Reaction Rate Orders Chapter 14 Rate Laws Review


![Integrated Rate Laws Manipulating this equation produces… [A]t ln [A]0 = −kt ln [A]t Integrated Rate Laws Manipulating this equation produces… [A]t ln [A]0 = −kt ln [A]t](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-4.jpg)
![First-Order Processes ln [A]t = -kt + ln [A]0 Therefore, if a reaction is First-Order Processes ln [A]t = -kt + ln [A]0 Therefore, if a reaction is](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-5.jpg)
![Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = −kt 1/2 Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = −kt 1/2](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-10.jpg)

![Second-Order Processes 1 1 = −kt + [A]t [A]0 So if a process is Second-Order Processes 1 1 = −kt + [A]t [A]0 So if a process is](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-13.jpg)
![Second-Order Processes • Graphing ln [NO 2] vs. t yields: • The plot is Second-Order Processes • Graphing ln [NO 2] vs. t yields: • The plot is](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-15.jpg)
![Second-Order Processes • Graphing ln 1/[NO 2] vs. t, however, gives this plot. Time Second-Order Processes • Graphing ln 1/[NO 2] vs. t, however, gives this plot. Time](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-16.jpg)
![Zeroth Order processes • [A] vs time when graphed creates straight line • The Zeroth Order processes • [A] vs time when graphed creates straight line • The](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-17.jpg)

- Slides: 18

Reaction Rate Orders Chapter 14

Rate Laws Review • The overall reaction order can be found by adding the exponents on the reactants in the rate law. Rate = k[A]m[B]n

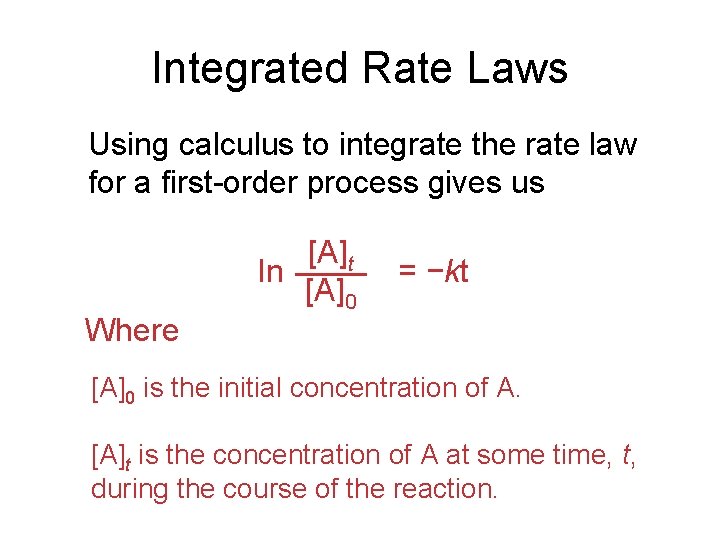
Integrated Rate Laws Using calculus to integrate the rate law for a first-order process gives us Where [A]t ln [A]0 = −kt [A]0 is the initial concentration of A. [A]t is the concentration of A at some time, t, during the course of the reaction.
![Integrated Rate Laws Manipulating this equation produces At ln A0 kt ln At Integrated Rate Laws Manipulating this equation produces… [A]t ln [A]0 = −kt ln [A]t](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-4.jpg)
Integrated Rate Laws Manipulating this equation produces… [A]t ln [A]0 = −kt ln [A]t − ln [A]0 = − kt ln [A]t = − kt + ln [A]0 …which is in the form y = mx + b
![FirstOrder Processes ln At kt ln A0 Therefore if a reaction is First-Order Processes ln [A]t = -kt + ln [A]0 Therefore, if a reaction is](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-5.jpg)
First-Order Processes ln [A]t = -kt + ln [A]0 Therefore, if a reaction is first-order, a plot of ln [A] vs. t will yield a straight line, and the slope of the line will be -k.

First-Order Processes Consider the process in which methyl isonitrile is converted to acetonitrile. CH 3 NC CH 3 CN

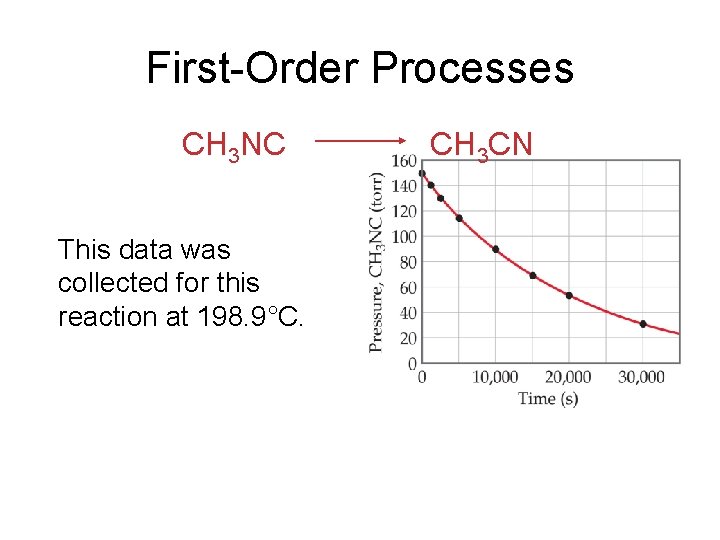
First-Order Processes CH 3 NC This data was collected for this reaction at 198. 9°C. CH 3 CN

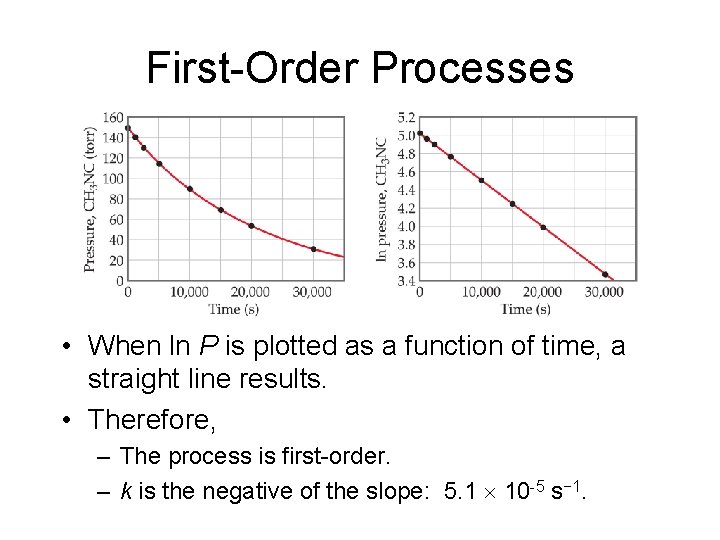
First-Order Processes • When ln P is plotted as a function of time, a straight line results. • Therefore, – The process is first-order. – k is the negative of the slope: 5. 1 10 -5 s− 1.

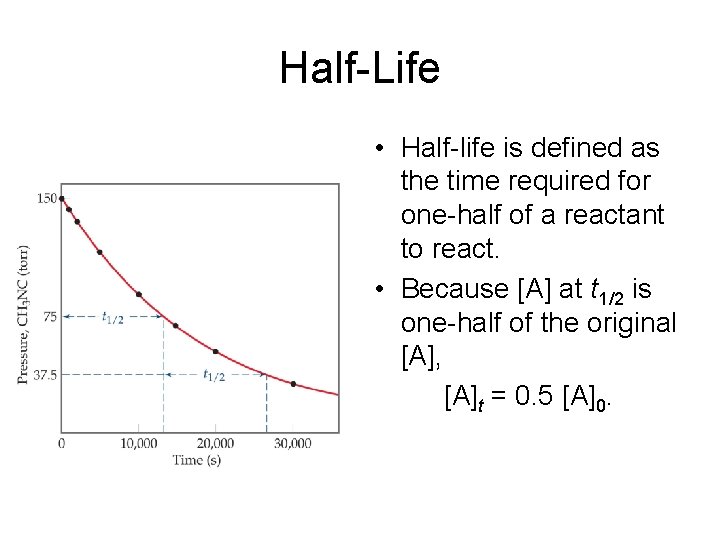
Half-Life • Half-life is defined as the time required for one-half of a reactant to react. • Because [A] at t 1/2 is one-half of the original [A], [A]t = 0. 5 [A]0.
![HalfLife For a firstorder process this becomes 0 5 A0 ln kt 12 Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = −kt 1/2](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-10.jpg)
Half-Life For a first-order process, this becomes 0. 5 [A]0 ln = −kt 1/2 [A]0 ln 0. 5 = −kt 1/2 − 0. 693 = −kt 1/2 NOTE: For a first-order process, the half-life does not depend on [A]0. 0. 693 = t 1/2 k

Half Life • Since the half life is independent of the original concentration, half life of a first order reaction can be used as a representation of the rate constant because the half life and rate constant are inversely proportional to each other. – The shorter the half life, the faster the reaction rate – Real life examples of first order reactions: • Radioactive decay

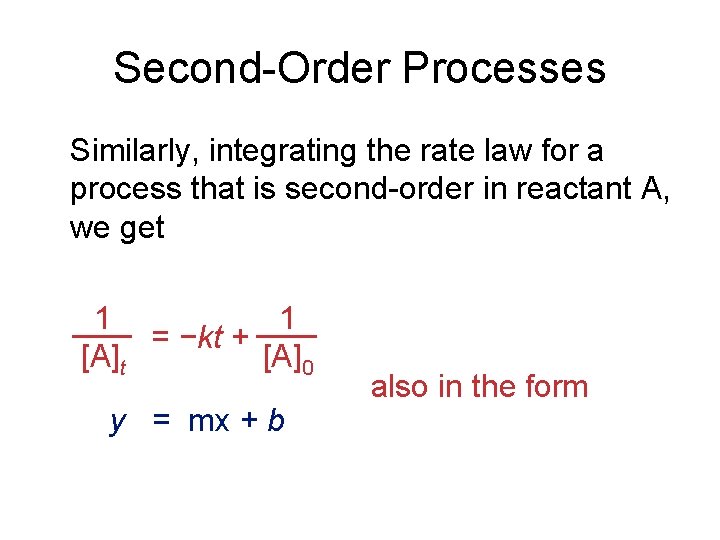
Second-Order Processes Similarly, integrating the rate law for a process that is second-order in reactant A, we get 1 1 = −kt + [A]t [A]0 y = mx + b also in the form
![SecondOrder Processes 1 1 kt At A0 So if a process is Second-Order Processes 1 1 = −kt + [A]t [A]0 So if a process is](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-13.jpg)
Second-Order Processes 1 1 = −kt + [A]t [A]0 So if a process is second-order in A, a plot of 1/[A] vs. t will yield a straight line, and the slope of that line is k.

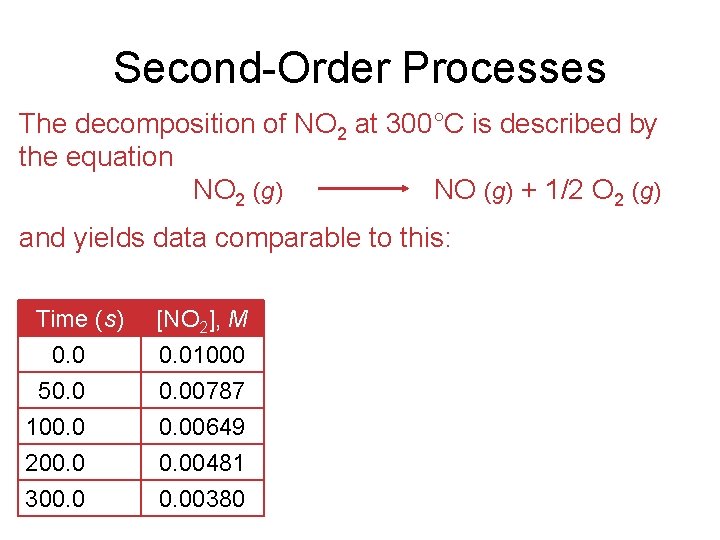
Second-Order Processes The decomposition of NO 2 at 300°C is described by the equation NO 2 (g) NO (g) + 1/2 O 2 (g) and yields data comparable to this: Time (s) 0. 0 50. 0 100. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 200. 0 300. 00481 0. 00380
![SecondOrder Processes Graphing ln NO 2 vs t yields The plot is Second-Order Processes • Graphing ln [NO 2] vs. t yields: • The plot is](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-15.jpg)
Second-Order Processes • Graphing ln [NO 2] vs. t yields: • The plot is not a straight line, so the process is not first-order in [A]. Time (s) 0. 0 50. 0 100. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 ln [NO 2] − 4. 610 − 4. 845 − 5. 038 200. 0 300. 00481 0. 00380 − 5. 337 − 5. 573
![SecondOrder Processes Graphing ln 1NO 2 vs t however gives this plot Time Second-Order Processes • Graphing ln 1/[NO 2] vs. t, however, gives this plot. Time](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-16.jpg)
Second-Order Processes • Graphing ln 1/[NO 2] vs. t, however, gives this plot. Time (s) 0. 0 50. 0 100. 0 [NO 2], M 0. 01000 0. 00787 0. 00649 1/[NO 2] 100 127 154 200. 0 300. 00481 0. 00380 208 263 • Because this is a straight line, the process is secondorder in [A].
![Zeroth Order processes A vs time when graphed creates straight line The Zeroth Order processes • [A] vs time when graphed creates straight line • The](https://slidetodoc.com/presentation_image_h2/846879ed0cb69c1c02336827c99d6f8e/image-17.jpg)
Zeroth Order processes • [A] vs time when graphed creates straight line • The negative slope is k • Reaction rate is independent of the concentration of a reactant – Changing its concentration has no effect on the speed of the reaction – Rate stays the constant

• The number of molecules involved in a collision can tell you the reaction rate order – Unimolecular reactions = first order – Bimolecular reactions = second order