Measuring Reaction Rate Unit 3 Reaction Rate Expressing









- Slides: 19

Measuring Reaction Rate Unit 3 – Reaction Rate

Expressing Reaction Rate It can be useful to know the rate of a chemical reaction. Here a few examples of real life chemical reactions where it is helpful to know their rates: In archaeology – the rate of degradation of carbon 14 In pharmacology – the speed at which a medication acts The environment – the speed at which a toxic substance breaks down In engineering – the speed at which asphalt hardens or concrete degrades The food industry - the speed at which cookie dough bakes

Expressing Reaction Rate Define rate of reaction. Here’s an example to help you put it into the context of chemical reactions: the average speed of an automobile, expressed in km/h, is a measure of the distance travelled by the car divided by the time it takes to cover that distance. A rate is usually defined as a change in quantity as a function of time.

Expressing Reaction Rate A reaction rate is a positive value that corresponds to the change in the quantity of a reactant or a product as a function of time, during a chemical change. The quantities can me expressed as a mass (g), moles or as a concentration (mol/L). The time is usually expressed in seconds (s), but may be expressed in minutes (min) or hours (h).

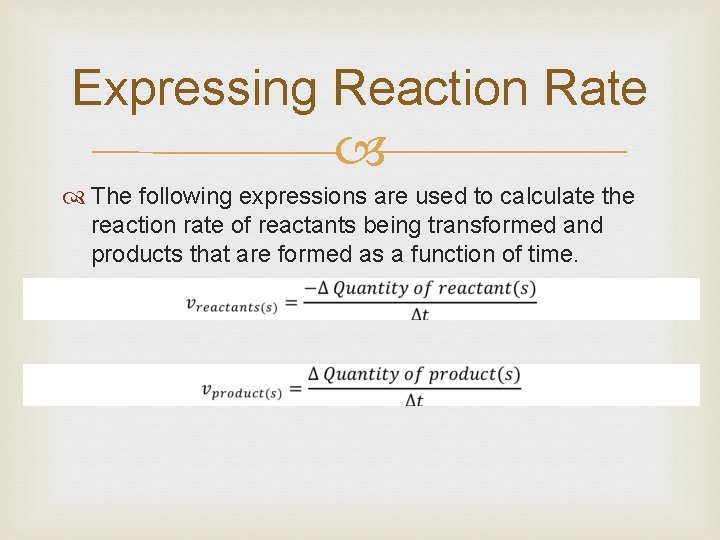
Expressing Reaction Rate The following expressions are used to calculate the reaction rate of reactants being transformed and products that are formed as a function of time.

Expressing Reaction Rate Chemical reactions do not all occur at the same rate. Some reactions are very rapid, and others may take weeks or years. Give two examples of slow reactions and two examples of fast reactions. Slow – the destruction of the ozone layer, ripening of fruit, rust formation Fast – explosion, air bag deployment

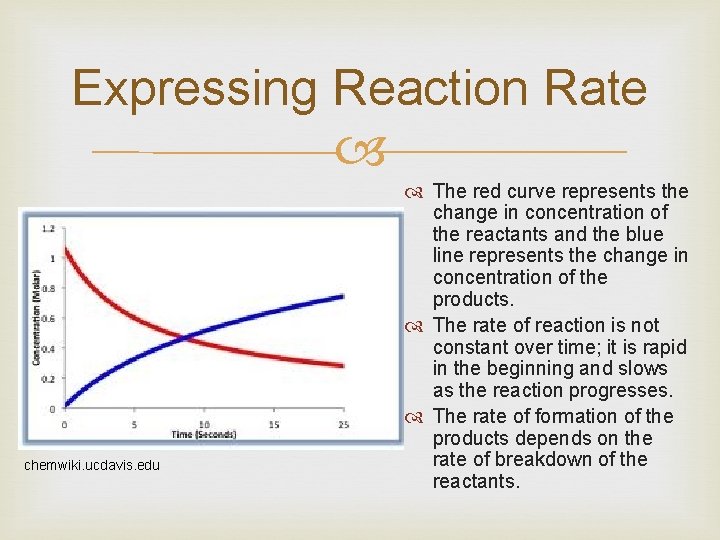
Expressing Reaction Rate chemwiki. ucdavis. edu The red curve represents the change in concentration of the reactants and the blue line represents the change in concentration of the products. The rate of reaction is not constant over time; it is rapid in the beginning and slows as the reaction progresses. The rate of formation of the products depends on the rate of breakdown of the reactants.

Reaction Rate in Terms of Stoichiometry The coefficients in a balanced chemical reaction indicate the proportions of reactants that react to form products. We can use these proportions to express reaction rate in terms of a given reactant or product.

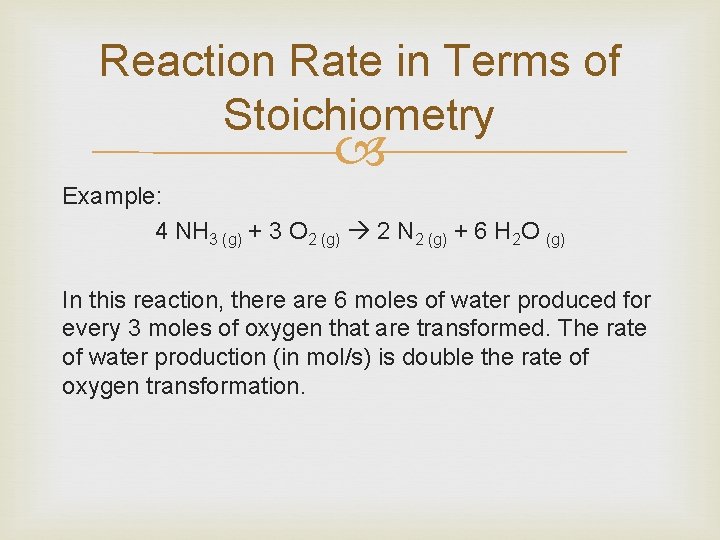
Reaction Rate in Terms of Stoichiometry Example: 4 NH 3 (g) + 3 O 2 (g) 2 N 2 (g) + 6 H 2 O (g) In this reaction, there are 6 moles of water produced for every 3 moles of oxygen that are transformed. The rate of water production (in mol/s) is double the rate of oxygen transformation.

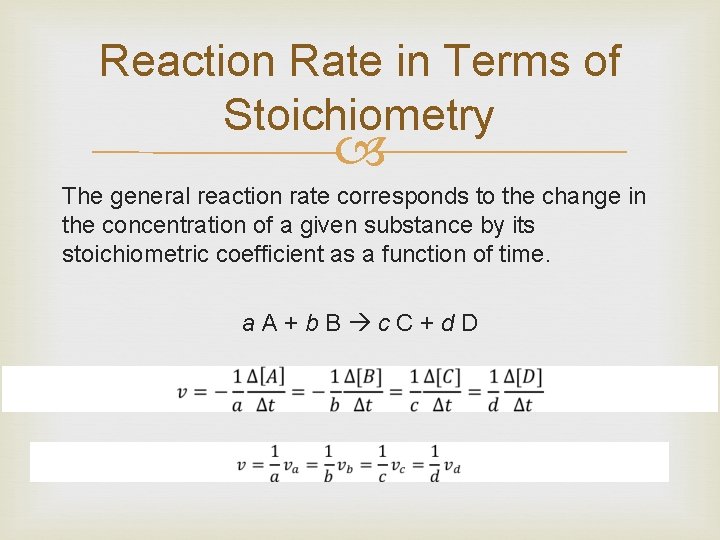
Reaction Rate in Terms of Stoichiometry The general reaction rate corresponds to the change in the concentration of a given substance by its stoichiometric coefficient as a function of time. a. A+b. B c. C+d. D

Ways to Measure Reaction Rate In order to calculate the reaction rate we must be able to find the change in the quantity of a reactant or a product over time. The change can be measured in a variety of ways, depending on the phase of the substance being measured. Question: What are the measurably parameters for each of the phases of matter?

Ways to Measure Reaction Rate Answer: Solid – mass, number of particles Liquid – mass, volume, number of particles Gas – mass, volume, pressure, concentration, number of particles Aqueous solution – concentration, p. H (if an acid or base)

Ways to Measure Reaction Rate Demo - Changing different parameters in time to calculate reaction rate Colour change over time p. H change over time Change in the mass of Mg over time Some rates of reaction can be measured qualitatively rather than quantitatively. The down side to this is that you cannot determine a precise rate of reaction, however, you can compare the time the reaction takes in terms of variables.

Ways to Measure Reaction Rate Reaction 1: Potassium hydroxide + glucose The hydroxide ions react with the glucose to form glycoside, which reacts with methylene blue, making the solution transparent. Shaking the solution dissolves the oxygen, which reacts with the methylene blue. When the solution is allowed to rest, it becomes transparent as the oxygen is used up by the reaction. The longer the solution is shaken, the longer it will take for it to become transparent again. In this reaction we cannot determine the reaction rate precisely, but we can compare the time it takes for the oxygen to disappear as a function of its initial concentration.

Ways to Measure Reaction Rate Reaction 2: Sodium thiosulfate + hydrochloric acid Na 2 S 2 O 3 (aq) + 2 HCl (aq) 2 Na. Cl (aq) + H 2 O (l) + SO 2 (aq) + S (s) In this reaction we measured the p. H at the beginning of the reaction and at the end of the reaction and determined the time it took the solution to become opaque. Measuring the p. H allows us to determine the quantity of H+ ions at the beginning of the reaction and the H+ ions at the end of the reaction. This will give us a rate for the change in concentration of H+ over time. We will go into more detail about p. H in the next unit (unit 4 - equilibrium).

Ways to Measure Reaction Rate Reaction 3: Magnesium + hydrochloric acid Mg (s) + HCl (aq) Mg. Cl 2 (aq) + H 2 (g) For this reaction we can measure the rate of reaction in a few different ways. If we are considering the rate of transformation of the Mg, we can measure the rate in g/s or mol/s. We could measure the rate of the formation of H 2 (g) as well. For this we would use stoichiometry and the ideal gas law to determine the rate of formation in mol/s or L/s.

Average Reaction Rate and Instantaneous Reaction Rate Remember: a reaction rate corresponds to the change in quantity of a reactant or product as a function of time. Reactions tend to take place quickly at the beginning of a reaction and slow down as the reaction progresses. For this reason it can be useful to calculate the instantaneous reaction rate.

Average Reaction Rate and Instantaneous Reaction Rate The instantaneous reaction rate corresponds to the rate at a precise time in the reaction. The average reaction rate corresponds to the change in the quantity of a reactant or product as a function of a given period of time.

Average Reaction Rate and Instantaneous Reaction Rate Experimentally, reaction rates are determined from a graph. In order to calculate the average rate: Graph the secant of the curve from two points of the chosen time period. The rate corresponds to the slope of the secant. In order to calculate the instantaneous rate: Graph the tangent to the curve at the precise point for the chosen time. The rate corresponds to the slop of the tangent. It can be difficult to determine the tangent at a precise point, so graph a secant of the curve that intersects two points on either side, at an equal distance, from the desired time. This secant is parallel to the tangent; therefore the slope of the secant represents the slope of the tangent.