Topic 8 Reaction Rate The reaction rate of



- Slides: 5

Topic 8: Reaction Rate The reaction rate of a chemical reaction is how fast the reaction occurs. Factors that affect reaction rate: • Temperature (higher temp usually produces faster reaction) • Stirring (usually increases reaction rate) • Size of reactants (smaller amounts react more quickly) • Concentration of reactants (higher concentrations react faster)

Catalysts These are substances that speed up reaction rate but do not take part in the reaction themselves. It does not affect the chemical reaction in any way except how fast it reaches completion. • Enzymes: natural catalysts, (produced by living things) Examples: laundry detergents, saliva, ear wax, tears Inhibitors These are substances that slow down reaction rate but do not take part in the reaction themselves Examples: food preservatives, car paint http: //www. youtube. com/watch? v=XX 9 Xo 6 zm_k. M http: //www. youtube. com/watch? v=Ott. RV 5 yk. P 7 A

Corrosion When a metal is exposed to air and moisture it corrodes. This is the oxidation of the metal. Oxidation produces rust, (iron oxide). When rust is formed it weakens a metal because as the rust “falls” off the metal more of the metal is exposed resulting in more rust until there is no more of the original metal left behind. When this happens the metal has been completely corroded.

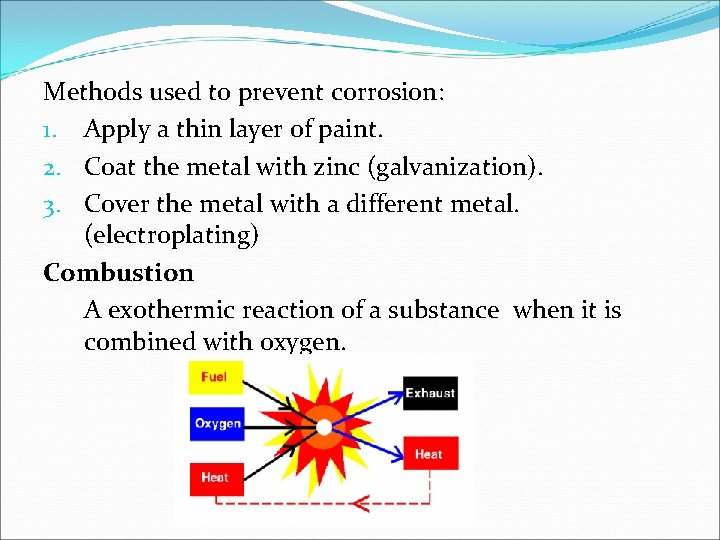
Methods used to prevent corrosion: 1. Apply a thin layer of paint. 2. Coat the metal with zinc (galvanization). 3. Cover the metal with a different metal. (electroplating) Combustion A exothermic reaction of a substance when it is combined with oxygen.

Products of Combustion If carbon is burned: CO 2, H 2 O and energy If nitrogen is burned: NO 2, H 2 O, and energy If sulfur is burned: SO 2, H 2 O, and energy Many of the pollutants created by human consumption of fuel results from combustion reactions. Ex. Smog, car/industry exhaust