CHAPTER 16 RATE OF A REACTION REACTION RATE













- Slides: 18

CHAPTER 16 RATE OF A REACTION

REACTION RATE The amount of time it takes to use up the reactants (decrease in reactant concentration) OR The amount of time it takes to make products (increase in product concentration)

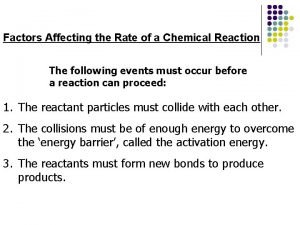
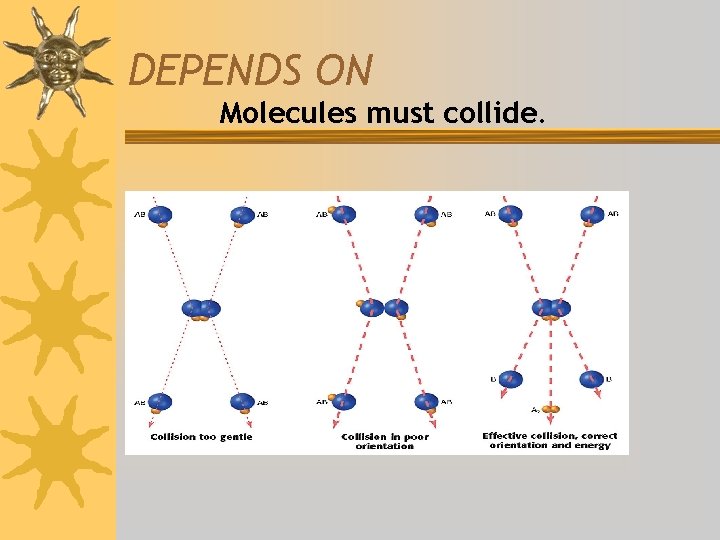
DEPENDS ON Molecules must collide.

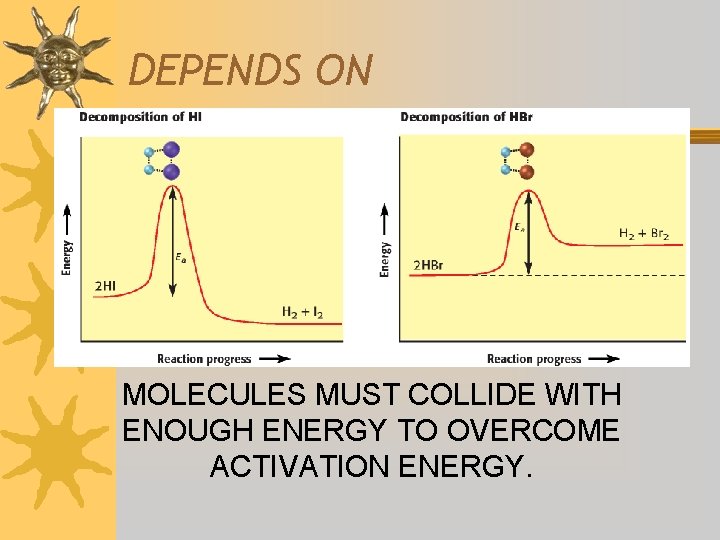
DEPENDS ON MOLECULES MUST COLLIDE WITH ENOUGH ENERGY TO OVERCOME ACTIVATION ENERGY.

Activation Energy and Chemical Reactions

DEPENDS ON MOLECULES MUST COLLIDE WITH THE CORRECT GEOMETRY

FACTORS AFFECTING RATE

WAYS OF INCREASING RATE 1. Types of Reactants – certain bonds are easier to break than others 2. Surface Area – the more surface a molecule can touch → the faster the reaction 3. Temperature – the hotter the molecules → the more they move → the more they collide & change into product

WAYS OF INCREASING RATE 4. Concentration/Molarity – the higher the concentration → the more molecules there are → the more collisions take place creating product

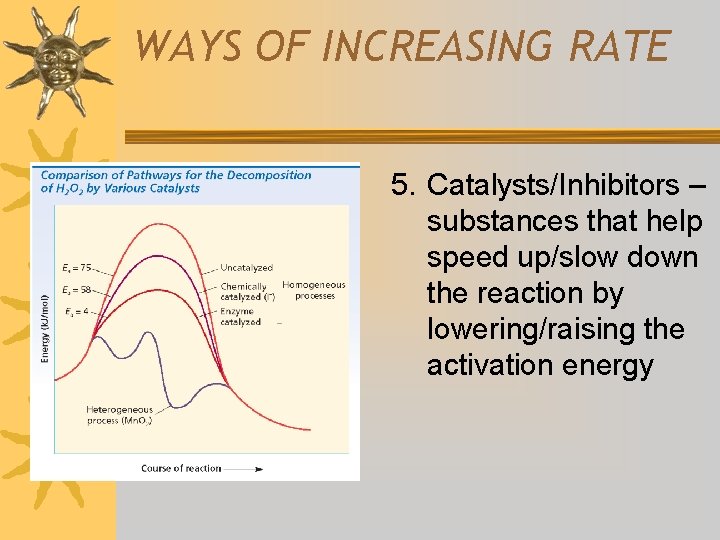
WAYS OF INCREASING RATE 5. Catalysts/Inhibitors – substances that help speed up/slow down the reaction by lowering/raising the activation energy

Multiple Choice 1. To be effective, a collision requires A. enough energy only. B. favorable orientation only. C. enough energy and a favorable orientation. D. a reaction mechanism.

Multiple Choice 1. To be effective, a collision requires A. enough energy only. B. favorable orientation only. C. enough energy and a favorable orientation. D. a reaction mechanism.

Multiple Choice 2. If a collision between molecules is very gentle, the molecules are A. more likely to be oriented favorably. B. less likely to be oriented favorably. C. likely to react. D. likely to rebound without reacting.

Multiple Choice 2. If a collision between molecules is very gentle, the molecules are A. more likely to be oriented favorably. B. less likely to be oriented favorably. C. likely to react. D. likely to rebound without reacting.

Multiple Choice 3. A species that changes the rate of a reaction but is neither consumed nor changed is A. a catalyst. B. an activated complex. C. an intermediate. D. a reactant.

Multiple Choice 3. A species that changes the rate of a reaction but is neither consumed nor changed is A. a catalyst. B. an activated complex. C. an intermediate. D a reactant.

Short Answer 4. Two molecules collide but bounce apart unchanged. What two reasons could account for their failure to react?

Short Answer 4. Two molecules collide but bounce apart unchanged. What two reasons could account for their failure to react? Answer: They had insufficient energy for bonds to break, or they did not collide in a favorable orientation.
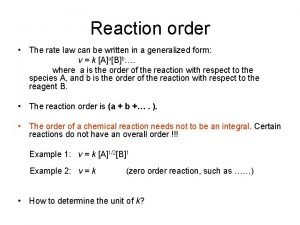
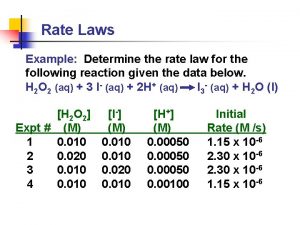
 Rate law
Rate law Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half life formula
Half life formula Equilibrium reaction rate
Equilibrium reaction rate Rate law orders
Rate law orders Overall rate law of a reaction
Overall rate law of a reaction How to calculate rate of reaction
How to calculate rate of reaction Determine
Determine How to calculate the instantaneous rate of reaction
How to calculate the instantaneous rate of reaction Rate law for first order reaction
Rate law for first order reaction Inital rate
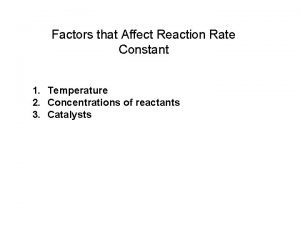
Inital rate Factors affecting rate constant
Factors affecting rate constant Factors affecting the rate of chemical reaction
Factors affecting the rate of chemical reaction Overall rate law of a reaction
Overall rate law of a reaction What is catalyst and how it affects reaction rate
What is catalyst and how it affects reaction rate Maxwell boltzmann distribution catalyst
Maxwell boltzmann distribution catalyst Average rate of reaction formula
Average rate of reaction formula How to determine the rate law of a reaction
How to determine the rate law of a reaction