Drinking Water Wastewater Regulation and Treatment Drinking Water



























- Slides: 46

Drinking Water & Wastewater Regulation and Treatment

Drinking Water Regulations • 1893 - Interstate Quarantine Act – Result: Prohibition of Common Drinking Cup on Interstate Carriers – created a market for Dixie® cups • 1914 - Microbiological Standard – 2 coliforms / 100 ml • 1925 - New Microbiological Standard – 1 coliform / 100 ml • 1942 - Maximum Concentrations for Constituents: Lead Selenium Copper Zinc Phenolics* Fluoride Barium Magnesium Chloride Total Solids Arsenic Hexavalent Chromium Iron + Manganese Sulfate Alkalinity *Phenol = carbolic acid, addition of methyl group forms cresols, o, m, or p

Drinking Water Regulations 1962 Limits for: Alkyl benzene sulfonates (synthetic detergents) Carbon-chloroform extract (organic residues) [Adsorption on activated carbon, chloroform extraction, gravity quantification] Barium Nitrate Cadmium Silver Cyanide Radioactivity Safe Drinking Water Act of 1974 Enacted over concern about organic materials in drinking water Established Maximum Contaminant Levels (MCLs) for several substances [Enforceable] Federal Guidelines - Secondary MCLs (SMCLs), [Nonenforceable]

1975 - National Interim Primary Drinking Water Regulations Amended repeatedly, now include: • Microbiological Contaminants – Total and Fecal coliforms, E. coli, Turbidity • Radioactive Contaminants – Beta/photon emitters, Alpha emitters, Combined radium • Inorganic Contaminants – 15 elements or materials • Synthetic Organic Contaminants including Pesticides and Herbicides; Volatile Organics – 54 compounds and groups of compounds

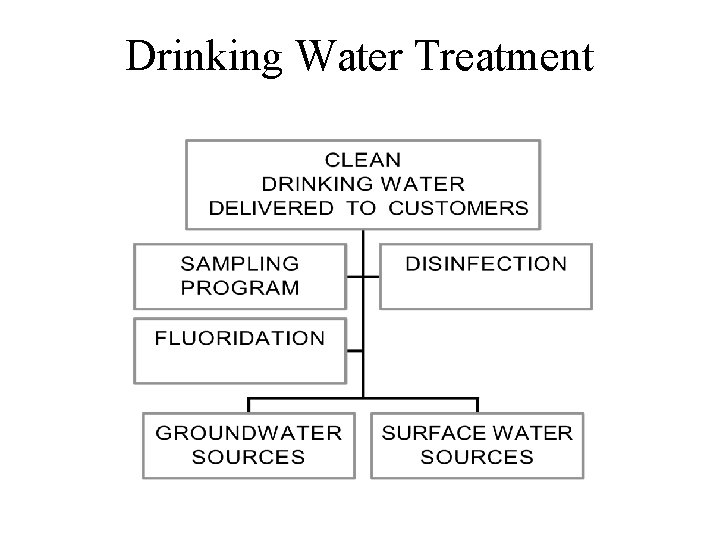
Drinking Water Treatment

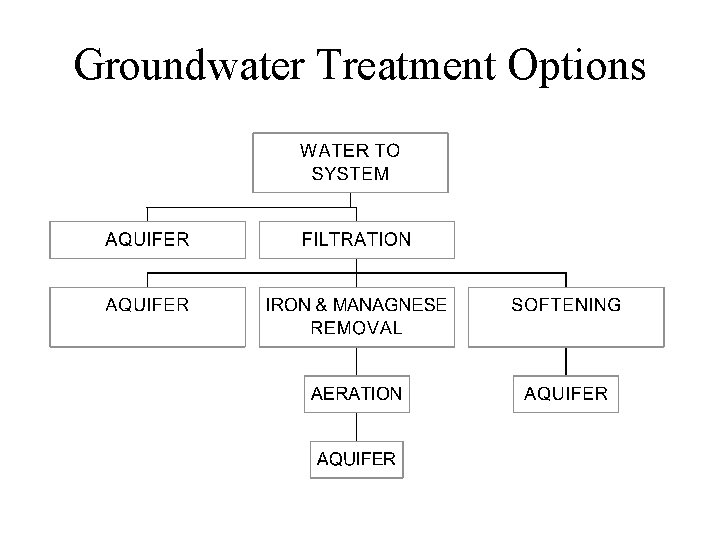
Groundwater Treatment Options

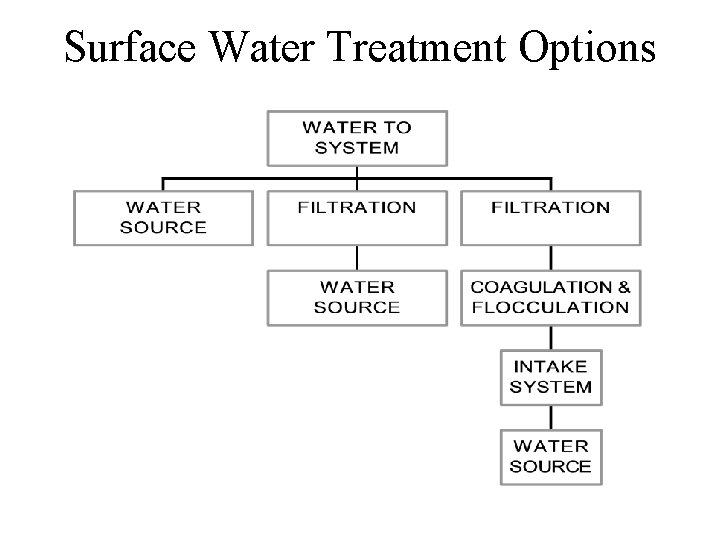
Surface Water Treatment Options

Drinking Water Treatment Units or Processes • • • INTAKES AERATION COAGULATION & FLOCCULATION CLARIFICATION FILTRATION DISINFECTION SOFTENING TASTE & ODOR CONTROL IRON & MANGANESE REMOVAL TRACE METALS & ORGANICS

AERATION (Usually for Groundwater) • ADDS OXYGEN • REMOVES: – Carbon Dioxide – Methane Hydrogen Sulfide Taste & Odors • REMOVAL MAY BE BY: – Oxidation • Iron & Manganese Volatilization Organics

Coagulation, Flocculation & Clarification Coagulation changes the electrical charge of suspended particles and colloids; allows attachment to each other. Coagulants are usually cations: Alum, Ferric Sulfate, Lime (Ca. O) Chemical/Physical process of mixing special purpose chemical from flow and removing the resulting product – Silts/Clays, Viruses, Bacteria – Fulvic & Humic Acids, Minerals, Organic Particulates Flocculation is the agglomeration of particles into settleable particles Clarification - sedimentation of floc particles, allows longer filter runs, settling velocity of the floc allows particle removal before the water leaves the basin

Drinking Water Filtration • Rapid Sand Filters are most commonly used for surface water • May be gravity or pressure flow – Some States prohibit pressure flow • Fine-to-course (back wash result) • Course-to-fine (multi-media) – Anthracite, Sand, Course Garnet, Fine Garnet • Rapid Sand filters may clean 1 - 2 gpm/ft 2 • Alternatives include: microscreens, diatomaceous earth filters, cartridge filters

DISINFECTION • CT concept is the current basis for disinfection theory: CT = K C = disinfectant concentration T= contact time K= proportionality constant, variable with different organisms Ohio regulation = 30 min contact time, 0. 2 mg/l Cl 2 residual • Chlorination is most common in the U. S. – Effective, low cost, proven technology – Reactions with natural aquatic organics produce trihalomethanes -- suspected carcinogens • Ozonation is popular in France, Germany, Canada, and Russia • Chlorine Dioxide gaining acceptance in Europe and U. S.

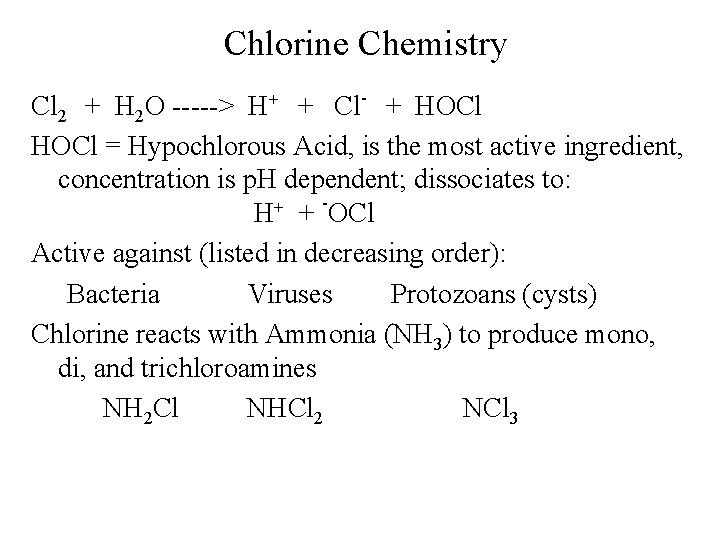
Chlorine Chemistry Cl 2 + H 2 O -----> H+ + Cl- + HOCl = Hypochlorous Acid, is the most active ingredient, concentration is p. H dependent; dissociates to: H+ + -OCl Active against (listed in decreasing order): Bacteria Viruses Protozoans (cysts) Chlorine reacts with Ammonia (NH 3) to produce mono, di, and trichloroamines NH 2 Cl NHCl 2 NCl 3

Chlorine Residuals & Chlorine Demand • Free Chlorine: Cl 2, HOCl, -OCl • Combined Chlorine: Chloramines • Free Chlorine has strong disinfecting powers but is quickly dissipated • Combined Chlorine is slower acting but remains in solution longer and provides longer-term protection • Chlorine Demand = the difference between the amount of chlorine applied and the amount of free, combined, or total chlorine remaining at the end of the contact period • Anything oxidizable can produce a chlorine demand, including pathogens, organics, particulates, sulfides, ammonia, etc.

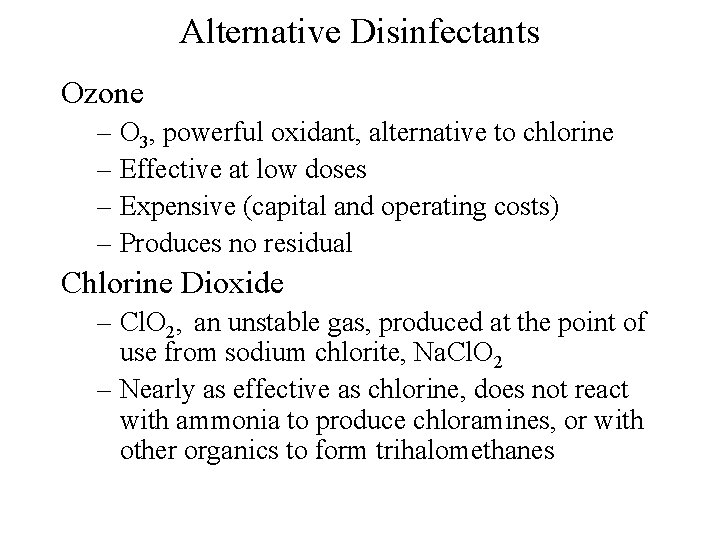
Alternative Disinfectants Ozone – O 3, powerful oxidant, alternative to chlorine – Effective at low doses – Expensive (capital and operating costs) – Produces no residual Chlorine Dioxide – Cl. O 2, an unstable gas, produced at the point of use from sodium chlorite, Na. Cl. O 2 – Nearly as effective as chlorine, does not react with ammonia to produce chloramines, or with other organics to form trihalomethanes

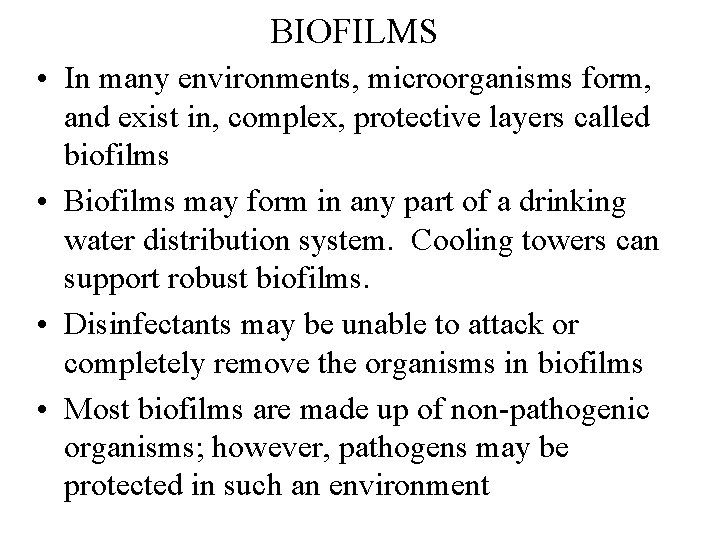
BIOFILMS • In many environments, microorganisms form, and exist in, complex, protective layers called biofilms • Biofilms may form in any part of a drinking water distribution system. Cooling towers can support robust biofilms. • Disinfectants may be unable to attack or completely remove the organisms in biofilms • Most biofilms are made up of non-pathogenic organisms; however, pathogens may be protected in such an environment

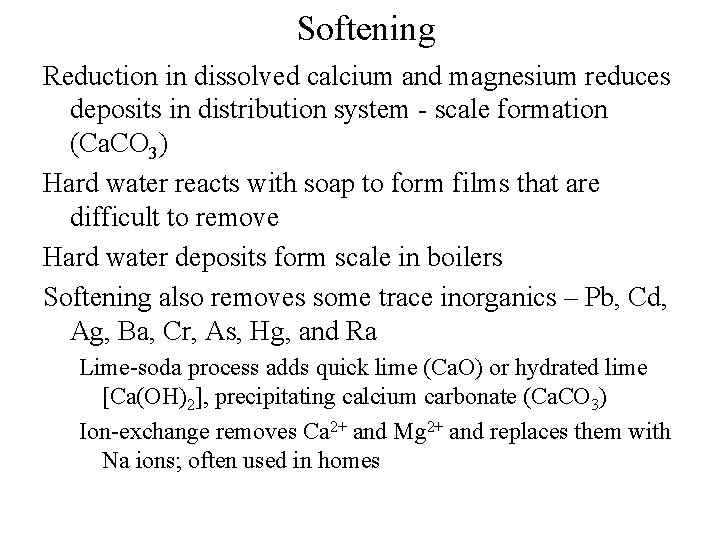
Softening Reduction in dissolved calcium and magnesium reduces deposits in distribution system - scale formation (Ca. CO 3) Hard water reacts with soap to form films that are difficult to remove Hard water deposits form scale in boilers Softening also removes some trace inorganics – Pb, Cd, Ag, Ba, Cr, As, Hg, and Ra Lime-soda process adds quick lime (Ca. O) or hydrated lime [Ca(OH)2], precipitating calcium carbonate (Ca. CO 3) Ion-exchange removes Ca 2+ and Mg 2+ and replaces them with Na ions; often used in homes

Taste & Odor Control • Very low concentrations of metals, salts, or organics may produce detectable levels in sensitive people - iron, copper, manganese, and zinc, magnesium chloride and bicarbonate, chlorinated organics; fungal and algal metabolites; hydrogen sulfide, other sulfur compounds • Activated carbon is often very effective in removing organics • Oxidation (chlorine, chlorine dioxide, ozone)

Iron & Manganese Removal Iron and manganese cause staining and leave noticeable residuals at very low concentrations Fe >0. 2 mg/l Mn >0. 1 mg/l Iron promotes growth of “iron bacteria” in mains that increase friction and power consumption Oxidation produces less soluble compounds and precipitation is often used for removal Trace Metals Iron, Cadmium, Lead, Copper, Zinc may come from the plumbing system; others may be from the aquifer Corrosion control processes may be the most effective means of reducing these concentrations - precipitation of a layer of calcium carbonate often provides a nonreactive surface

Turbidity A measure of suspended particulates in water - clays, microorganisms, organics Highly turbid waters are difficult to disinfect because of high demands - large amounts of materials to be oxidized; organisms protected from exposure to disinfectants Coagulation and flocculation, and filtration are common removal methods Trace Organics Solvents, hydrocarbons, etc. may come from the aquifer Modified organics may be the result of disinfection processes Humic and fulvic acids are poorly defined and are common in natural waters TOC and TOX are broad tests of water quality Precipitation, filtration, adsorption, and oxidation may all remove some of the materials

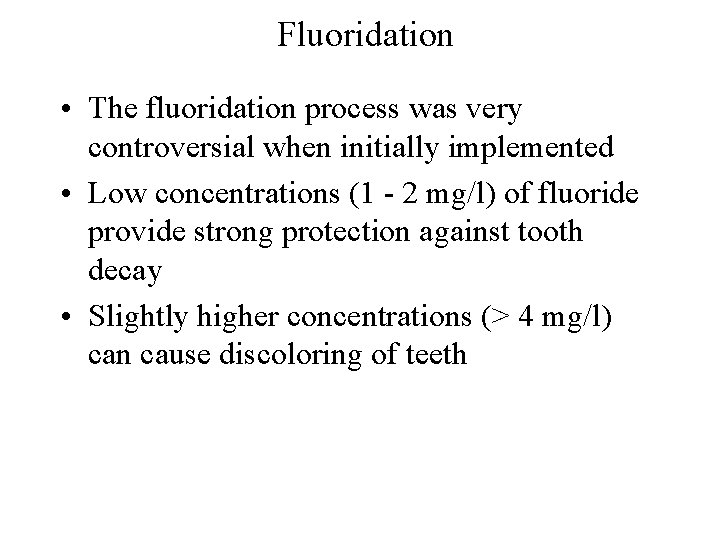
Fluoridation • The fluoridation process was very controversial when initially implemented • Low concentrations (1 - 2 mg/l) of fluoride provide strong protection against tooth decay • Slightly higher concentrations (> 4 mg/l) can cause discoloring of teeth

CROSS CONNECTIONS – Accidental contamination of drinking water can occur during routine plumbing modification, sewer backups, floods, earthquakes, careless homeowners, etc. FOAMING AGENTS – Surfactants (the active part of detergents) can get into surface water through incomplete sewage treatment – Groundwater sources include septic tank systems

Nitrate & Nitrite • Nitrate is common in natural waters at 1 to 2 mg/l • Nitrate (NO 3) is reduced to nitrite (NO 2) in the digestive system - reduction is more complete in infants than adults - because of more alkaline conditions in system • Excess nitrite produces methemoglobinemia in infants by oxidizing hemoglobin to methemoglobin which cannot carry oxygen • Nitrosamines formation (suspected carcinogens) can also occur from nitrate or nitrite

• TOTAL DISSOLVED SOLIDS – High TDS may increase corrosivity because of increase conductance – High sodium may be of health concern • CORROSIVITY – Decreases life of plumbing system – Solubilized metals, perhaps in toxic quantities - lead and cadmium are of most concern – Copper, iron, and zinc produce tastes and stains – Corrosion can shield microorganisms from disinfection processes – Water may be characterized as passive or aggressive

CLEAN WATER ACT - Background • Rivers and Harbors Act of 1899 – Prohibited discharge of refuse without a permit from the Secretary of the Army • Water Pollution Control Act of 1948 – Gave primary responsibilities to the States – Provided construction funds, Money never appropriated • Water Pollution Control Act Amendments of 1956 – Authorized Grants for construction – Provided funds for research into Health Effects • Other minor Acts in 1961, 1965, 1966, 1970

• Federal Water Pollution Control Act - 1972 - PL 92 -500 – Goal of “fishable/swimmable” water – Construction Grants for Sewage Treatment Facilities • BPT - Best Practicable Treatment • BAT - Best Available Treatment – Concentrated on Oxygen Demand, Suspended Solids – 1976 - NRDC v. Train - Consent Decree - resulted in. . . • Clean Water Act - 1977 - PL 95 -217 • Wetlands Resources Act - 1986 • Water Quality Act Amendments of 1987 – Required EPA regulations on storm water runoff – Required State nonpoint source management programs

TITLE I -RESEARCH AND RELATED PROGRAMS • Sec. 101 - Declaration of Goals and Policy -Objective: Restore and maintain the chemical, physical, and biological integrity of the Nation's waters. • Goals: 1. Eliminate pollutant discharges into navigable waters by 1985. 2. Interim goal to protect fish, shellfish, and wildlife and provide for recreation in and on the water by July 1, 1983. 3. Prohibit the discharge of toxic pollutants in toxic amounts.

TITLE III - STANDARDS AND ENFORCEMENT • 304 - Information and Guidelines - provides for development of water quality criteria. Defines conventional pollutants - including, but not limited to, biological oxygen demand, suspended solids, fecal coliforms, and p. H, --- specifically excluded thermal. • 305 - Water Quality Inventory - requires States to provide a water quality description, an inventory of point -source dischargers, and an estimate of costs of improving quality. • 306 - National Standards of Performance - requires a list of categories of sources and establishment of new source performance standards for those categories

TITLE IV: PERMITS AND LICENSES Sec 402 - National Pollutant Discharge Elimination System - (NPDES) establishes requirements for issuing permits and State implementation of the program. – Excludes: Irrigation return flows, Storm water runoff from Oil, Gas and Mining operations – Anti-Backsliding - renewed permits must be as stringent as the original – Storm water is included by October 1, 1993

ADDITIONS, AMENDMENTS • LIMITATIONS ON DISCHARGE OF RAW SEWAGE BY NEW YORK CITY – North River Plant - required to have advanced preliminary treatment by Aug, 1986 – Red Hook Plant - required advanced preliminary treatment by Aug, 1987 • BOSTON HARBOR AND ADJACENT WATERS – Authorization for constructing waste treatment works for providing secondary treatment

Oil Pollution Act • Revised penalties for oil discharges – Administrative penalties of $125, 000 for violations of regulations or discharges – Civil penalties of $25, 000/ day for discharges, or $1, 000/ barrel of oil – Gross negligence or misconduct minimum penalty of $100, 000

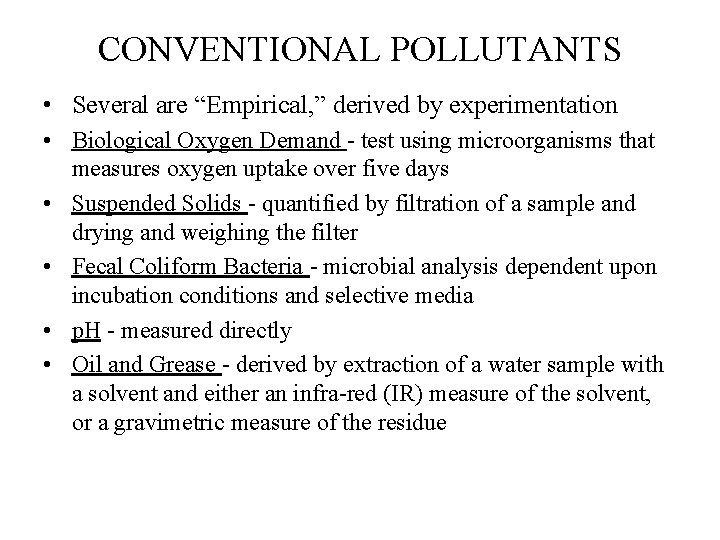
CONVENTIONAL POLLUTANTS • Several are “Empirical, ” derived by experimentation • Biological Oxygen Demand - test using microorganisms that measures oxygen uptake over five days • Suspended Solids - quantified by filtration of a sample and drying and weighing the filter • Fecal Coliform Bacteria - microbial analysis dependent upon incubation conditions and selective media • p. H - measured directly • Oil and Grease - derived by extraction of a water sample with a solvent and either an infra-red (IR) measure of the solvent, or a gravimetric measure of the residue

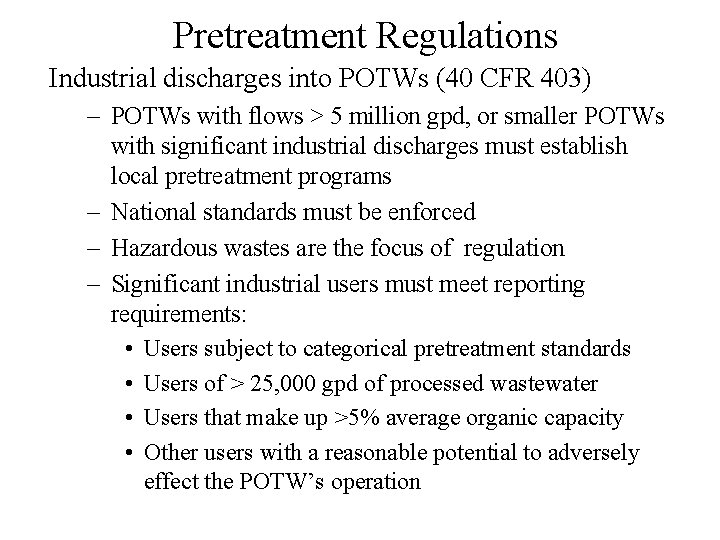
Pretreatment Regulations Industrial discharges into POTWs (40 CFR 403) – POTWs with flows > 5 million gpd, or smaller POTWs with significant industrial discharges must establish local pretreatment programs – National standards must be enforced – Hazardous wastes are the focus of regulation – Significant industrial users must meet reporting requirements: • Users subject to categorical pretreatment standards • Users of > 25, 000 gpd of processed wastewater • Users that make up >5% average organic capacity • Other users with a reasonable potential to adversely effect the POTW’s operation

Wastewater Treatment • Collection System – Sewage • Domestic (sanitary) • Industrial • Mixed – Stormwater • Separate Systems • Combined Systems • Infiltration (20 to 3, 000 gal/acre/day)

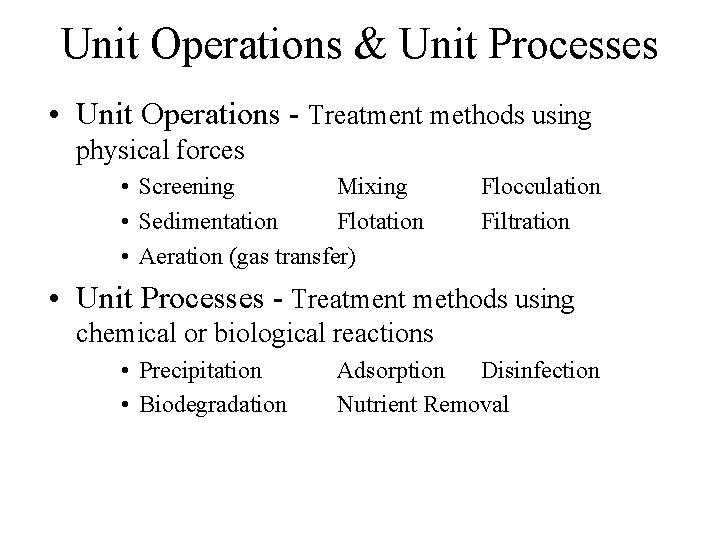
Unit Operations & Unit Processes • Unit Operations - Treatment methods using physical forces • Screening Mixing • Sedimentation Flotation • Aeration (gas transfer) Flocculation Filtration • Unit Processes - Treatment methods using chemical or biological reactions • Precipitation • Biodegradation Adsorption Disinfection Nutrient Removal

Treatment Levels • Primary Treatment - (preliminary), physical unit operations – Removal of constituents that cause maintenance or operational problems -- debris, grit, oil and grease, • Secondary Treatment - chemical and biological unit processes – Removal of biodegradable organics and suspended solids • Tertiary Treatment - (advanced), combinations of all three methods – Removal of residual nutrients, toxics, specific contaminants

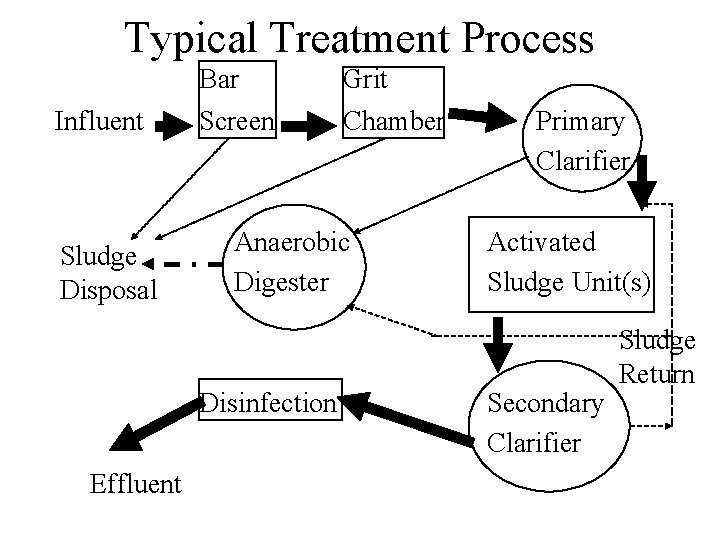
Typical Treatment Process Influent Sludge Disposal Bar Screen Anaerobic Digester Disinfection Effluent Grit Chamber Primary Clarifier Activated Sludge Unit(s) Secondary Clarifier Sludge Return

Industrial Wastewater Treatment - Differences • Equalization - hydraulic residence time at least equal to activated sludge unit, may be several times longer • Oil Separation – Dissolved Air Flotation, Dissolved Gas Flotation – Corrugated Plate Interceptors • Sludges may be hazardous by regulation

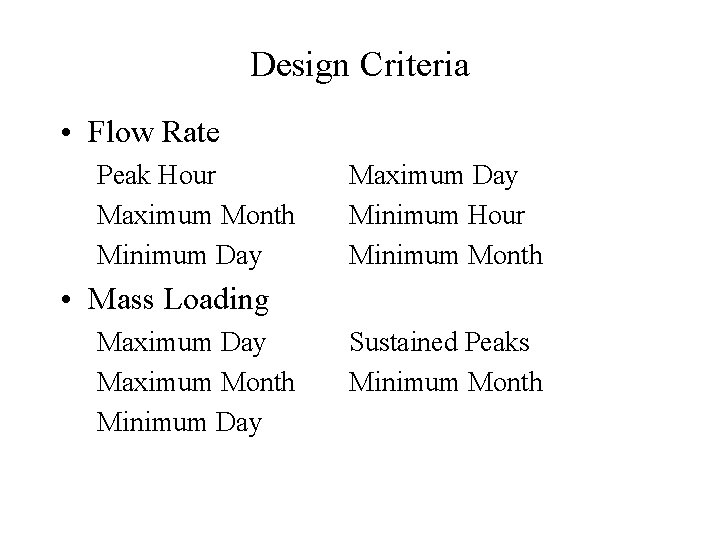
Design Criteria • Flow Rate Peak Hour Maximum Month Minimum Day Maximum Day Minimum Hour Minimum Month • Mass Loading Maximum Day Maximum Month Minimum Day Sustained Peaks Minimum Month

Wastewater Daily Flow Pattern Midnight Noon Midnight

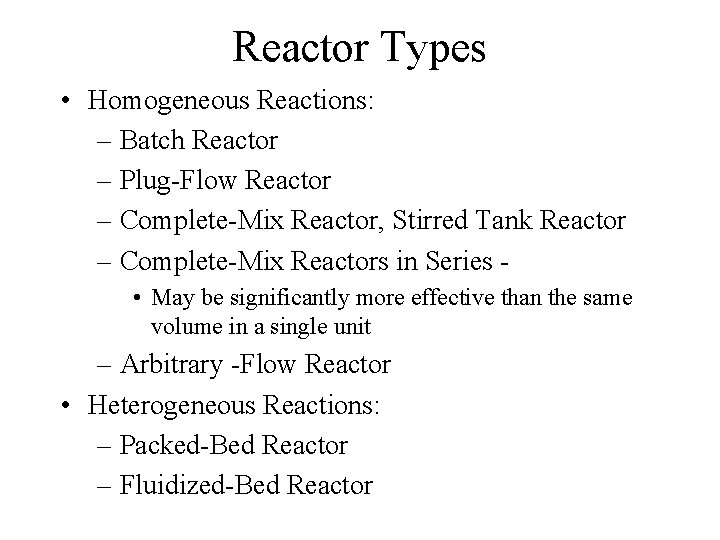
Reactor Types • Homogeneous Reactions: – Batch Reactor – Plug-Flow Reactor – Complete-Mix Reactor, Stirred Tank Reactor – Complete-Mix Reactors in Series • May be significantly more effective than the same volume in a single unit – Arbitrary -Flow Reactor • Heterogeneous Reactions: – Packed-Bed Reactor – Fluidized-Bed Reactor

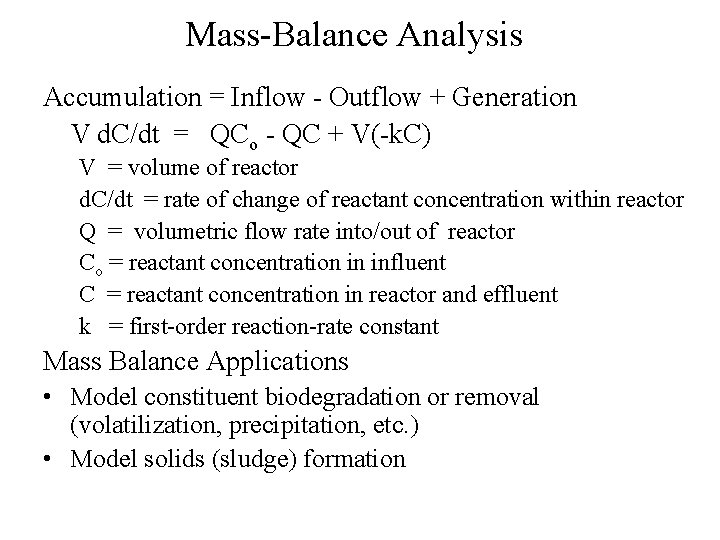
Mass-Balance Analysis Accumulation = Inflow - Outflow + Generation V d. C/dt = QCo - QC + V(-k. C) V = volume of reactor d. C/dt = rate of change of reactant concentration within reactor Q = volumetric flow rate into/out of reactor Co = reactant concentration in influent C = reactant concentration in reactor and effluent k = first-order reaction-rate constant Mass Balance Applications • Model constituent biodegradation or removal (volatilization, precipitation, etc. ) • Model solids (sludge) formation

Common Operational and Design Practices • Gravity flow through system – Only pump the water one time • Parallel units – Allow operational flexibility and maintenance • Minimize human contact with materials

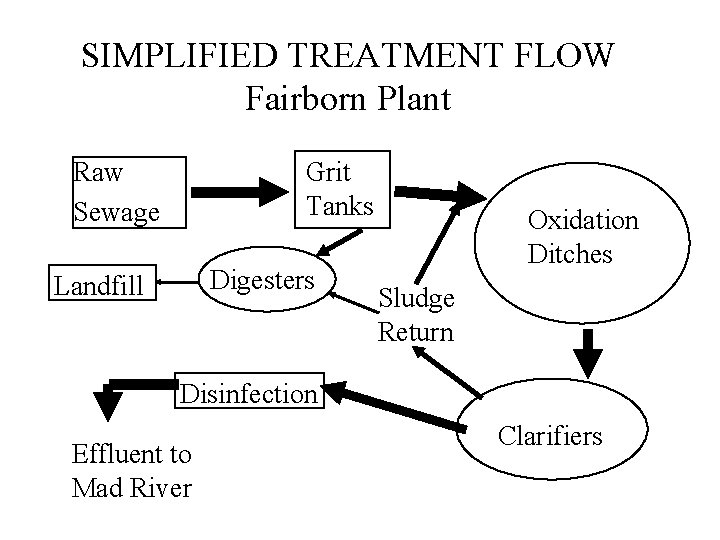
SIMPLIFIED TREATMENT FLOW Fairborn Plant Raw Sewage Grit Tanks Digesters Landfill Oxidation Ditches Sludge Return Disinfection Effluent to Mad River Clarifiers

Fairborn NPDES Permit Requirements • Sampling Stations – Plant Outfall – Waste Sludge – Raw Sewage Influent – Upstream at State Route 235 – Downstream - 200 ft south of lift station at River Mile 8. 6 • Samples: – Composite samples of at least three grab samples proportionate in volume to the sewage flow rate at the time of sampling. . . intervals of at least 30 min. , but not more than 2 hours

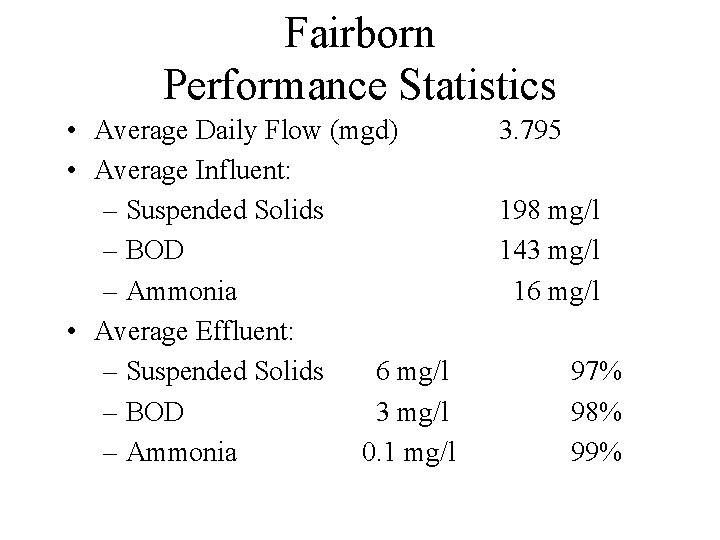
Fairborn Performance Statistics • Average Daily Flow (mgd) • Average Influent: – Suspended Solids – BOD – Ammonia • Average Effluent: – Suspended Solids 6 mg/l – BOD 3 mg/l – Ammonia 0. 1 mg/l 3. 795 198 mg/l 143 mg/l 16 mg/l 97% 98% 99%