Measurement SI UNITS AND ENGLISH UNITS UNIT CONVERSION
















- Slides: 19

Measurement SI UNITS AND ENGLISH UNITS UNIT CONVERSION SCIENTIFIC NOTATION

The English System n n The English system of measurement is the one that we are most familiar with in the US. Some of the common units of measurement in this system are as follows: 1 pound= 16 ounces 8 ounces= 1 cup 1 foot= 12 inches

The English System n n n The English system grew out of the common way that people measured things, by using body parts or available items for measurement. For example, 1 foot was the length of someone’s foot. Gallon is the word that people used for pail, and any cup was used to measure 1 cup.

The English System n n n n Units of Length 12 in = 1 ft 3 ft = 1 yd 5280 ft = 1 mi Units of Weight 16 oz = 1 lb 2000 lbs = 1 ton n n n Capacity 3 teaspoons (tsp)= 1 tablespoon (tbsp) 16 tbsp = 1 cup (c ) 8 oz = 1 c 2 c = 1 pint (pt) 2 pt = 1 quart (qt) 4 qt = 1 gallon (gal)

SI Units n n n SI units stands for the Le Systeme International d’Unites. This is the modern metric system. Scientists all over the world use this system so everyone is familiar with the units. It’s like communicating in the same language. The base unit in this system is 10.

SI Units n n Length The base SI unit to measure length is the meter (m). n Mass The base SI unit to measure mass is the gram (g). Volume The base SI unit to measure volume is the liter (L).

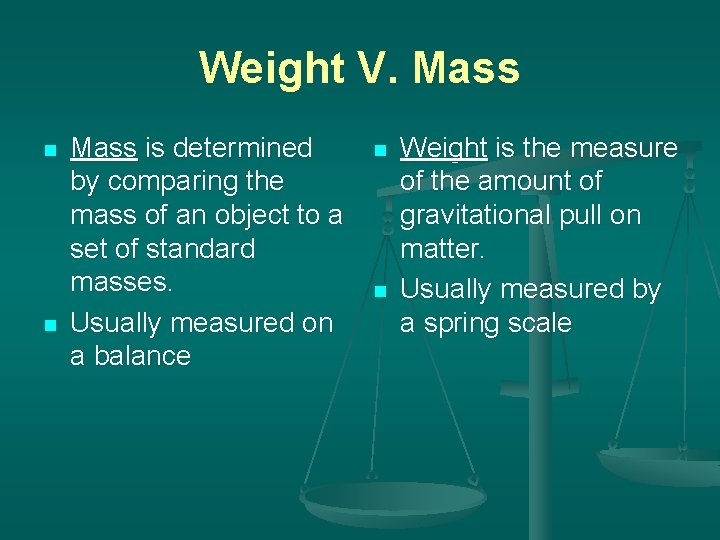
Weight V. Mass n n Mass is determined by comparing the mass of an object to a set of standard masses. Usually measured on a balance n n Weight is the measure of the amount of gravitational pull on matter. Usually measured by a spring scale

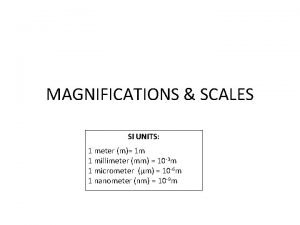
Prefixes in SI Units n n The same prefixes are used for length, mass, and volume. The prefixes are based on units of 10. kilo- k 1 kg = 1000 g hecto- h 1 h. L = 100 L deka-da 1 dam = 10 m

Prefixes in SI Units n n Additional prefixes are as follows: deci- d 1 dm = 0. 1 m centi- c 1 cm = 0. 01 m milli- m 1 m. L = 0. 001 L micro-μ 1 μm = 0. 000001 m nano-n 1 nm= 10 -9 m The prefixes determine whether the value is greater or less than the base unit.

Precison n n Precision is defined as how close a set of measurements are to each other. Precision is important because scientists must have data that can be reproduced in any setting anywhere in the world. n n Precision can vary due to the type of instrument you are using. Precision also depends upon the measurer’s technique.

Accuracy n n Accuracy is how close the measurement is to the actual or accepted value. Example: Think of a bulls eye in a dart game. If I hit the bulls eye, I am accurate! n n The actual value may be able to be discerned by looking it up. For examples: densities of various substances are widely known and reported

Unit Conversions n n n Converting between units in the SI system and the English system is a useful skill and can be achieved by various methods. One method is called the factor label method or unit analysis. This method uses fractions that equal 1 to convert between units. Another method is called the ladder method and simply involves moving the decimal left or right.

Using Units of Measure n n Area- amount of surface included within a set of boundaries, measured in square units of length x width = area Volume- amount of space occupied by an object is its volume, measured in cubic length for solids and liters for liquids length x width x height = volume

Using Units of Measure n Density- measure of the amount of matter that occupies a given space Formula: density = mass/ volume n Density of solids is expressed in grams per cubic centimeters, and the density of liquids is grams per milliliter. ( 1 cm 3 = 1 m. L)

Temperature n n Measure of the average vibrations of the particles that make up a material. Temperature is often measured on the Celsius scale in science ( C). n n Temperature is measured in Kelvin (K) in the SI system of measurement. To convert between Celsius and Kelvin: K= C + 273 Absolute zero is 0 K.

Scientific Notation n n Scientific notation is a shorthand method of writing numbers that are extremely large or small. In this type of notation, the number is expressed as a decimal between numbers 1 and 10 with a power of ten multiplier. n n n Some examples are listed below: The speed of light is 3 x 108 m/s Avogadro’s number is 6. 022 x 1023 atoms/mol Mass of the earth 5. 9724 x 1024 kg Diameter of atom 1 x 10 -10 m

Scientific Notation n Putting numbers into scientific method: If the number is smaller than 1, move the decimal to the right until you get to a number between 1 and 10. The number of places you move the decimal is the negative exponent. n n n Putting numbers into scientific method: If the number is greater than 1, move the decimal to the left until you get to a number between 1 and 10. The number of places you move the decimal is the positive exponent.

Scientific Notation n Taking numbers out of scientific notation: If the exponent is positive, move the decimal to the right. If the exponent is negative, move the decimal to the left.

Using Scientific Notation n n To put these numbers in your calculator: Look for an EE button. It may be a second function! Enter the decimal then EE then the exponent! NO NEED FOR 10 or the X button n It is better if you avoid doing this… 7. 25 x 10^12 Because the calculator must follow the order of operations, so it will do the exponent first, then multiply or divide through…
 Metric system order
Metric system order Liberia measurement system
Liberia measurement system Conversion of units of measurement
Conversion of units of measurement Si unit to english unit
Si unit to english unit Ladder method conversion
Ladder method conversion Conversion measurement table
Conversion measurement table Units, physical quantities, and vectors
Units, physical quantities, and vectors Mussd
Mussd Si unit for magnification
Si unit for magnification Metric system word problems
Metric system word problems Conversion of units
Conversion of units Gallon man chart
Gallon man chart Momentum units physics
Momentum units physics 3 units of linear measurements in metric system
3 units of linear measurements in metric system Customary units of measurement
Customary units of measurement Typical room height metric unit
Typical room height metric unit Us customary units of measurement
Us customary units of measurement Units of measurement microscope
Units of measurement microscope Units of measurement in physics
Units of measurement in physics Customary and metric system
Customary and metric system