Reaction Rates I Expressing Reaction Rates or the









![Reaction Rates V. Reaction Orders Trial 1 2 3 Initial [A] (in M) Initial Reaction Rates V. Reaction Orders Trial 1 2 3 Initial [A] (in M) Initial](https://slidetodoc.com/presentation_image_h/09590658ddbc31b6e81cdf308d7d8d09/image-40.jpg)




- Slides: 61

Reaction Rates I. Expressing Reaction Rates -____, or the ____ at which a _____ occurs, is expressed in terms of ______ in ______ of a ____ or _______ per unit ____ What is the reaction rate of the following reaction, in moles/liter·second, if the concentration of NO is 0. 000 moles/liter at t 1 = 0. 00 seconds and 0. 010 moles/liter 2 seconds after the reaction begins?

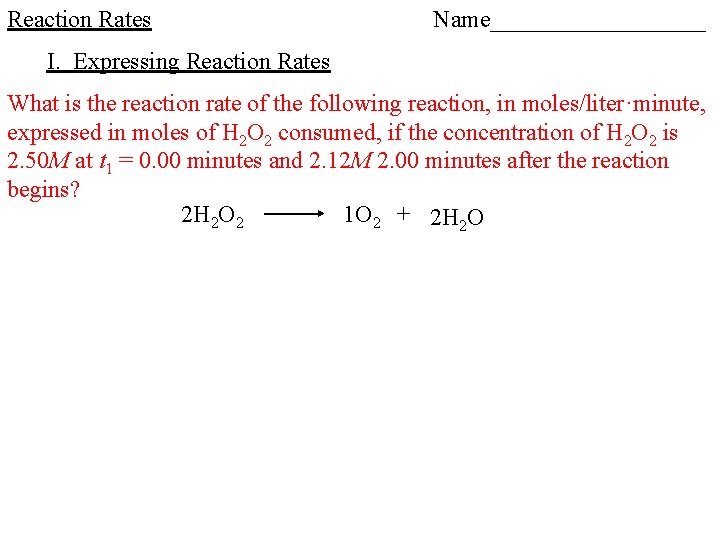
Reaction Rates I. Expressing Reaction Rates -____ can be expressed as the ____ at which a ____ is produced or the ____ at which a ____ is consumed What is the reaction rate of the following reaction, in moles/liter·second, if the concentration of C 4 H 9 Cl is 0. 220 M at t 1 = 0. 00 seconds and 0. 100 M 4. 00 seconds after the reaction begins?

Reaction Rates I. Expressing Reaction Rates What is the reaction rate of the following reaction, in moles/liter·second, expressed in moles of H 2 consumed, if the concentration of H 2 is 0. 030 M at t 1 = 0. 00 seconds and 0. 020 M 4. 00 seconds after the reaction begins?

Reaction Rates I. Expressing Reaction Rates What is the reaction rate of the following reaction, in moles/liter·second, expressed in moles of HCl produced, if the concentration of HCl is 0. 000 M at t 1 = 0. 00 seconds and 0. 020 M 4. 00 seconds after the reaction begins?

Reaction Rates Name_________ I. Expressing Reaction Rates What is the reaction rate of the following reaction, in moles/liter·minute, expressed in moles of H 2 O 2 consumed, if the concentration of H 2 O 2 is 2. 50 M at t 1 = 0. 00 minutes and 2. 12 M 2. 00 minutes after the reaction begins? 2 H 2 O 2 1 O 2 + 2 H 2 O

Reaction Rates I. Expressing Reaction Rates What is the reaction rate of the following reaction, in moles/liter·minute, expressed in moles of O 2 produced, if the concentration of H 2 O 2 is 1. 82 M at t 1 = 0. 00 minutes and 1. 48 M 5. 00 minutes after the reaction begins? 2 H 2 O 2 1 O 2 + 2 H 2 O

Reaction Rates II. The Collision Theory -the ______ states that, in order for a ________ to take place, the ______, or _____ must _______ in order to _____

Reaction Rates II. The Collision Theory -according to the ______, ___ and ___ molecules must _______ in order to _____, but in the reaction of ________ and ______, only a _______ of the _____ produce ____

Reaction Rates II. The Collision Theory

Reaction Rates II. The Collision Theory

Reaction Rates II. The Collision Theory -according to the ______, the _____ of _________ must __ _______, __ _______ with the correct ______, and __ _______ with sufficient ______ to form the _______

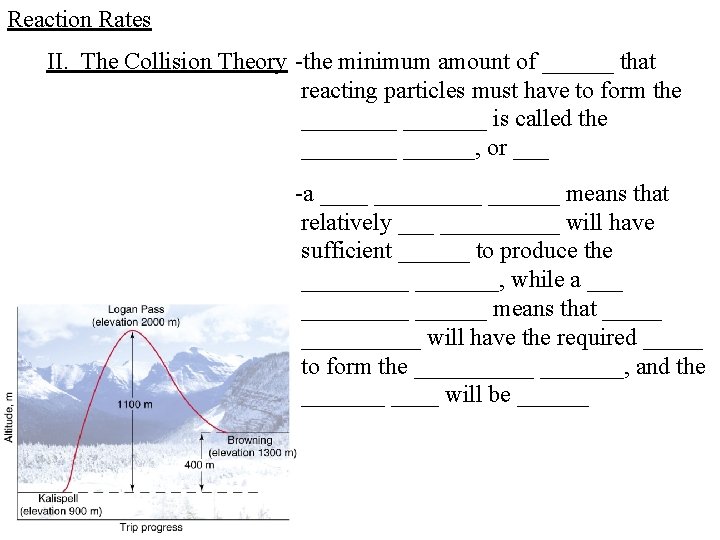
Reaction Rates II. The Collision Theory -the minimum amount of ______ that reacting particles must have to form the _______ is called the ______, or ___ -a _________ means that relatively __________ will have sufficient ______ to produce the _______, while a _________ means that __________ will have the required _____ to form the _______, and the _______ will be ______

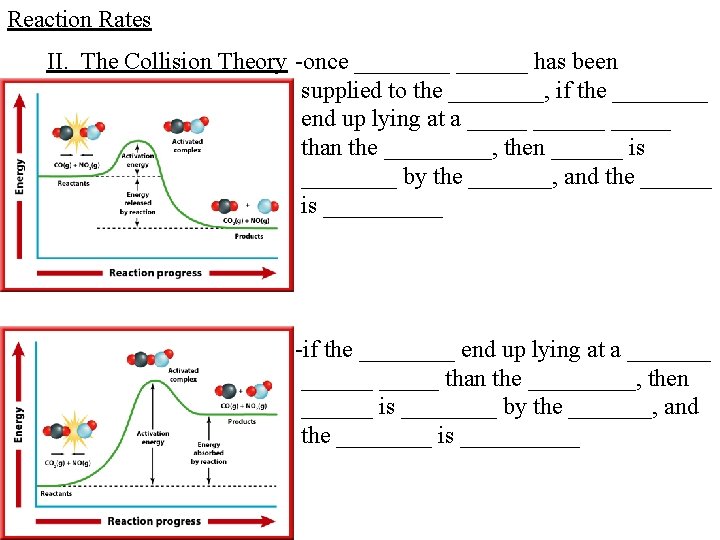
Reaction Rates II. The Collision Theory -once ______ has been supplied to the ____, if the ____ end up lying at a ______ than the _____, then ______ is ____ by the _______, and the ______ is _____ -if the ____ end up lying at a _______ than the _____, then ______ is ____ by the _______, and the ____ is _____

Reaction Rates III. Factors Affecting Reaction Rates A. The Nature of the Reactants -one factor that affects the ____ of chemical _____ is the _______ of the ____

Reaction Rates III. Factors Affecting Reaction Rates B. Concentration -when the _______ of the _____ is _____, reactions ______ -since _____ is necessary for _________ to take place, _____ the ______ of the _________ increases the likelihood that the _____ of one ____ will _______ with the _____ of the other _____

Reaction Rates III. Factors Affecting Reaction Rates B. Concentration

Reaction Rates III. Factors Affecting Reaction Rates C. Surface Area -if the _______ of the _____ of reactant is _____ by _____ particle _____, the _______ will ____, since the greater _____ allows the _____ of one ____ to _______ with _____ particles of the other ____ per unit time

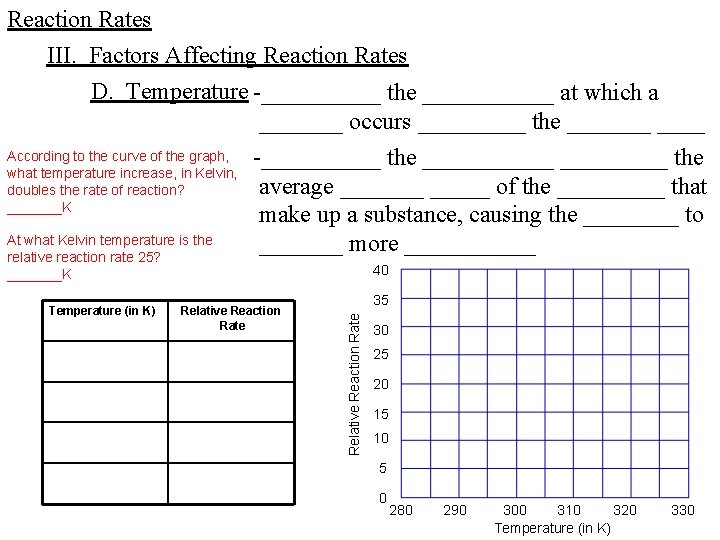
Reaction Rates III. Factors Affecting Reaction Rates D. Temperature -_____ the ______ at which a _______ occurs _____ the _______ According to the curve of the graph, -_____ the ______ the what temperature increase, in Kelvin, average _______ of the _____ that doubles the rate of reaction? _______K make up a substance, causing the ____ to At what Kelvin temperature is the _______ more ______ relative reaction rate 25? 40 _______K Relative Reaction Rate 35 Relative Reaction Rate Temperature (in K) 30 25 20 15 10 5 0 280 290 300 310 320 Temperature (in K) 330

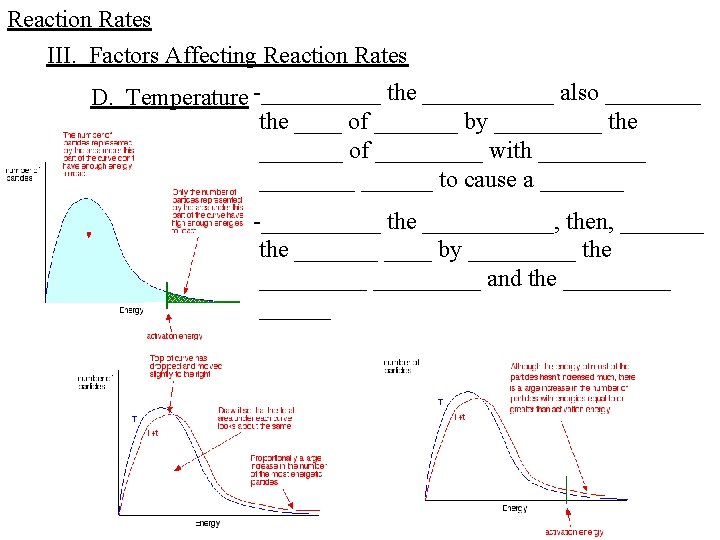
Reaction Rates III. Factors Affecting Reaction Rates D. Temperature -_____ the ______ also ____ the ____ of _______ by _____ the _______ of _____ with _____ ______ to cause a _______ -_____ the ______, then, _______ the _______ by _____ the _________ and the ______

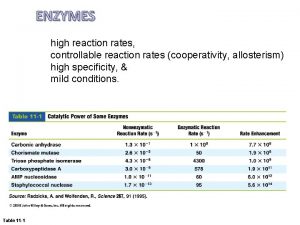
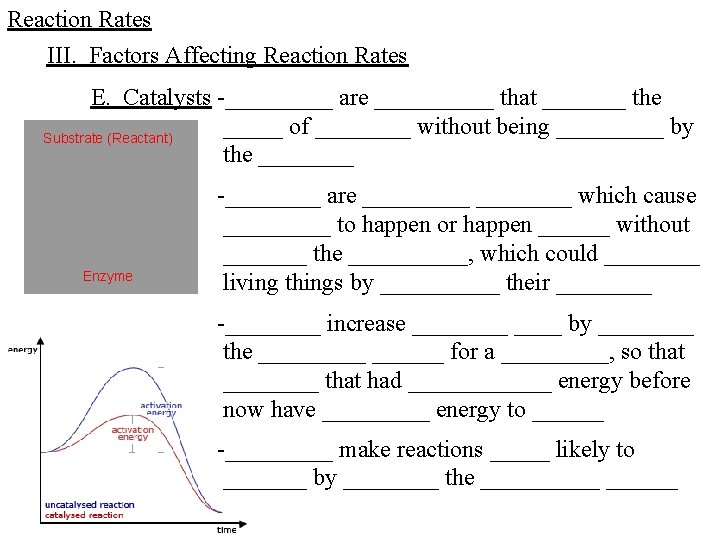
Reaction Rates III. Factors Affecting Reaction Rates E. Catalysts -_____ are _____ that _______ the _____ of ____ without being _____ by Substrate (Reactant) the ____ Enzyme -____ are _____ which cause _____ to happen or happen ______ without _______ the _____, which could ____ living things by _____ their ____ -____ increase ____ by ____ the ______ for a _____, so that ____ that had ______ energy before now have _____ energy to ______ -_____ make reactions _____ likely to _______ by ____ the ______

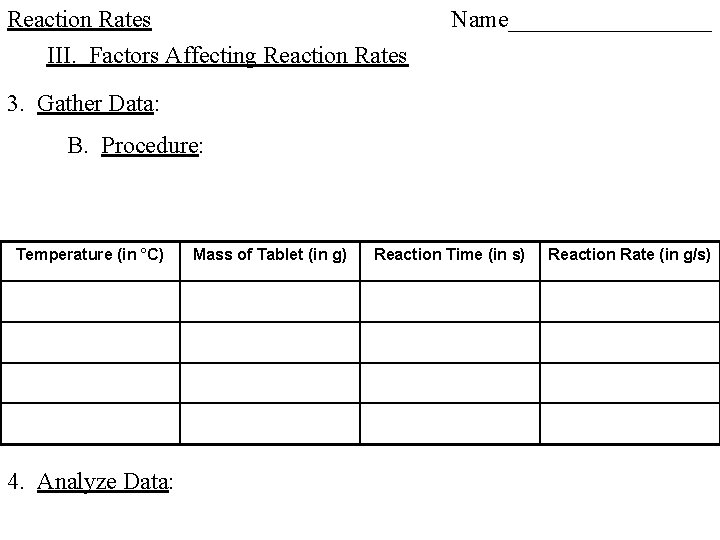
Reaction Rates III. Factors Affecting Reaction Rates 1. Hypothesis: 2. Prediction: 3. Gather Data: A. Safety: B. Procedure:

Reaction Rates III. Factors Affecting Reaction Rates 3. Gather Data: B. Procedure:

Reaction Rates III. Factors Affecting Reaction Rates Name_________ 3. Gather Data: B. Procedure: Temperature (in °C) 4. Analyze Data: Mass of Tablet (in g) Reaction Time (in s) Reaction Rate (in g/s)

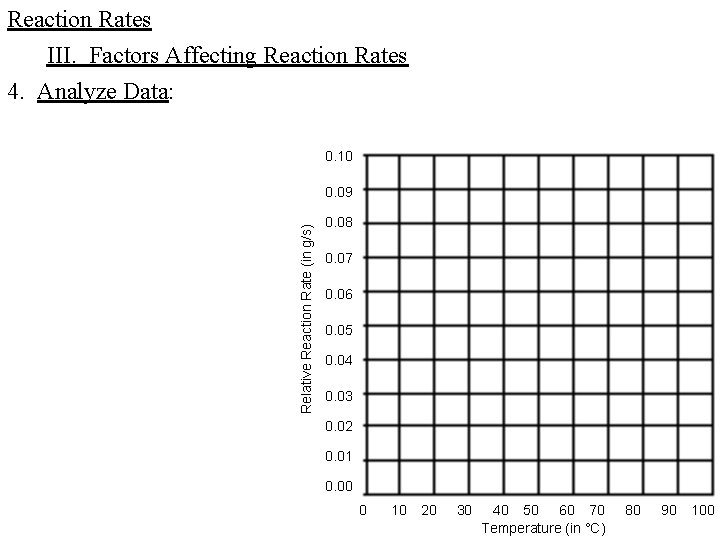
Reaction Rates III. Factors Affecting Reaction Rates 4. Analyze Data: 0. 10 Relative Reaction Rate (in g/s) 0. 09 0. 08 0. 07 0. 06 0. 05 0. 04 0. 03 0. 02 0. 01 0. 00 0 10 20 30 40 50 60 70 Temperature (in °C) 80 90 100

Reaction Rates III. Factors Affecting Reaction Rates 4. Analyze Data:

Reaction Rates III. Factors Affecting Reaction Rates 5. Draw Conclusions: ________________________________________

Reaction Rates IV. Reaction Rate Laws -when we divide the ____ in ____________, _____ by the _______ in _____, we get an ________ -chemical reactions tend to _____ as ____ are _____, because in order for a reaction to proceed, _____ must _______, and as _____ are _____ there are ________ left to _______

Reaction Rates IV. Reaction Rate Laws -________ the results of the _______ in terms of a ______ between the _____ of a _____ and the _____________ -in the reaction __ __, there is only _________ between the ____ and ____, so the _____ for the reaction is _____ = __ ____, where ____ is the _______ of the _____ __ and __ is the _________ ________, which depends on _________, especially the ______ -the _____, then, is _____________ to the _______

Reaction Rates V. Reaction Orders -in the reaction __ __, the _____ = __ ___, and it is understood that ____ means the same as ____, and the _____ __ is the ______ -the _____ for the _______ of _____ is _____ = __ ______, and the ____ is said to be _____ in _____ -for _____ with _____ than _______, the _____ is _____ = __ ____ where __ is the _______ for __ and __ is the _______ for __

Reaction Rates V. Reaction Orders -for the ____ that has the _________, the reaction is described as _______ in ___, and _____ overall -an _______ of evaluating ______is the _______ of _______, in which the _______ of the _____ are _______ and the effect on the _____ is observed

Reaction Rates V. Reaction Orders -the _____ for this type of reaction is _________. From the data, you can see that, while ____ was held constant, the _____ has ____ in Trial 2 compared to Trial 1, at the same time ____ has ____, so the ______ __ must equal __, or because ___ = __, __ = __ -in Trial 3 compared to Trial 2, ____ is _______, and the _______, so the ______ __ must equal __, or ___ = __, and the _______ is _________ and the ________ is _______

Reaction Rates V. Reaction Orders Given the following experimental data, use the method of initial rates to determine the rate law for the reaction and the overall reaction order.

Reaction Rates V. Reaction Orders Given the following experimental data, use the method of initial rates to determine the rate law for the reaction and the overall reaction order.

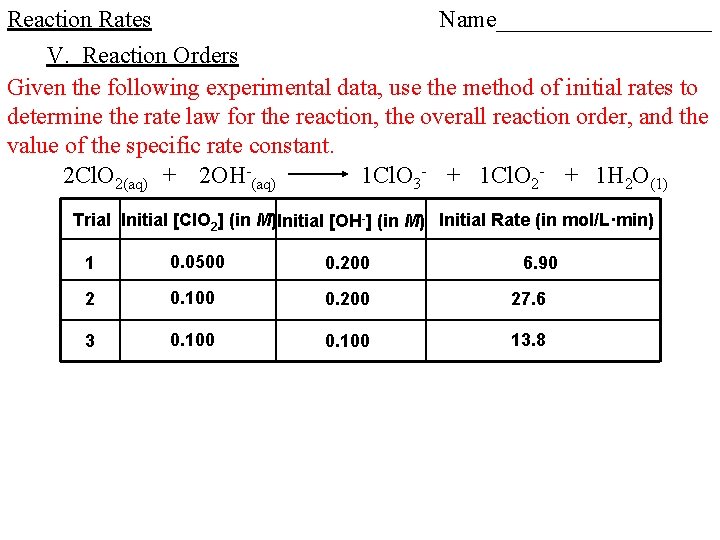
Reaction Rates V. Reaction Orders Given the following experimental data, use the method of initial rates to determine the rate law for the reaction, the overall reaction order, and the value of the specific rate constant.

Reaction Rates Name_________ V. Reaction Orders Given the following experimental data, use the method of initial rates to determine the rate law for the reaction, the overall reaction order, and the value of the specific rate constant. 2 Cl. O 2(aq) + 2 OH-(aq) 1 Cl. O 3 - + 1 Cl. O 2 - + 1 H 2 O(1) Trial Initial [Cl. O 2] (in M)Initial [OH-] (in M) Initial Rate (in mol/L·min) 1 0. 0500 0. 200 2 0. 100 0. 200 27. 6 3 0. 100 13. 8 6. 90

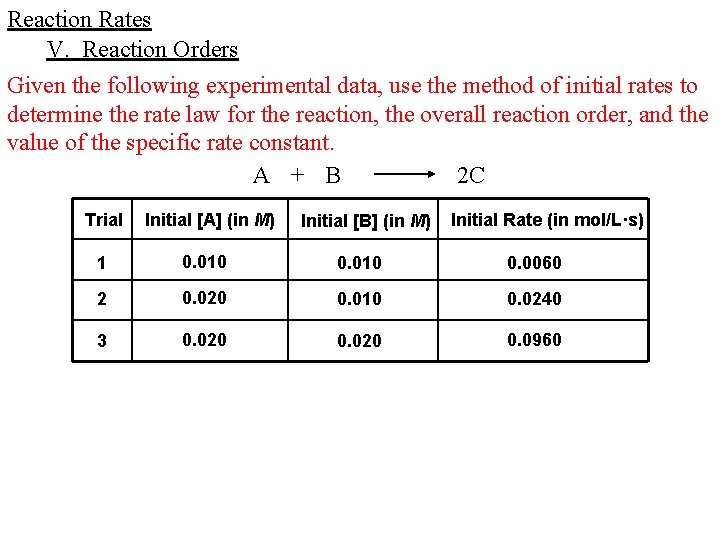
Reaction Rates V. Reaction Orders Given the following experimental data, use the method of initial rates to determine the rate law for the reaction, the overall reaction order, and the value of the specific rate constant. A + B 2 C Trial Initial [A] (in M) Initial [B] (in M) Initial Rate (in mol/L·s) 1 0. 010 0. 0060 2 0. 020 0. 010 0. 0240 3 0. 020 0. 0960

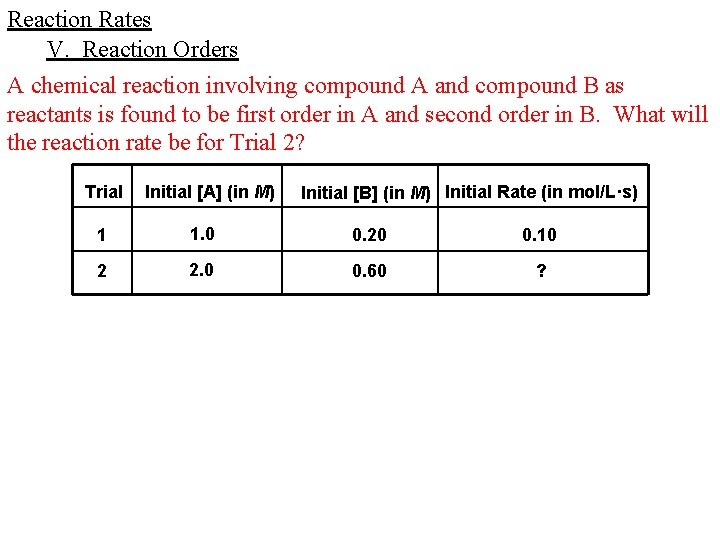
Reaction Rates V. Reaction Orders Given the following experimental data, use the method of initial rates to determine the rate law for the reaction, the overall reaction order, and the value of the specific rate constant. A + B products Trial Initial [A] (in M) Initial [B] (in M) Initial Rate (in mol/L·hr) 1 0. 010 0. 020 2 0. 015 0. 020 0. 030 3 0. 015 0. 040 0. 240

Reaction Rates V. Reaction Orders A chemical reaction involving compound A and compound B as reactants is found to be first order in A and second order in B. What will the reaction rate be for Trial 2? Trial Initial [A] (in M) Initial [B] (in M) Initial Rate (in mol/L·s) 1 1. 0 0. 20 0. 10 2 2. 0 0. 60 ?

Reaction Rates V. Reaction Orders -most _________ obey ____ of ______: _____, or _______, and the ______ of the ________, vary with the ______ of the ____ -if a ____ with ____ or _________ was _______ to be _______, the _____ would be _______ or ________ -in ______ reactions, the _____ is _____, and the ______ of __ are ____
![Reaction Rates V Reaction Orders Trial 1 2 3 Initial A in M Initial Reaction Rates V. Reaction Orders Trial 1 2 3 Initial [A] (in M) Initial](https://slidetodoc.com/presentation_image_h/09590658ddbc31b6e81cdf308d7d8d09/image-40.jpg)
Reaction Rates V. Reaction Orders Trial 1 2 3 Initial [A] (in M) Initial Rate (in mol/L·s)

Reaction Rates V. Reaction Orders -plot the data from the table on the graph below: 10. 00 Reaction Rate x 10 -11 (in mol/L·s) 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0 . 0 10 00 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. Concentration [A] x 10 -3 (in mol/L)

Reaction Rates V. Reaction Orders -if a ____ with ____ or _________ was _______ to be _______, the _____ would be _______ or ________ -in ______ reactions, the _____ is _______, and the ______ of __ are ____ Trial 1 2 3 4 Initial [A] (in M) Initial [B] (in M) Initial Rate (in mol/L·s)

Reaction Rates V. Reaction Orders -plot the data from the table on the graph below: 10. 00 Reaction Rate x 10 -11 (in mol/L·s) 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0 . 0 10 00 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. Concentration [A] x 10 -3 (in mol/L)

Reaction Rates V. Reaction Orders -if a ____ with ____ or _________ was _______ to be _______, the _____ would be _______ or ________ -in ______ reactions, the _____ is ________, and the ______ of __ are _______ Trial 1 2 3 4 Initial [A] (in M) Initial [B] (in M) Initial Rate (in mol/L·s)

Reaction Rates V. Reaction Orders -plot the data from the table on the graph below: 10. 00 Reaction Rate x 10 -11 (in mol/L·s) 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0 . 0 10 00 9. 00 8. 00 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 1. 00 0. Concentration [A] x 10 -3 (in mol/L)

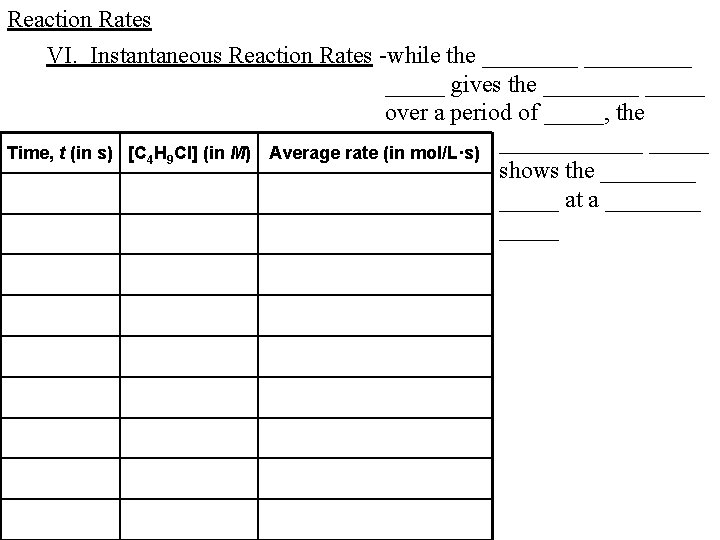
Reaction Rates VI. Instantaneous Reaction Rates -while the _________ gives the _____ over a period of _____, the ______ Time, t (in s) [C 4 H 9 Cl] (in M) Average rate (in mol/L·s) shows the _____ at a _____

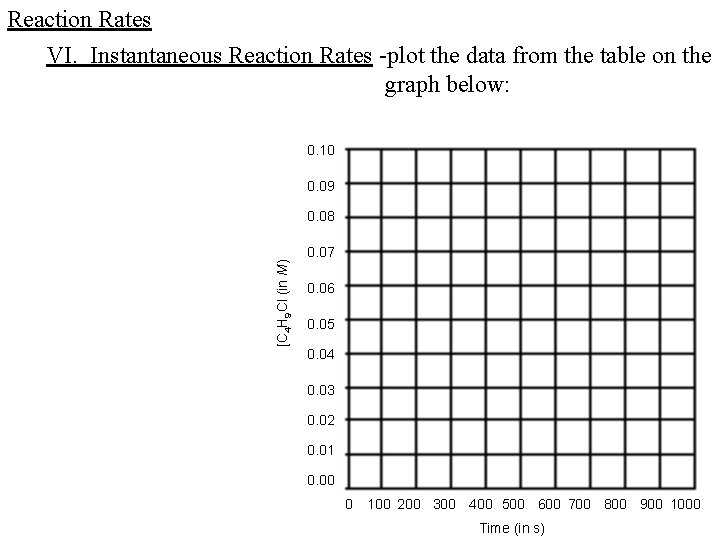
Reaction Rates VI. Instantaneous Reaction Rates -plot the data from the table on the graph below: 0. 10 0. 09 [C 4 H 9 Cl (in M) 0. 08 0. 07 0. 06 0. 05 0. 04 0. 03 0. 02 0. 01 0. 00 0 100 200 300 400 500 600 700 800 900 1000 Time (in s)

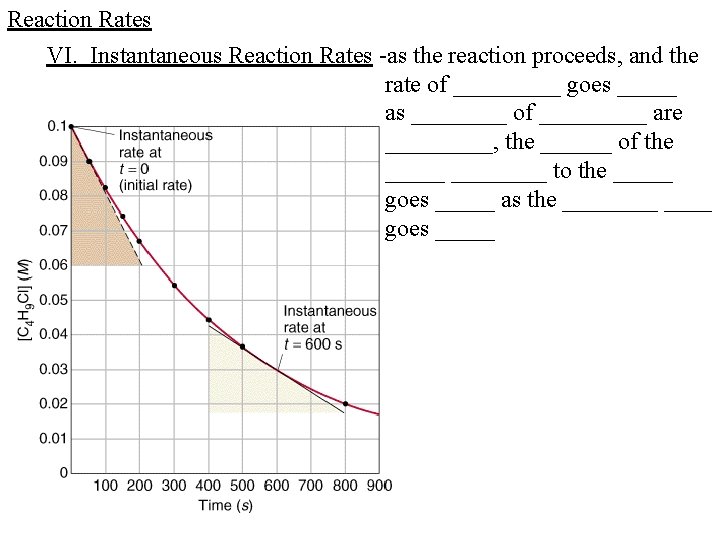
Reaction Rates VI. Instantaneous Reaction Rates -as the reaction proceeds, and the rate of _____ goes _____ as ____ of _____ are _____, the ______ of the ________ to the _____ goes _____ as the ____ goes _____

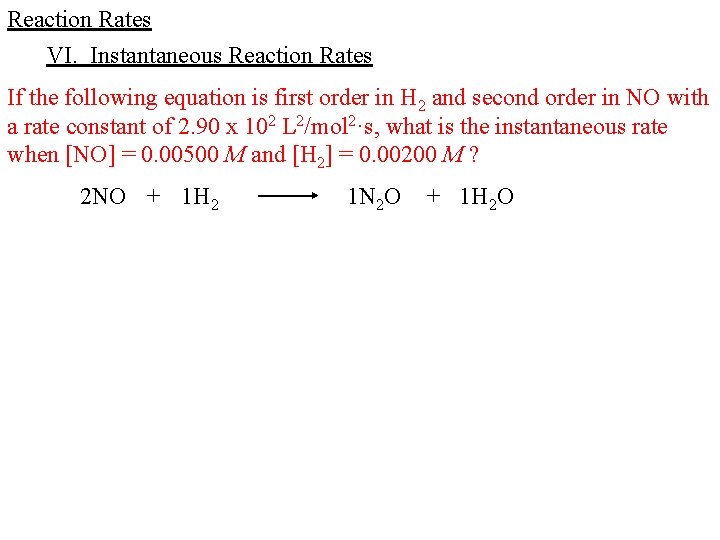
Reaction Rates VI. Instantaneous Reaction Rates If the following equation is first order in H 2 and second order in NO with a rate constant of 2. 90 x 102 L 2/mol 2·s, what is the instantaneous rate when [NO] = 0. 00200 M and [H 2] = 0. 00400 M ?

Reaction Rates VI. Instantaneous Reaction Rates If the following equation is first order in H 2 and second order in NO with a rate constant of 2. 90 x 102 L 2/mol 2·s, what is the instantaneous rate when [NO] = 0. 00500 M and [H 2] = 0. 00200 M ? 2 NO + 1 H 2 1 N 2 O + 1 H 2 O

Reaction Rates VI. Instantaneous Reaction Rates If the following equation is first order in H 2 and second order in NO with a rate constant of 2. 90 x 102 L 2/mol 2·s, what is the instantaneous rate when [NO] = 0. 0100 M and [H 2] = 0. 00125 M ? 2 NO + 1 H 2 1 N 2 O + 1 H 2 O

Reaction Rates VI. Instantaneous Reaction Rates If the following equation is first order in H 2 and second order in NO with a rate constant of 2. 90 x 102 L 2/mol 2·s, what is the instantaneous rate when [NO] = 0. 00446 M and [H 2] = 0. 00282 M ? 2 NO + 1 H 2 1 N 2 O + 1 H 2 O

Reaction Rates VI. Instantaneous Reaction Rates If the following equation is first order in A and second order in B with a specific rate constant of 4. 75 x 10 -7 L 2/mol 2·s, what is the instantaneous rate when [A] = 0. 355 M and [B] = 0. 0122 M ? A + B products

Reaction Rates III. Factors Affecting Reaction Rates 1. Hypothesis: 2. Prediction: 3. Gather Data: A. Safety: The acids used in this lab are corrosive and cause irritation and damage to the skin, eyes, and mucous membranes. Avoid contact. Use caution. Goggles and aprons mandatory. Gloves recommended. B. Procedure:

Reaction Rates III. Factors Affecting Reaction Rates 3. Gather Data: B. Procedure:

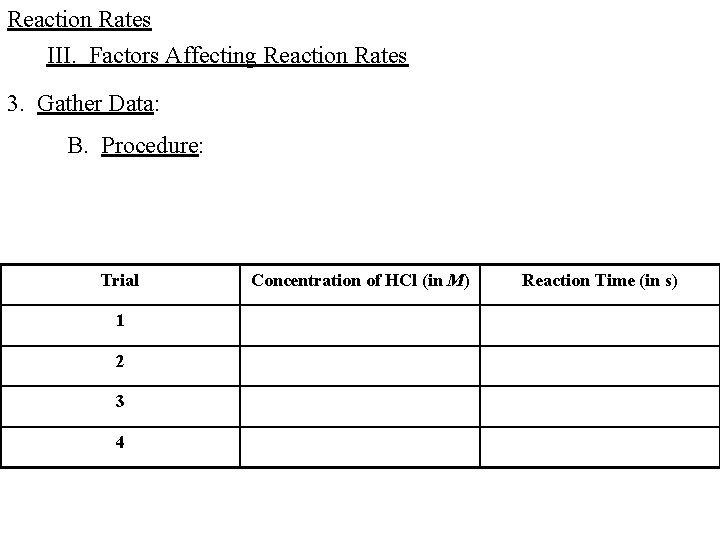
Reaction Rates III. Factors Affecting Reaction Rates 3. Gather Data: B. Procedure: Trial 1 2 3 4 Concentration of HCl (in M) Reaction Time (in s)

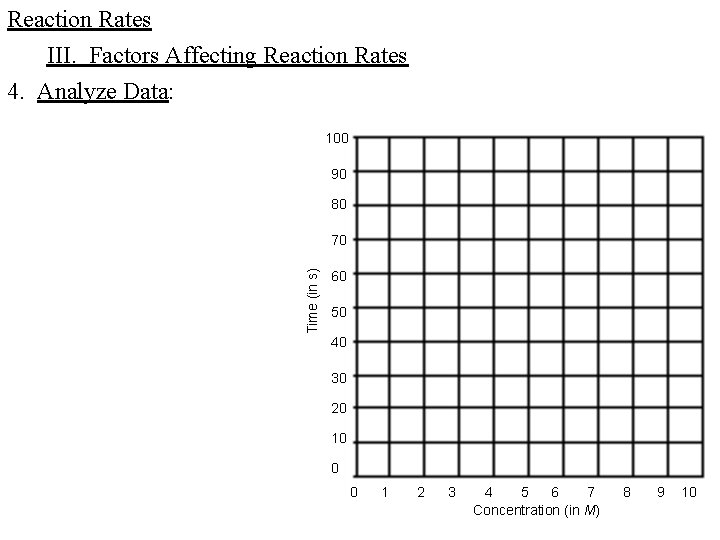
Reaction Rates III. Factors Affecting Reaction Rates 4. Analyze Data: 100 90 80 Time (in s) 70 60 50 40 30 20 10 0 0 1 2 3 4 5 6 7 Concentration (in M) 8 9 10

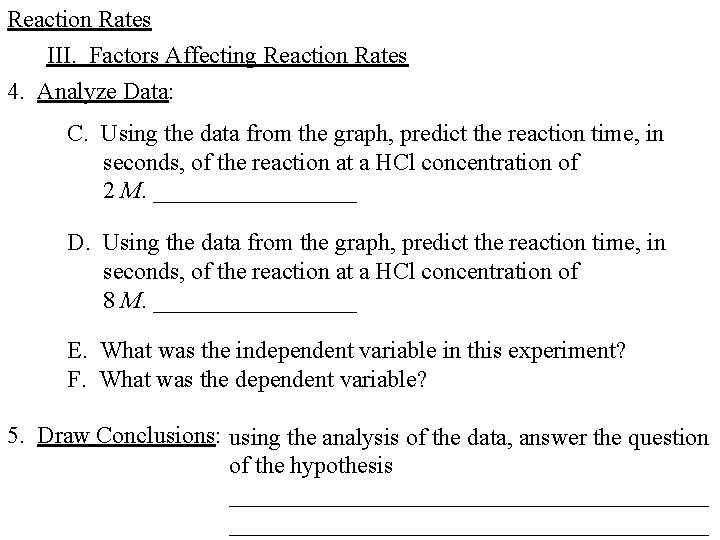
Reaction Rates III. Factors Affecting Reaction Rates 4. Analyze Data: C. Using the data from the graph, predict the reaction time, in seconds, of the reaction at a HCl concentration of 2 M. _________ D. Using the data from the graph, predict the reaction time, in seconds, of the reaction at a HCl concentration of 8 M. _________ E. What was the independent variable in this experiment? F. What was the dependent variable? 5. Draw Conclusions: using the analysis of the data, answer the question of the hypothesis ________________________________________

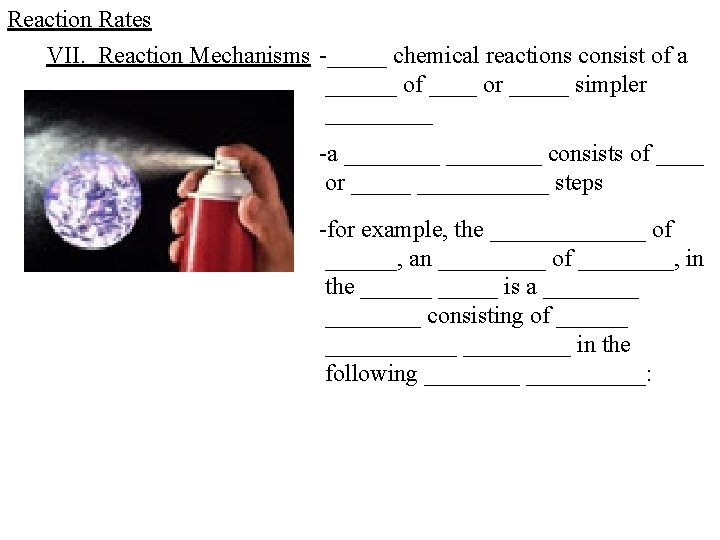
Reaction Rates VII. Reaction Mechanisms -_____ chemical reactions consist of a ______ of ____ or _____ simpler _____ -a ________ consists of ____ or ___________ steps -for example, the _______ of ______, an _____ of ____, in the ______ is a ________ consisting of ___________ in the following __________:

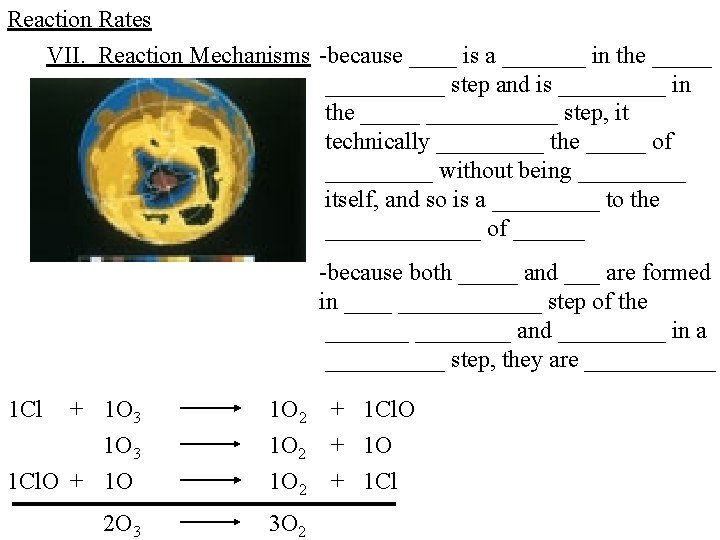
Reaction Rates VII. Reaction Mechanisms -because ____ is a _______ in the __________ step and is _____ in the ___________ step, it technically _____ the _____ of _____ without being _____ itself, and so is a _____ to the _______ of ______ -because both _____ and ___ are formed in ________ step of the ________ and _____ in a _____ step, they are ______ 1 Cl + 1 O 3 1 Cl. O + 1 O 1 O 2 2 O 3 3 O 2 + 1 Cl. O + 1 Cl

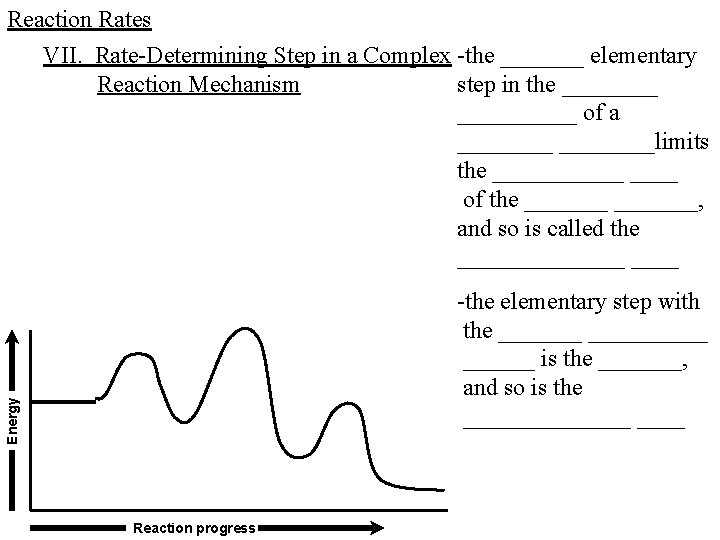
Reaction Rates VII. Rate-Determining Step in a Complex -the _______ elementary Reaction Mechanism step in the __________ of a ________limits the ______ of the _______, and so is called the _______ Energy -the elementary step with the __________ is the _______, and so is the _______ Reaction progress
 Expressing reaction rates
Expressing reaction rates Expressing reaction rates
Expressing reaction rates Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates What is a unit ratio
What is a unit ratio Ratios and proportions guided notes
Ratios and proportions guided notes Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Rate of reaction quiz
Rate of reaction quiz Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Did a chemical reaction occur
Did a chemical reaction occur Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Hệ hô hấp
Hệ hô hấp Ng-html
Ng-html Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Tư thế worm breton
Tư thế worm breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là V cc cc
V cc cc Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Chúa yêu trần thế
Chúa yêu trần thế Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công của trọng lực
Công của trọng lực Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Phản ứng thế ankan
Phản ứng thế ankan Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể điện thế nghỉ
điện thế nghỉ Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Một số thể thơ truyền thống
Một số thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Activity formula
Activity formula Rate of reaction formula
Rate of reaction formula Verb expressing emotion
Verb expressing emotion A group of words with complete thought.
A group of words with complete thought.