Rates and Rate Laws Reaction Rate The change

![2 NO 2(g) 2 NO(g) + O 2(g) [NO 2] t Reaction Rates: 4. 2 NO 2(g) 2 NO(g) + O 2(g) [NO 2] t Reaction Rates: 4.](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-4.jpg)
![Solving an Integrated Rate Law Time (s) [H 2 O 2] (mol/L) 0 1. Solving an Integrated Rate Law Time (s) [H 2 O 2] (mol/L) 0 1.](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-12.jpg)
![Time vs. [H 2 O 2] Regression results: y = ax + b a Time vs. [H 2 O 2] Regression results: y = ax + b a](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-13.jpg)
![Time vs. ln[H 2 O 2] Time (s) ln[H 2 O 2] 0 0 Time vs. ln[H 2 O 2] Time (s) ln[H 2 O 2] 0 0](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-14.jpg)
![Time vs. 1/[H 2 O 2] Regression results: y = ax + b a Time vs. 1/[H 2 O 2] Regression results: y = ax + b a](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-15.jpg)
![And the winner is… Time vs. ln[H 2 O 2] 1. As a result, And the winner is… Time vs. ln[H 2 O 2] 1. As a result,](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-16.jpg)
- Slides: 19

Rates and Rate Laws

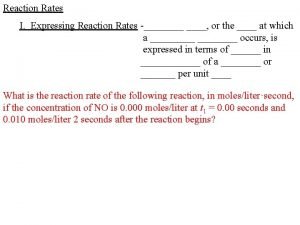
Reaction Rate The change in concentration of a reactant or product per unit of time

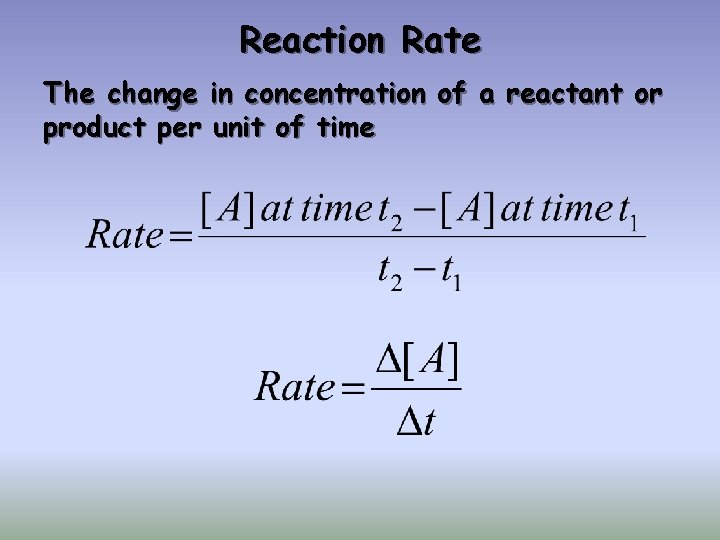
2 NO 2(g) 2 NO(g) + O 2(g) Reaction Rates: 1. Can measure disappearance of reactants 2. Can measure appearance of products 3. Are proportional stoichiometrically
![2 NO 2g 2 NOg O 2g NO 2 t Reaction Rates 4 2 NO 2(g) 2 NO(g) + O 2(g) [NO 2] t Reaction Rates: 4.](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-4.jpg)
2 NO 2(g) 2 NO(g) + O 2(g) [NO 2] t Reaction Rates: 4. Are equal to the slope tangent to that point 5. Change as the reaction proceeds, if the rate is dependent upon concentration

Rate Laws Differential rate laws express (reveal) the relationship between the concentration of reactants and the rate of the reaction. The differential rate law is usually just called “the rate law. ” Integrated rate laws express (reveal) the relationship between concentration of reactants and time

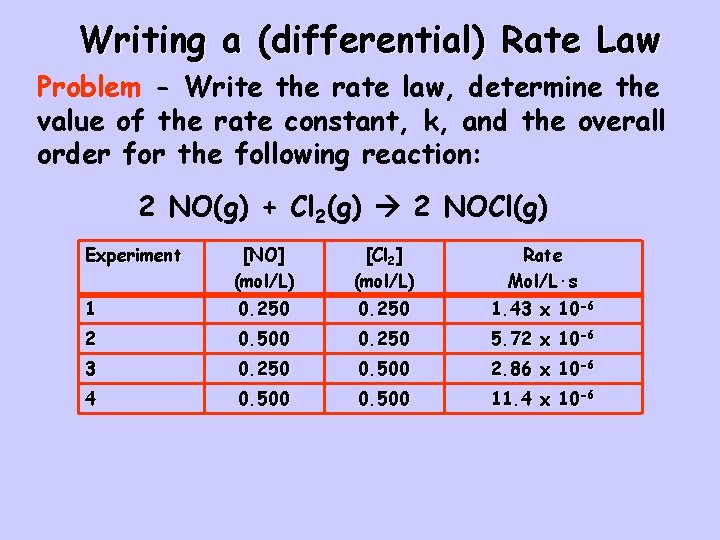
Writing a (differential) Rate Law Problem - Write the rate law, determine the value of the rate constant, k, and the overall order for the following reaction: 2 NO(g) + Cl 2(g) 2 NOCl(g) Experiment [NO] (mol/L) [Cl 2] (mol/L) Rate Mol/L·s 1 0. 250 1. 43 x 10 -6 2 0. 500 0. 250 5. 72 x 10 -6 3 0. 250 0. 500 2. 86 x 10 -6 4 0. 500 11. 4 x 10 -6

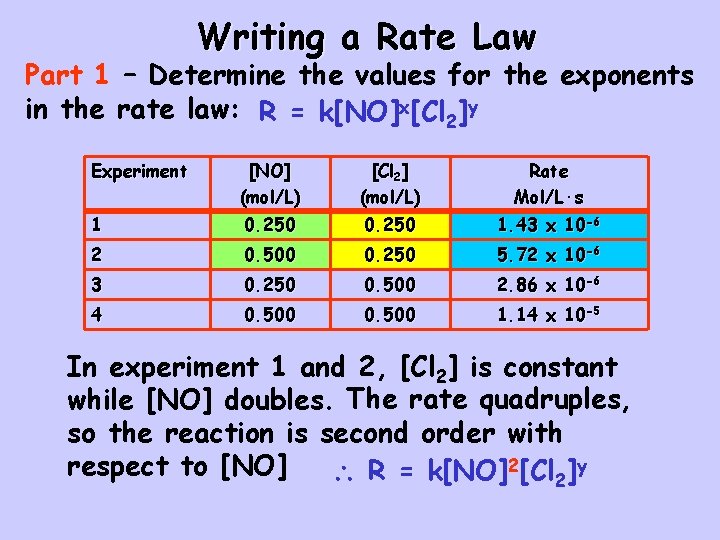
Writing a Rate Law Part 1 – Determine the values for the exponents in the rate law: R = k[NO]x[Cl 2]y Experiment [NO] (mol/L) [Cl 2] (mol/L) Rate Mol/L·s 1 0. 250 1. 43 x 10 -6 2 0. 500 0. 250 5. 72 x 10 -6 3 0. 250 0. 500 2. 86 x 10 -6 4 0. 500 1. 14 x 10 -5 In experiment 1 and 2, [Cl 2] is constant while [NO] doubles. The rate quadruples, so the reaction is second order with respect to [NO] R = k[NO]2[Cl 2]y

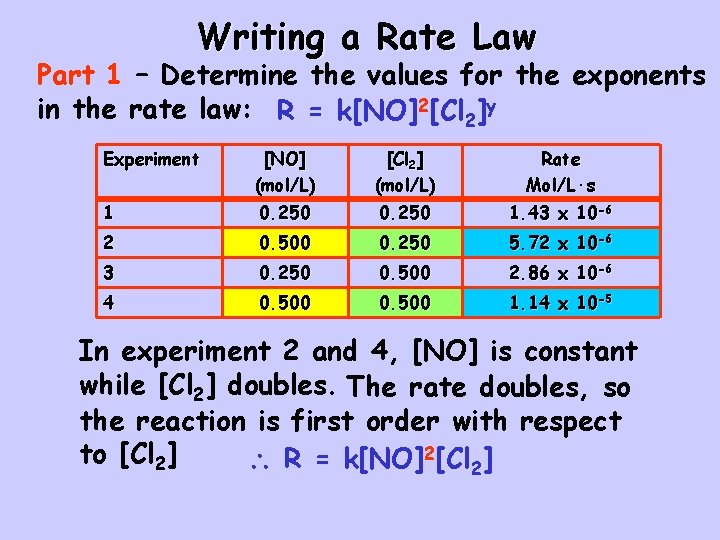
Writing a Rate Law Part 1 – Determine the values for the exponents in the rate law: R = k[NO]2[Cl 2]y Experiment [NO] (mol/L) [Cl 2] (mol/L) Rate Mol/L·s 1 0. 250 1. 43 x 10 -6 2 0. 500 0. 250 5. 72 x 10 -6 3 0. 250 0. 500 2. 86 x 10 -6 4 0. 500 1. 14 x 10 -5 In experiment 2 and 4, [NO] is constant while [Cl 2] doubles. The rate doubles, so the reaction is first order with respect to [Cl 2] R = k[NO]2[Cl 2]

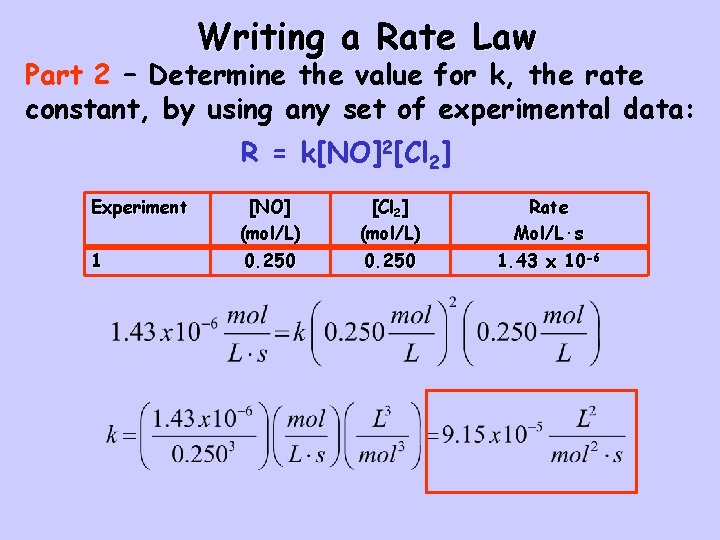
Writing a Rate Law Part 2 – Determine the value for k, the rate constant, by using any set of experimental data: R = k[NO]2[Cl 2] Experiment [NO] (mol/L) [Cl 2] (mol/L) Rate Mol/L·s 1 0. 250 1. 43 x 10 -6

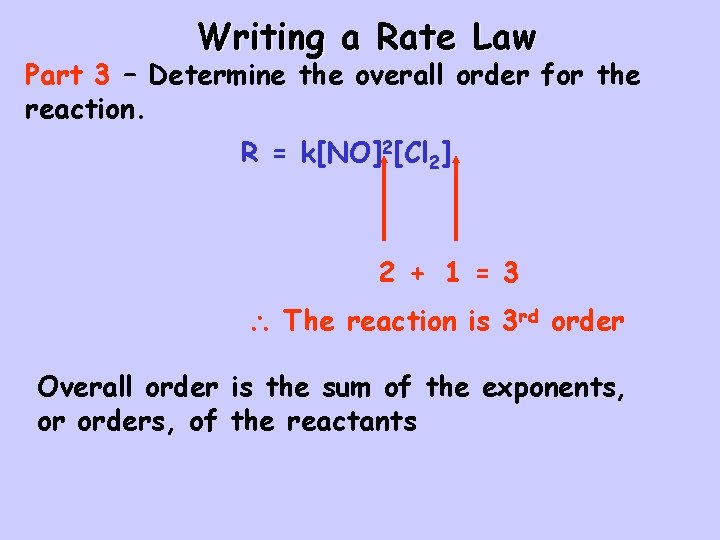
Writing a Rate Law Part 3 – Determine the overall order for the reaction. R = k[NO]2[Cl 2] 2 + 1 = 3 The reaction is 3 rd order Overall order is the sum of the exponents, or orders, of the reactants

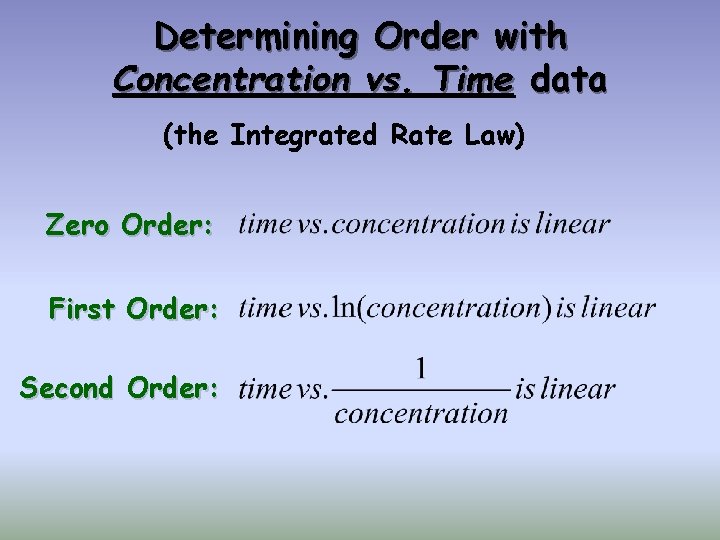
Determining Order with Concentration vs. Time data (the Integrated Rate Law) Zero Order: First Order: Second Order:
![Solving an Integrated Rate Law Time s H 2 O 2 molL 0 1 Solving an Integrated Rate Law Time (s) [H 2 O 2] (mol/L) 0 1.](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-12.jpg)
Solving an Integrated Rate Law Time (s) [H 2 O 2] (mol/L) 0 1. 00 120 0. 91 300 0. 78 600 0. 59 1200 0. 37 1800 0. 22 2400 0. 13 3000 0. 082 3600 0. 050 Problem: Find the integrated rate law and the value for the rate constant, k A graphing calculator with linear regression analysis greatly simplifies this process!! (Click here to download my Rate Laws program for the. Ti-83 and Ti-84)
![Time vs H 2 O 2 Regression results y ax b a Time vs. [H 2 O 2] Regression results: y = ax + b a](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-13.jpg)
Time vs. [H 2 O 2] Regression results: y = ax + b a = -2. 64 x 10 -4 b = 0. 841 r 2 = 0. 8891 r = -0. 9429 Time (s) [H 2 O 2] 0 1. 00 120 0. 91 300 0. 78 600 0. 59 1200 0. 37 1800 0. 22 2400 0. 13 3000 0. 082 3600 0. 050
![Time vs lnH 2 O 2 Time s lnH 2 O 2 0 0 Time vs. ln[H 2 O 2] Time (s) ln[H 2 O 2] 0 0](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-14.jpg)
Time vs. ln[H 2 O 2] Time (s) ln[H 2 O 2] 0 0 120 -0. 0943 300 -0. 2485 600 -0. 5276 Regression results: 1200 -0. 9943 y = ax + b a = -8. 35 x 10 -4 b = -. 005 r 2 = 0. 99978 r = -0. 9999 1800 -1. 514 2400 -2. 04 3000 -2. 501 3600 -2. 996
![Time vs 1H 2 O 2 Regression results y ax b a Time vs. 1/[H 2 O 2] Regression results: y = ax + b a](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-15.jpg)
Time vs. 1/[H 2 O 2] Regression results: y = ax + b a = 0. 00460 b = -0. 847 r 2 = 0. 8723 r = 0. 9340 Time (s) 1/[H 2 O 2] 0 1. 00 120 1. 0989 300 1. 2821 600 1. 6949 1200 2. 7027 1800 4. 5455 2400 7. 6923 3000 12. 195 3600 20. 000
![And the winner is Time vs lnH 2 O 2 1 As a result And the winner is… Time vs. ln[H 2 O 2] 1. As a result,](https://slidetodoc.com/presentation_image/124c9ce9967e4d21034ea1f83441e004/image-16.jpg)
And the winner is… Time vs. ln[H 2 O 2] 1. As a result, the reaction is 1 st order 2. The (differential) rate law is: 3. The integrated rate law is: 4. But…what is the rate constant, k ?

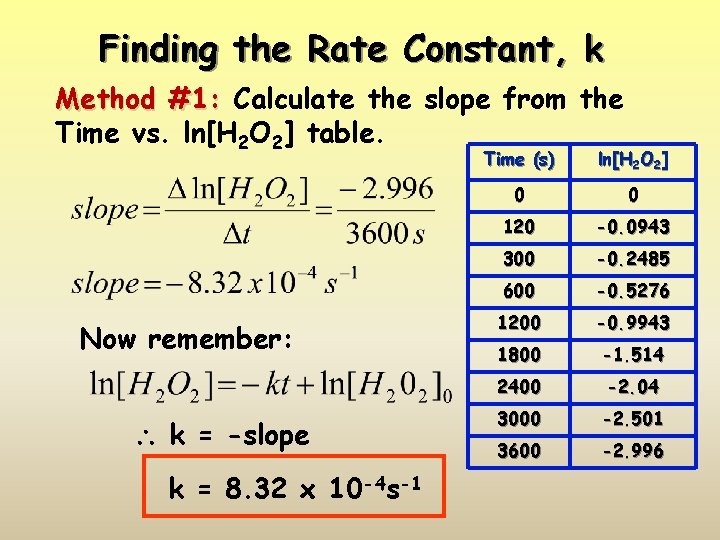
Finding the Rate Constant, k Method #1: Calculate the slope from the Time vs. ln[H 2 O 2] table. Now remember: k = -slope k = 8. 32 x 10 -4 s-1 Time (s) ln[H 2 O 2] 0 0 120 -0. 0943 300 -0. 2485 600 -0. 5276 1200 -0. 9943 1800 -1. 514 2400 -2. 04 3000 -2. 501 3600 -2. 996

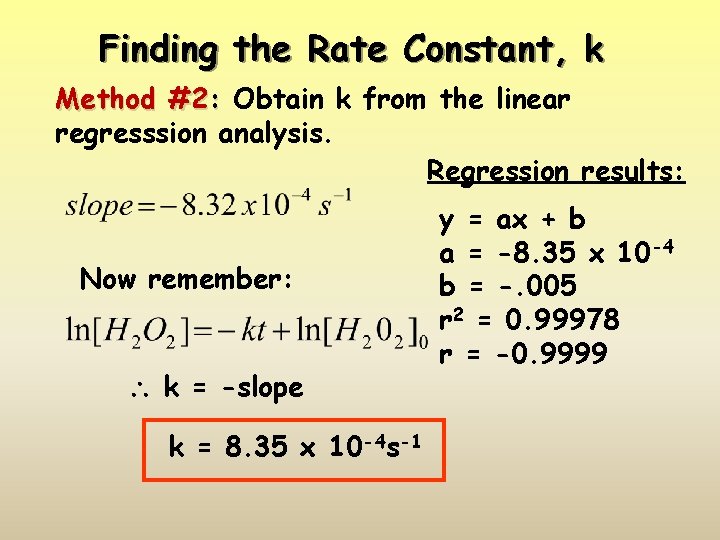
Finding the Rate Constant, k Method #2: Obtain k from the linear regresssion analysis. Regression results: Now remember: k = -slope k = 8. 35 x 10 -4 s-1 y = ax + b a = -8. 35 x 10 -4 b = -. 005 r 2 = 0. 99978 r = -0. 9999

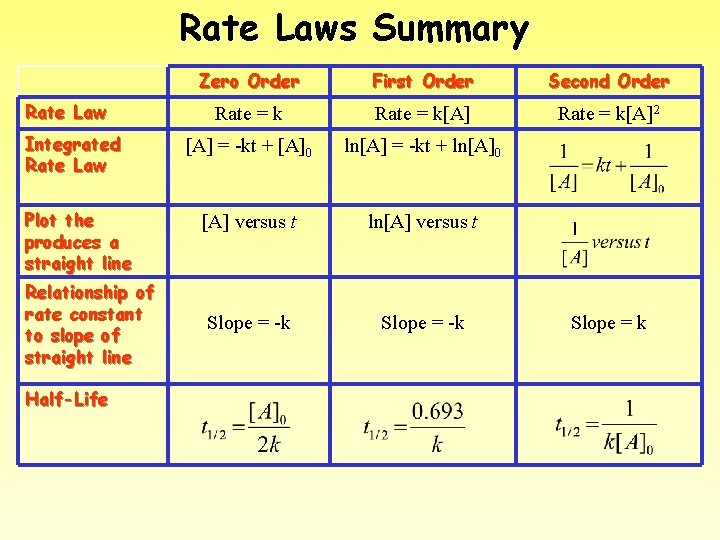
Rate Laws Summary Rate Law Integrated Rate Law Plot the produces a straight line Relationship of rate constant to slope of straight line Half-Life Zero Order First Order Second Order Rate = k[A]2 [A] = -kt + [A]0 ln[A] = -kt + ln[A]0 [A] versus t ln[A] versus t Slope = -k Slope = k
 A rate is a ratio
A rate is a ratio Equivalent ratios guided notes
Equivalent ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Reaction rate equation
Reaction rate equation Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Rates of change in the natural and social sciences
Rates of change in the natural and social sciences 1-4 practice extrema and average rates of change
1-4 practice extrema and average rates of change Linear models and rates of change
Linear models and rates of change Rate of change and slope lesson 3-2
Rate of change and slope lesson 3-2 Expressing reaction rates
Expressing reaction rates Rate of reaction quiz
Rate of reaction quiz Expressing reaction rates
Expressing reaction rates Reaction rates
Reaction rates Facts about montesquieu
Facts about montesquieu