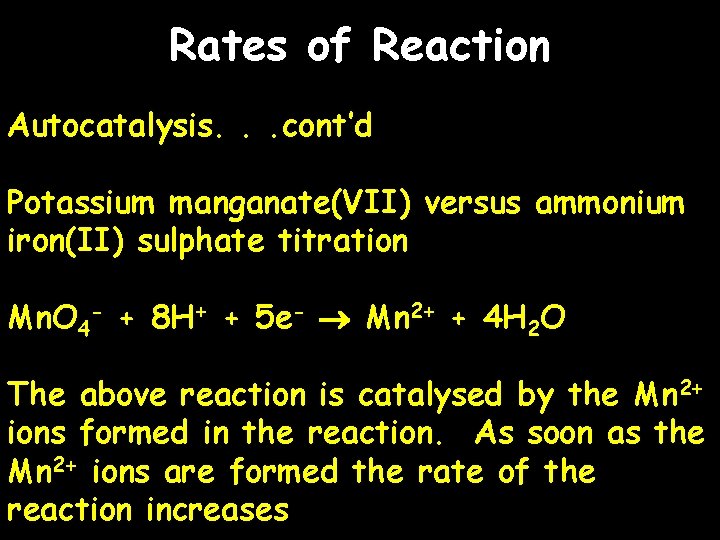
Rates of Reaction Autocatalysis. . . cont’d Potassium manganate(VII) versus ammonium iron(II) sulphate titration Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O The above reaction is catalysed by the Mn 2+ ions formed in the reaction. As soon as the Mn 2+ ions are formed the rate of the reaction increases

Rates of Reaction Mechanisms of Catalysis • Surface Adsorption Theory • Intermediate Formation Theory

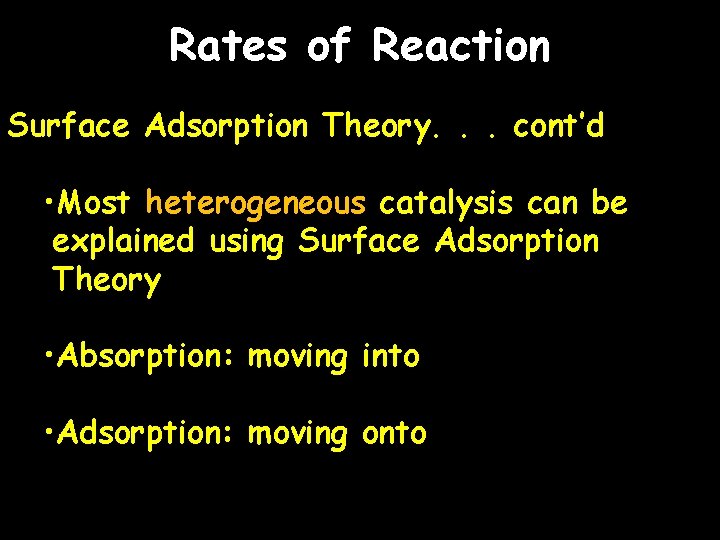
Rates of Reaction Surface Adsorption Theory. . . cont’d • Most heterogeneous catalysis can be explained using Surface Adsorption Theory • Absorption: moving into • Adsorption: moving onto

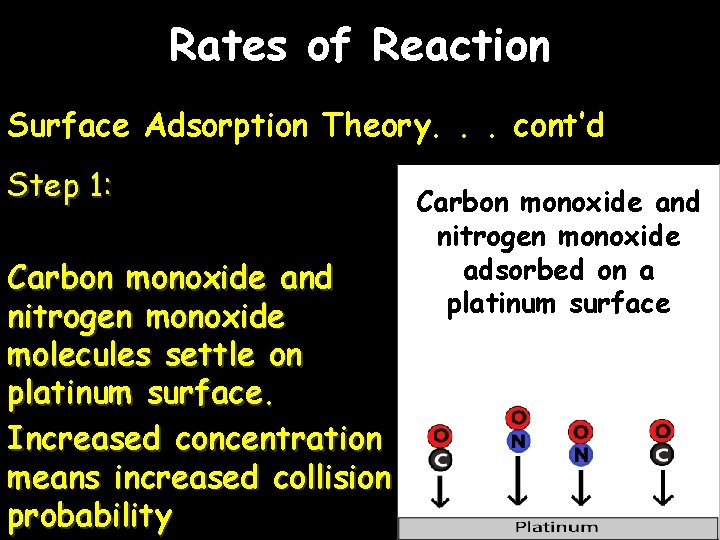
Rates of Reaction Surface Adsorption Theory. . . cont’d Step 1: Carbon monoxide and nitrogen monoxide molecules settle on platinum surface. Increased concentration means increased collision probability Carbon monoxide and nitrogen monoxide adsorbed on a platinum surface

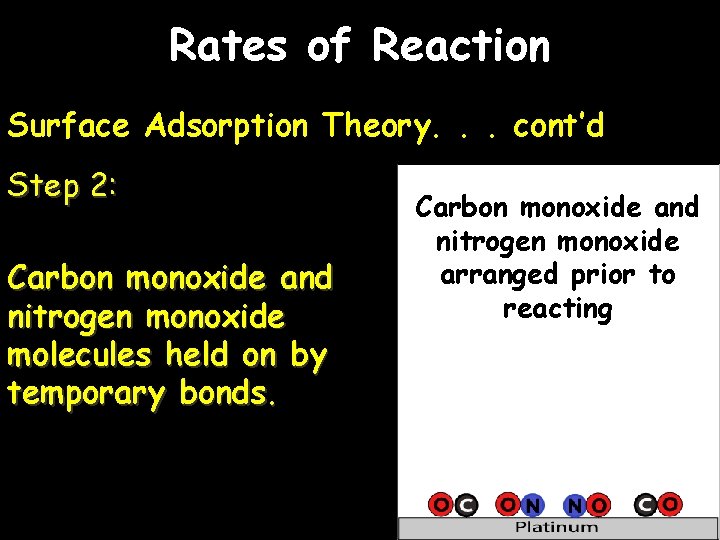
Rates of Reaction Surface Adsorption Theory. . . cont’d Step 2: Carbon monoxide and nitrogen monoxide molecules held on by temporary bonds. Carbon monoxide and nitrogen monoxide arranged prior to reacting

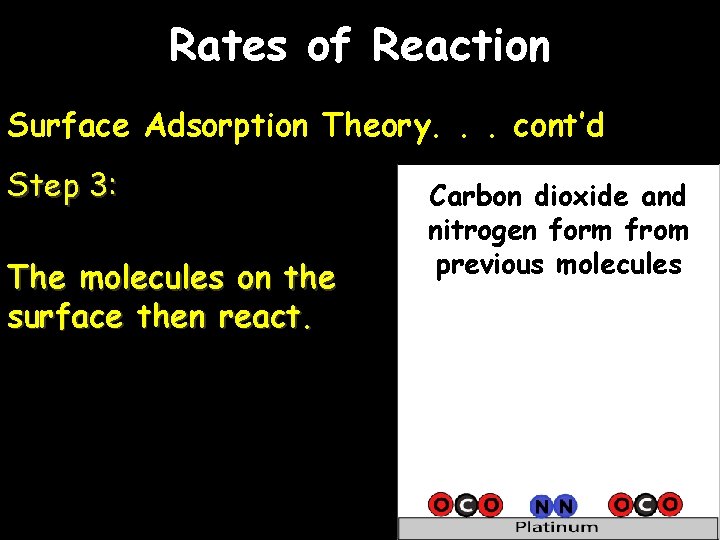
Rates of Reaction Surface Adsorption Theory. . . cont’d Step 3: The molecules on the surface then react. Carbon dioxide and nitrogen form from previous molecules

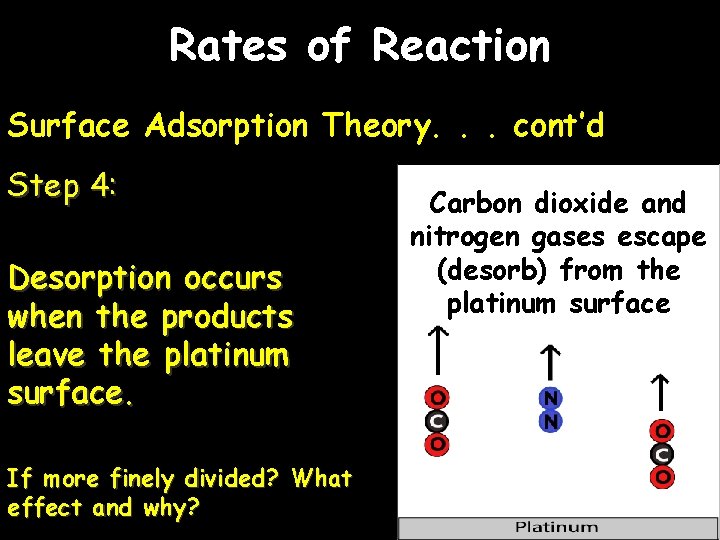
Rates of Reaction Surface Adsorption Theory. . . cont’d Step 4: Desorption occurs when the products leave the platinum surface. If more finely divided? What effect and why? Carbon dioxide and nitrogen gases escape (desorb) from the platinum surface

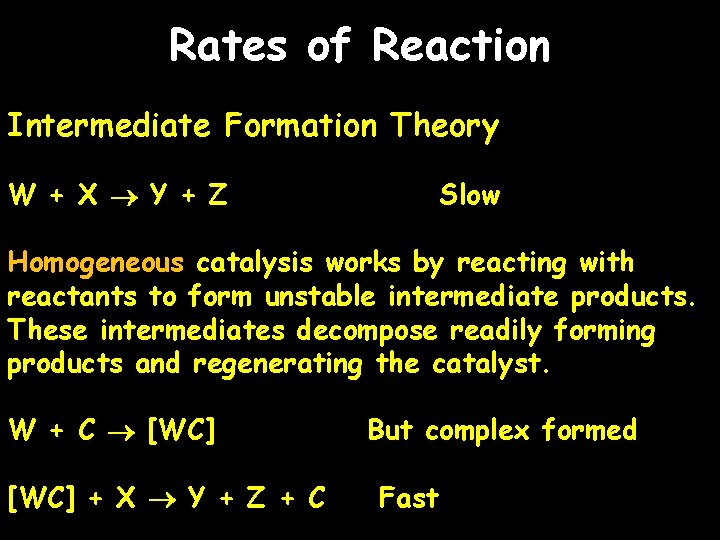
Rates of Reaction Intermediate Formation Theory W + X Y + Z Slow Homogeneous catalysis works by reacting with reactants to form unstable intermediate products. These intermediates decompose readily forming products and regenerating the catalyst. W + C [WC] + X Y + Z + C But complex formed Fast

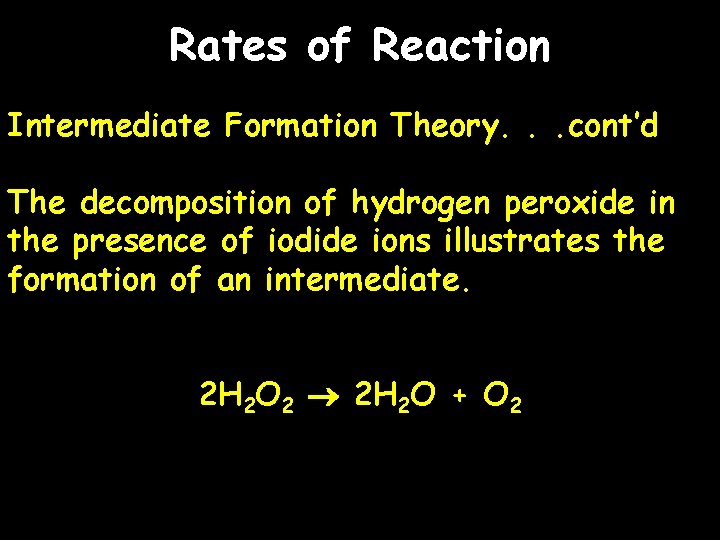
Rates of Reaction Intermediate Formation Theory. . . cont’d The decomposition of hydrogen peroxide in the presence of iodide ions illustrates the formation of an intermediate. 2 H 2 O 2 2 H 2 O + O 2

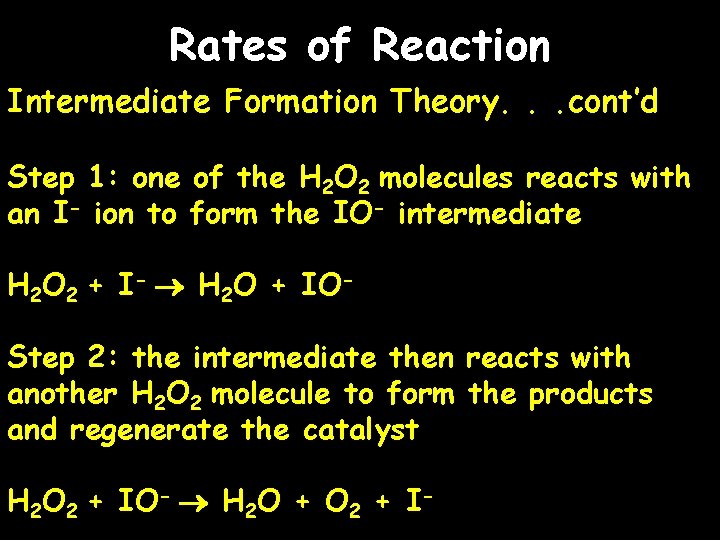
Rates of Reaction Intermediate Formation Theory. . . cont’d Step 1: one of the H 2 O 2 molecules reacts with an I- ion to form the IO- intermediate H 2 O 2 + I- H 2 O + IOStep 2: the intermediate then reacts with another H 2 O 2 molecule to form the products and regenerate the catalyst H 2 O 2 + IO- H 2 O + O 2 + I-

Rates of Reaction Collision Theory and Activation Energy For a reaction to occur, the reacting particles must collide with each other. A collision only results in the formation of products if a certain minimum energy is exceeded in the collision; Effective collision When an effective collision occurs bonds are broken, bonds are formed; Products

Rates of Reaction Collision Theory and Activation Energy. . . Not just important that collisions occur but rate dependent on number that are effective collisions (a collision that leads to the formation of products).

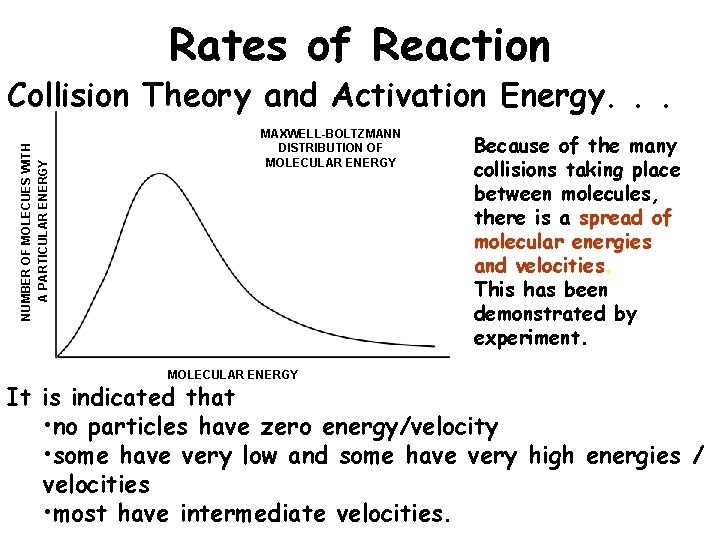
Rates of Reaction NUMBER OF MOLECUES WITH A PARTICULAR ENERGY Collision Theory and Activation Energy. . . MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY Because of the many collisions taking place between molecules, there is a spread of molecular energies and velocities. This has been demonstrated by experiment. It is indicated that • no particles have zero energy/velocity • some have very low and some have very high energies / velocities • most have intermediate velocities.

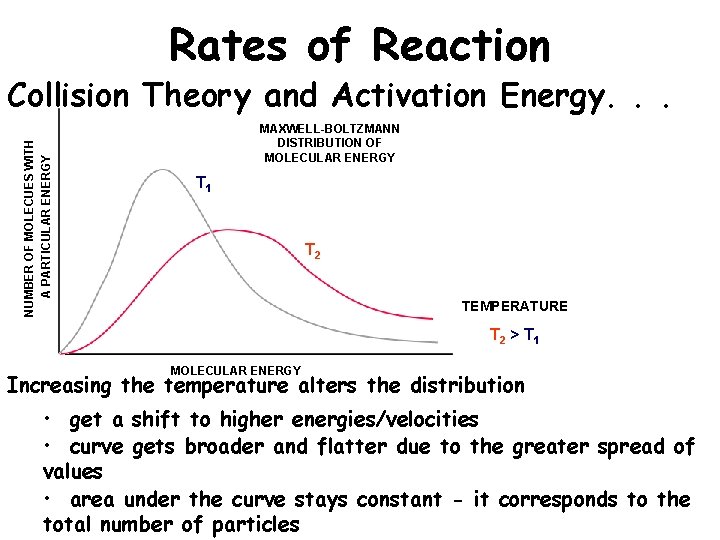
Rates of Reaction NUMBER OF MOLECUES WITH A PARTICULAR ENERGY Collision Theory and Activation Energy. . . MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY T 1 T 2 TEMPERATURE T 2 > T 1 MOLECULAR ENERGY Increasing the temperature alters the distribution • get a shift to higher energies/velocities • curve gets broader and flatter due to the greater spread of values • area under the curve stays constant - it corresponds to the total number of particles

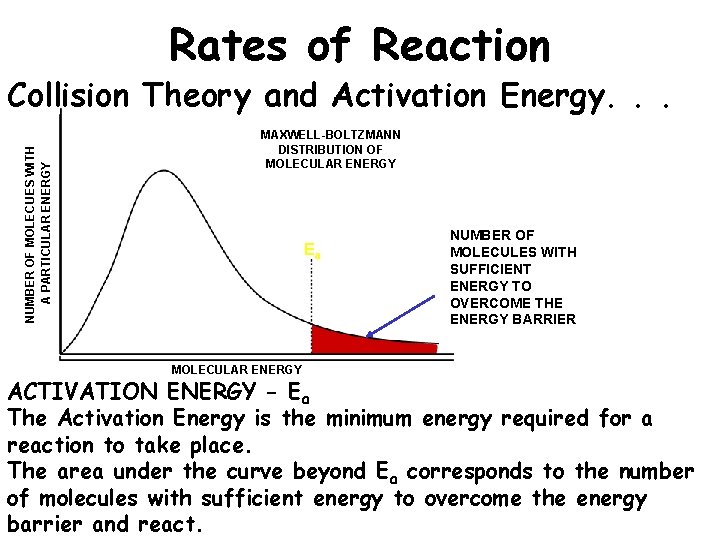
Rates of Reaction NUMBER OF MOLECUES WITH A PARTICULAR ENERGY Collision Theory and Activation Energy. . . MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY Ea MOLECULAR ENERGY NUMBER OF MOLECULES WITH SUFFICIENT ENERGY TO OVERCOME THE ENERGY BARRIER ACTIVATION ENERGY - Ea The Activation Energy is the minimum energy required for a reaction to take place. The area under the curve beyond Ea corresponds to the number of molecules with sufficient energy to overcome the energy barrier and react.

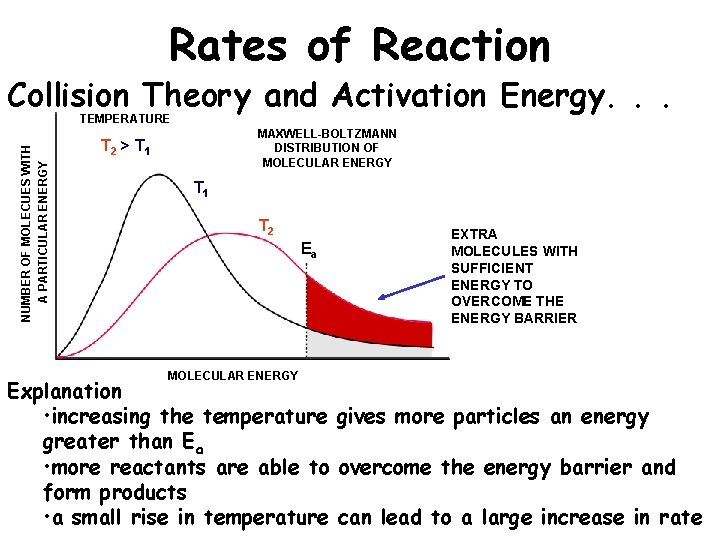
Rates of Reaction Collision Theory and Activation Energy. . . NUMBER OF MOLECUES WITH A PARTICULAR ENERGY TEMPERATURE MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY T 2 > T 1 T 2 Ea MOLECULAR ENERGY EXTRA MOLECULES WITH SUFFICIENT ENERGY TO OVERCOME THE ENERGY BARRIER Explanation • increasing the temperature gives more particles an energy greater than Ea • more reactants are able to overcome the energy barrier and form products • a small rise in temperature can lead to a large increase in rate

Rates of Reaction Collision Theory and Activation Energy. . . According to collision theory, to increase the rate of reaction you therefore need. . . • more frequent collisions • increase particle speed • have more particles present • more successful collisions • give particles more energy • lower the activation energy
 Unit of rate of reaction for first order reaction
Unit of rate of reaction for first order reaction Unit rate
Unit rate Ratios rates and unit rates guided notes
Ratios rates and unit rates guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Reaction rate
Reaction rate Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Reaction rates
Reaction rates Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Expressing reaction rates
Expressing reaction rates Bond equivalent yield
Bond equivalent yield Growth analysis
Growth analysis Spot rate and forward rate
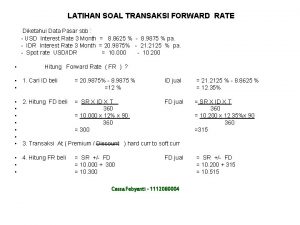
Spot rate and forward rate Transaksi forward
Transaksi forward Difference between rate and unit rate
Difference between rate and unit rate Cap rate interest rate relationship
Cap rate interest rate relationship Real exchange rate vs nominal exchange rate
Real exchange rate vs nominal exchange rate