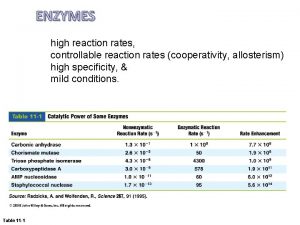
Unit 12 Reaction Rates 12 1 Reaction Rates














- Slides: 32

Unit 12: Reaction Rates

12. 1: Reaction Rates Learning Target: I can describe reaction rate in terms of changing concentration.

12. 1: Reaction Rates Reaction Time is defined as: Amount of time needed for the reaction to go from reactants to products. Measured in seconds or minutes.

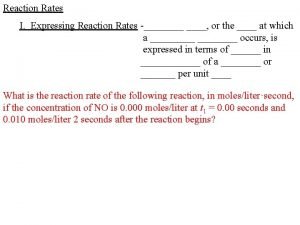
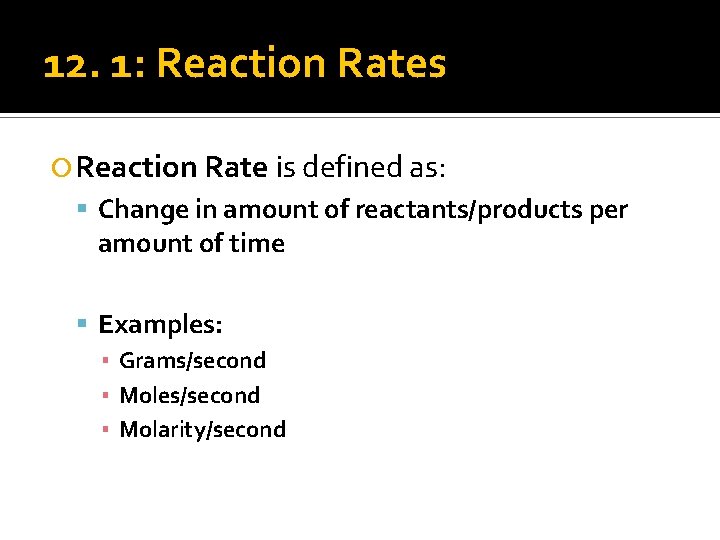
12. 1: Reaction Rates Reaction Rate is defined as: Change in amount of reactants/products per amount of time Examples: ▪ Grams/second ▪ Moles/second ▪ Molarity/second

12. 1: Reaction Rates Example 1: If 10 grams of Mg react with HCl in 150 seconds… What is the reaction time? What is the reaction rate?

12. 1: Reaction Rates Example 2: If 5 moles of O 2 reacts with H 2 in 2 seconds… What is the reaction time? What is the reaction rate?

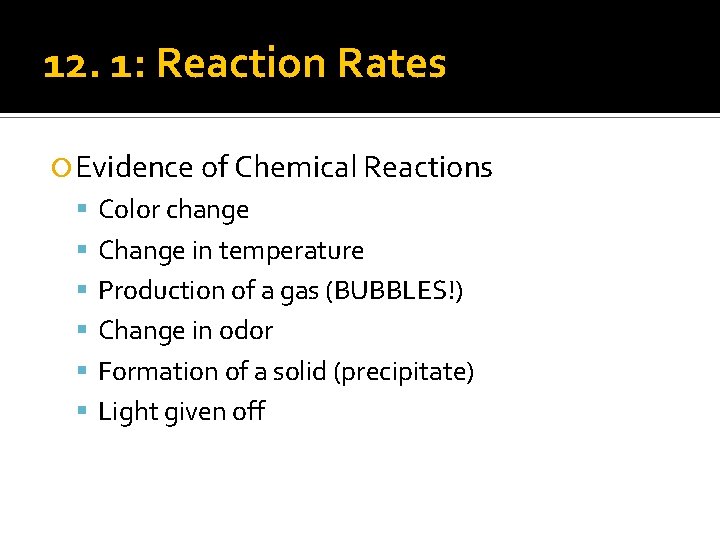
12. 1: Reaction Rates Evidence of Chemical Reactions Color change Change in temperature Production of a gas (BUBBLES!) Change in odor Formation of a solid (precipitate) Light given off

12. 2 Collision Theory Learning Targets: I can use collision theory to describe how chemical reactions take place. I can define collision energy, activation energy, activated complex and transition state.

12. 2 Collision Theory Review: Reactants are… ▪ Initial substances used up during a chemical reaction Products are… ▪ Substances formed after a chemical reaction In your notes, label the reactants and products in the chemical equation.

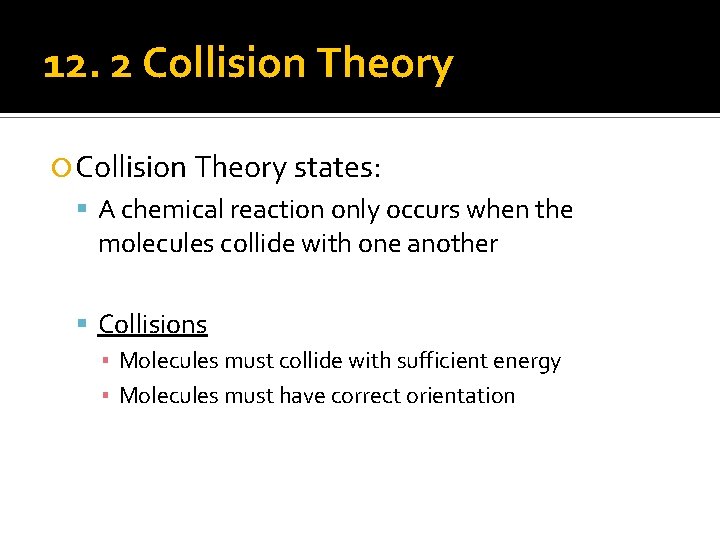
12. 2 Collision Theory states: A chemical reaction only occurs when the molecules collide with one another Collisions ▪ Molecules must collide with sufficient energy ▪ Molecules must have correct orientation

12. 2 Collision Theory Definitions you must know: Collision energy: molecules colliding with sufficient energy Activation energy: minimum energy required for a chemical reaction to proceed Transition energy: highest amount of energy during the reaction

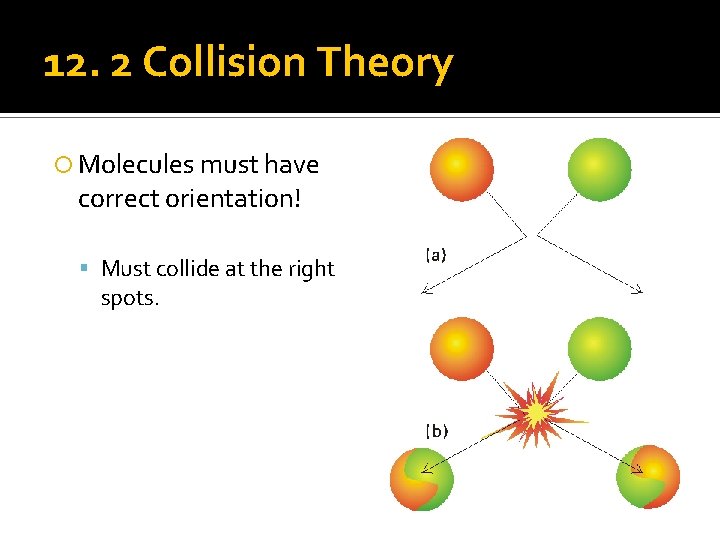
12. 2 Collision Theory Molecules must have correct orientation! Must collide at the right spots.

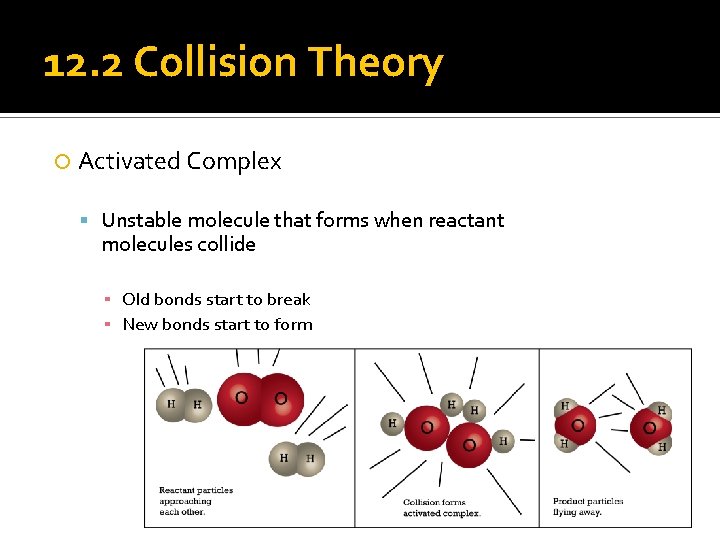
12. 2 Collision Theory Activated Complex Unstable molecule that forms when reactant molecules collide ▪ Old bonds start to break ▪ New bonds start to form

12. 3 Factors Affecting Rate Learning Target: I can list and describe the factors that affect reaction rates.

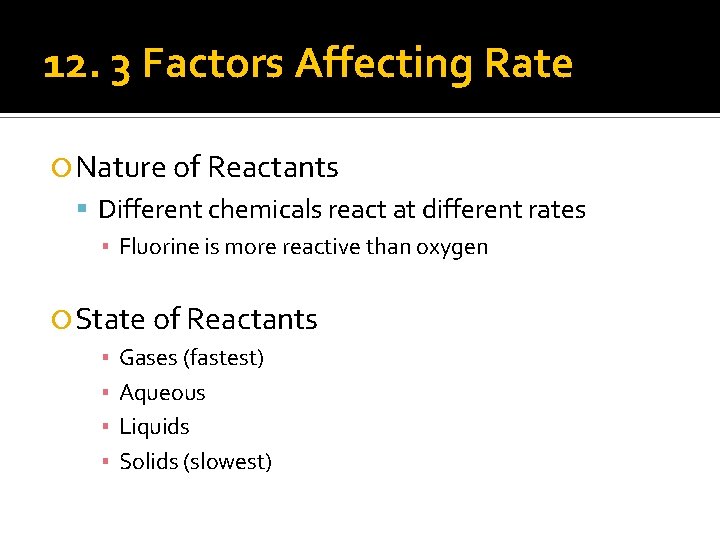
12. 3 Factors Affecting Rate Nature of Reactants Different chemicals react at different rates ▪ Fluorine is more reactive than oxygen State of Reactants ▪ ▪ Gases (fastest) Aqueous Liquids Solids (slowest)

12. 3 Factors Affecting Rate Surface Area Larger surface area, faster reaction rate Collisions only occur on the surface

12. 3 Factors Affecting Rate Concentration of Reactants Increasing the concentration (M) of reactants increases reaction rate More molecules = more collisions Elephant Toothpaste!

12. 3 Factors Affecting Rate Temperature Increasing temperature increases rate of reaction More molecules have sufficient energy to react Higher temp molecules moving more collisions!

12. 3 Factors Affecting Rate Catalyst Substance that lowers the activation energy. Lowers the hurdle of energy, more collisions have the right amount of energy. Not used up in reaction!

12. 3 Factors Affecting Rate Inhibitor Substance that interferes with a reaction Interferes with collisions, slower reaction

12. 4 Reaction Coordinate Diagram Learning Targets: Interpret reaction coordinate diagrams in terms of energy. Determine whether a reaction is exothermic or endothermic.

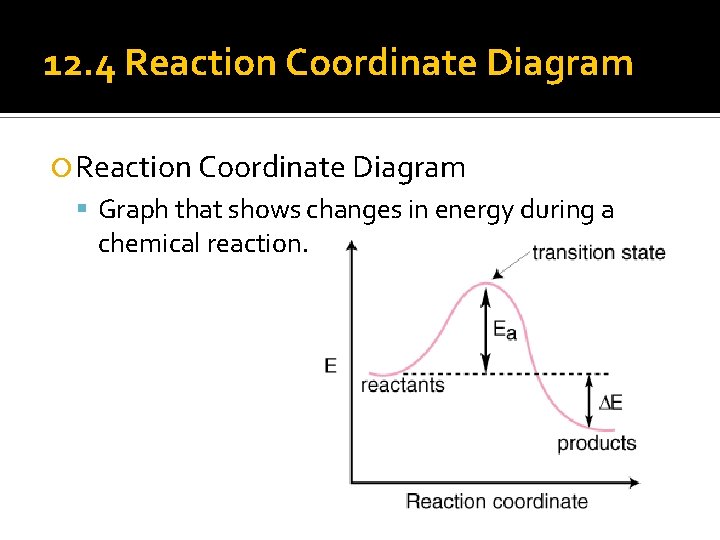
12. 4 Reaction Coordinate Diagram Graph that shows changes in energy during a chemical reaction.

12. 4 Reaction Coordinate Diagram Enthalpy (H) Potential Energy (bonds) of molecules Both reactants and products have enthalpy Δ H = Hproducts – Hreactants Ea = Hactivated complex – Hreactants

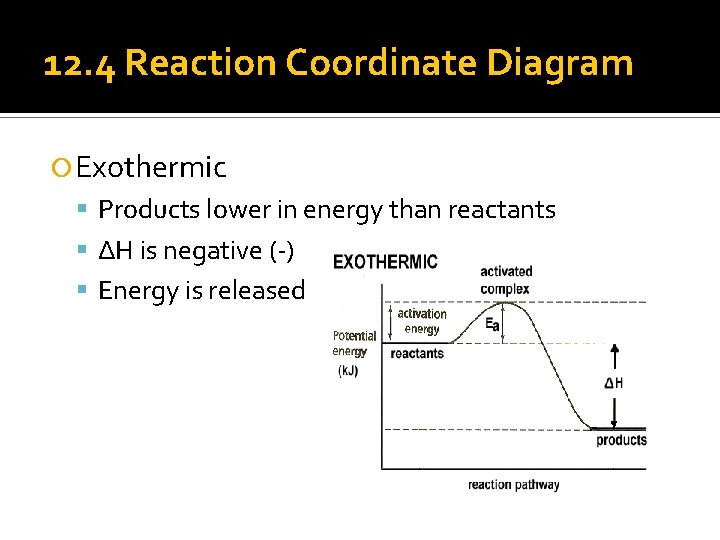
12. 4 Reaction Coordinate Diagram Exothermic Products lower in energy than reactants ΔH is negative (-) Energy is released

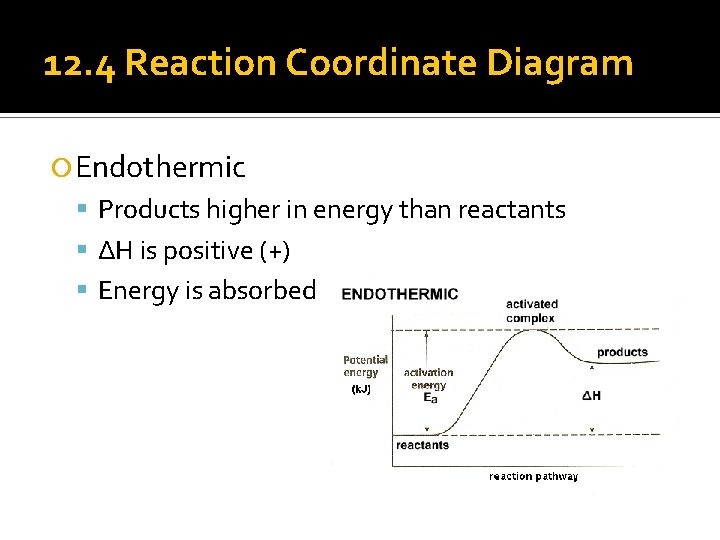
12. 4 Reaction Coordinate Diagram Endothermic Products higher in energy than reactants ΔH is positive (+) Energy is absorbed

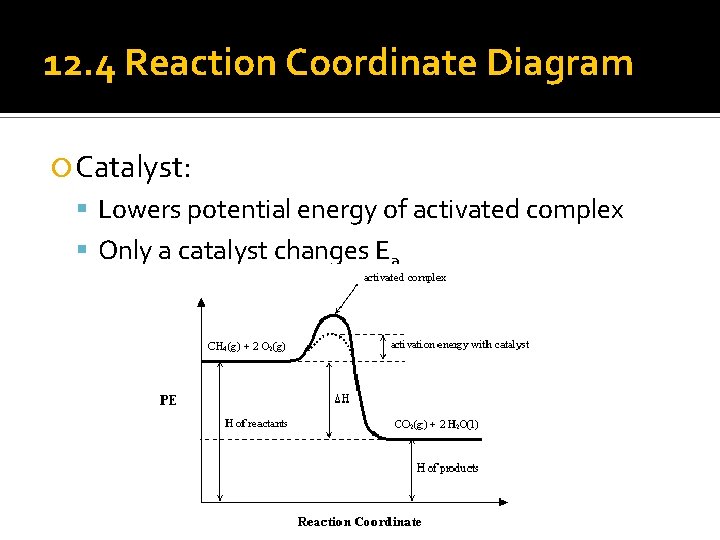
12. 4 Reaction Coordinate Diagram Catalyst: Lowers potential energy of activated complex Only a catalyst changes Ea

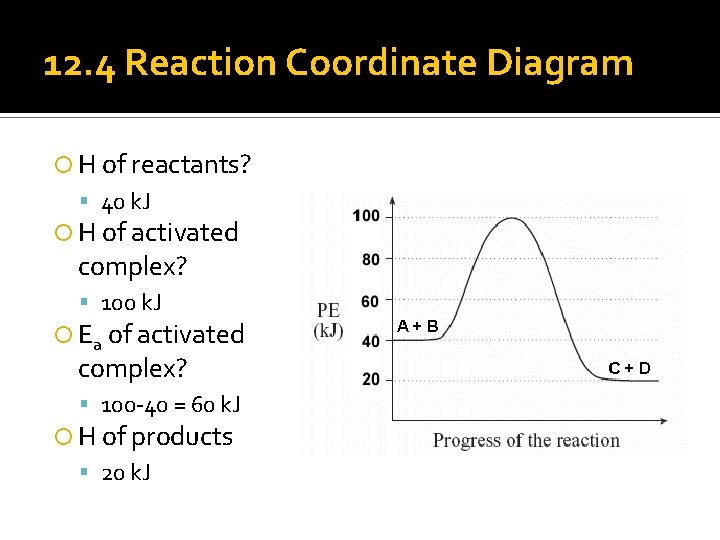
12. 4 Reaction Coordinate Diagram H of reactants? 40 k. J H of activated complex? 100 k. J Ea of activated complex? 100 -40 = 60 k. J H of products 20 k. J

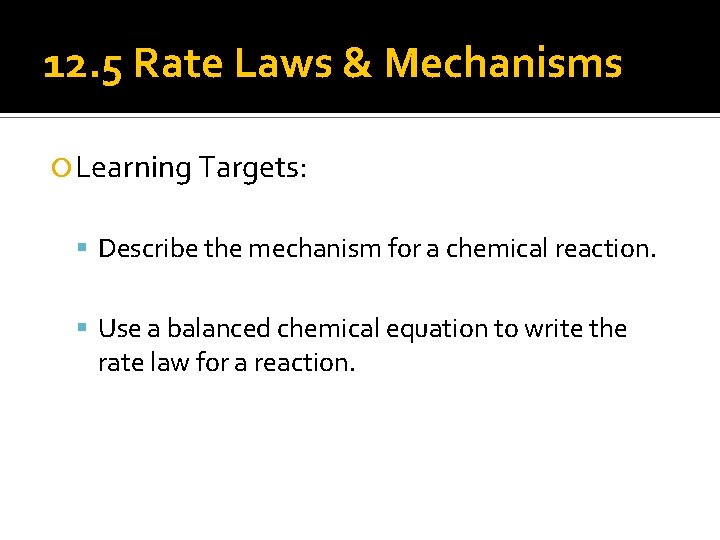
12. 5 Rate Laws & Mechanisms Learning Targets: Describe the mechanism for a chemical reaction. Use a balanced chemical equation to write the rate law for a reaction.

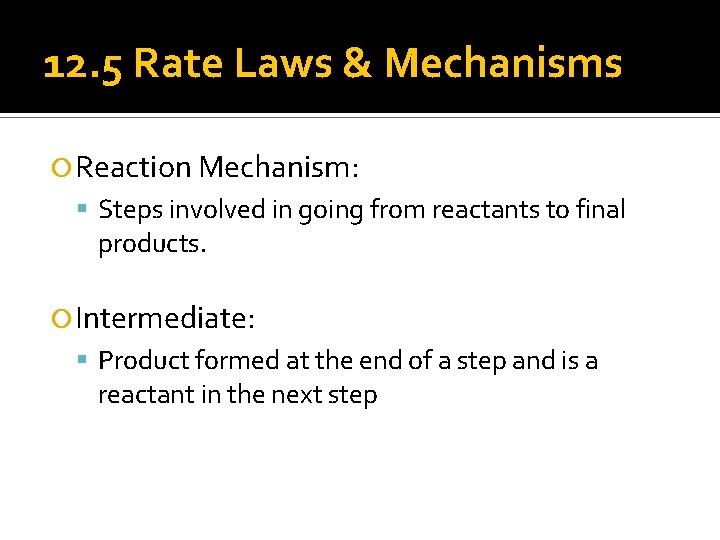
12. 5 Rate Laws & Mechanisms Reaction Mechanism: Steps involved in going from reactants to final products. Intermediate: Product formed at the end of a step and is a reactant in the next step

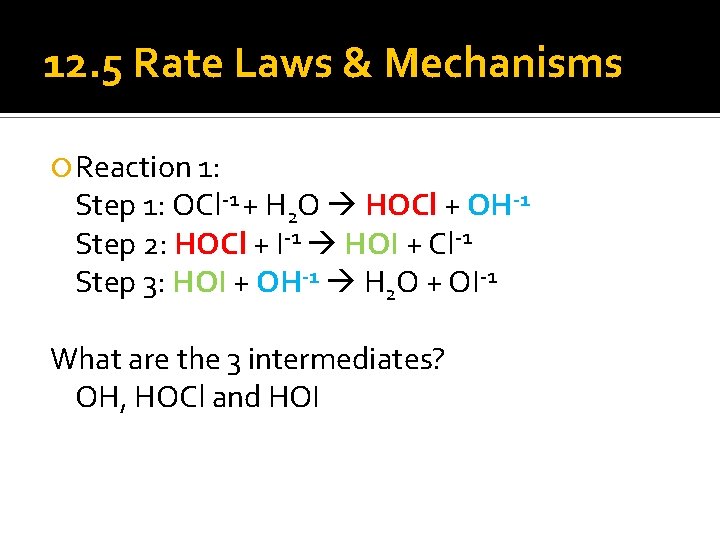
12. 5 Rate Laws & Mechanisms Reaction 1: Step 1: OCl-1 + H 2 O HOCl + OH-1 Step 2: HOCl + I-1 HOI + Cl-1 Step 3: HOI + OH-1 H 2 O + OI-1 What are the 3 intermediates? OH, HOCl and HOI

12. 5 Rate Laws & Mechanisms Rate-determining step Slowest step in the mechanism that controls speed of reaction

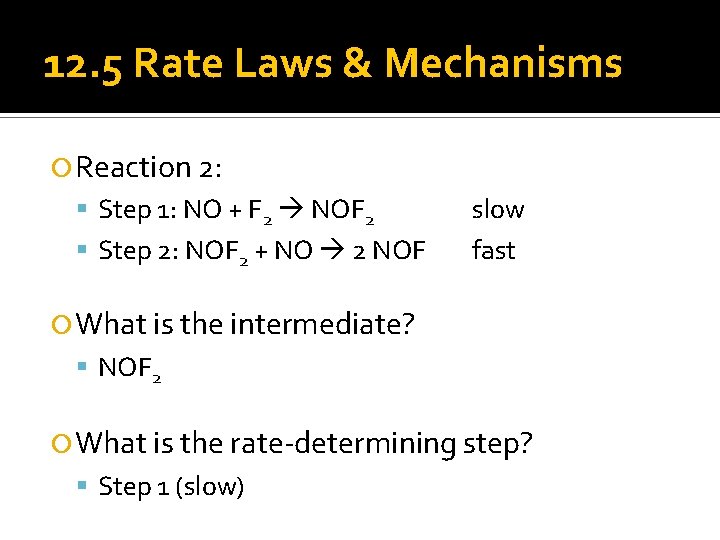
12. 5 Rate Laws & Mechanisms Reaction 2: Step 1: NO + F 2 NOF 2 Step 2: NOF 2 + NO 2 NOF slow fast What is the intermediate? NOF 2 What is the rate-determining step? Step 1 (slow)
 Is a ratio a rate
Is a ratio a rate Equivalent ratios definition
Equivalent ratios definition Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Reaction rate formula
Reaction rate formula Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium answer key
Chapter 18 reaction rates and equilibrium answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Reaction rate
Reaction rate Expressing reaction rates
Expressing reaction rates Reaction rates
Reaction rates Unit 6 review questions
Unit 6 review questions Unit rate with complex fractions
Unit rate with complex fractions Personal finance unit 2 lesson 2
Personal finance unit 2 lesson 2 Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Bomb power
Bomb power Metode pembiayaan langsung (direct financing method)
Metode pembiayaan langsung (direct financing method) Hyp opp adj
Hyp opp adj Si units mass
Si units mass Unit test unit test review algebra 2
Unit test unit test review algebra 2 Contoh soal perhitungan unit cost rumah sakit
Contoh soal perhitungan unit cost rumah sakit Unit process and unit operation
Unit process and unit operation Unit operation and unit process
Unit operation and unit process Kerangka konseptual akuntansi pemerintahan
Kerangka konseptual akuntansi pemerintahan What is nhf schedule of rates
What is nhf schedule of rates Ssema
Ssema Aswath damodaran risk free rate
Aswath damodaran risk free rate 6-2 pay periods and hourly rates
6-2 pay periods and hourly rates