Rate Laws Rate Laws n Chemists conduct experiments




![NO (g) + ½ Cl 2 (g) NOCl (g) Exp’t [NO]0 [Cl 2]0 Initial NO (g) + ½ Cl 2 (g) NOCl (g) Exp’t [NO]0 [Cl 2]0 Initial](https://slidetodoc.com/presentation_image_h2/475e4c2dca55b8bbafb232c06f4f58e1/image-9.jpg)

![Or, take Ratio of Rates Rate 2 = k[NH 4+]n[NO 2 -]m = k[0. Or, take Ratio of Rates Rate 2 = k[NH 4+]n[NO 2 -]m = k[0.](https://slidetodoc.com/presentation_image_h2/475e4c2dca55b8bbafb232c06f4f58e1/image-12.jpg)

- Slides: 14

Rate Laws

Rate Laws n Chemists conduct experiments to determine the rate law for a chemical reaction. n. A RATE LAW is a mathematical equation that shows how the reaction rate is related to the concentration of the reactants.

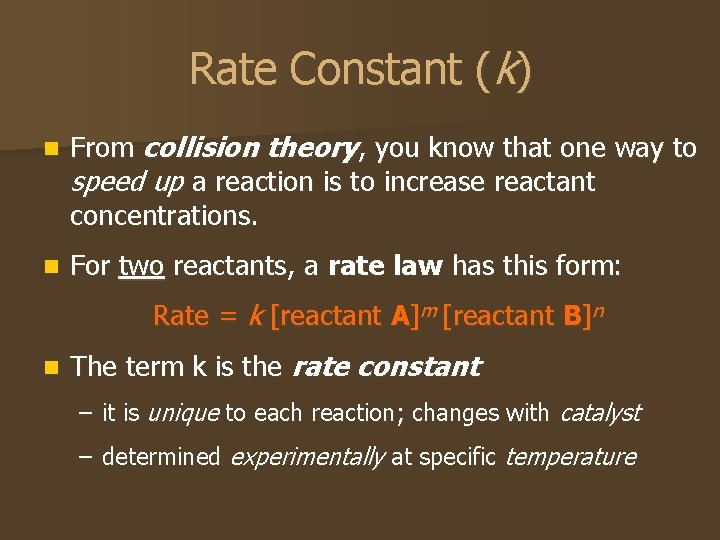
Rate Constant (k) n From collision theory, you know that one way to speed up a reaction is to increase reactant concentrations. n For two reactants, a rate law has this form: Rate = k [reactant A]m [reactant B]n n The term k is the rate constant – it is unique to each reaction; changes with catalyst – determined experimentally at specific temperature

Order of Reaction exponents m and n indicate the order of each reactant in the reaction. n The n For example, an exponent of 1 means the reaction is first order with respect to the given reactant. – Higher order, greater effect on reaction rate. sum of m + n gives the order of the overall reaction. n The

Reaction Order n 2 NO (g) n + O 2 (g) 2 NO 2 (g) Has rate equation: rate = k[NO]1[O 2]1 – Describe reaction – Describe reactants

Reaction Order n 2 NO (g) n + Cl 2 (g) 2 NOCl (g) Has rate equation: rate = k[NO]2[Cl 2] – Describe reaction – Describe reactants

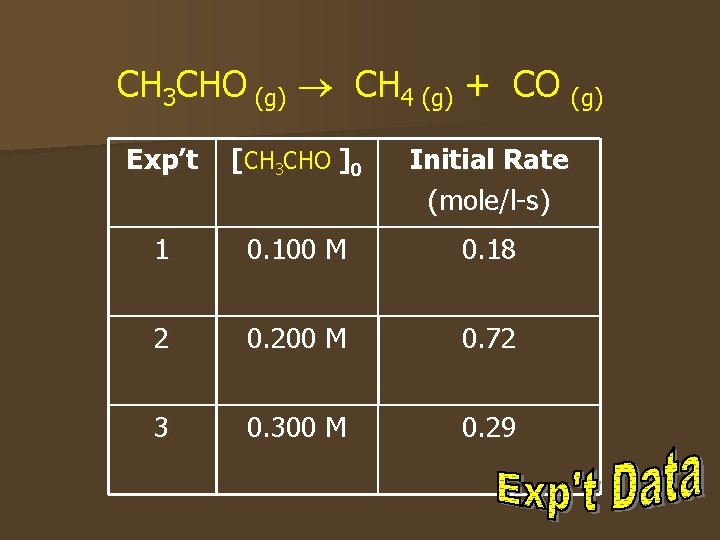
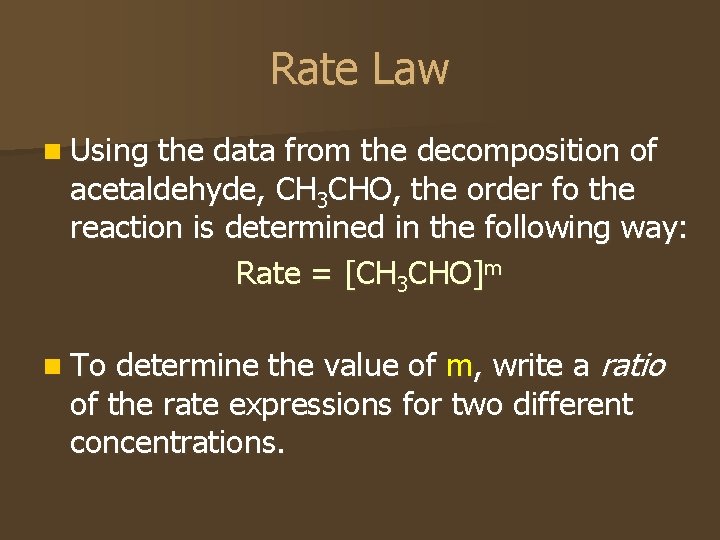
CH 3 CHO (g) CH 4 (g) + CO (g) Exp’t [CH 3 CHO ]0 Initial Rate (mole/l-s) 1 0. 100 M 0. 18 2 0. 200 M 0. 72 3 0. 300 M 0. 29

Rate Law n Using the data from the decomposition of acetaldehyde, CH 3 CHO, the order fo the reaction is determined in the following way: Rate = [CH 3 CHO]m determine the value of m, write a ratio of the rate expressions for two different concentrations. n To
![NO g ½ Cl 2 g NOCl g Expt NO0 Cl 20 Initial NO (g) + ½ Cl 2 (g) NOCl (g) Exp’t [NO]0 [Cl 2]0 Initial](https://slidetodoc.com/presentation_image_h2/475e4c2dca55b8bbafb232c06f4f58e1/image-9.jpg)
NO (g) + ½ Cl 2 (g) NOCl (g) Exp’t [NO]0 [Cl 2]0 Initial Rate (moles/L) (moles/L-s) 1 0. 250 1. 43 X 10 -6 2 0. 250 0. 500 2. 86 X 10 -6 3 0. 500 11. 4 X 10 -6

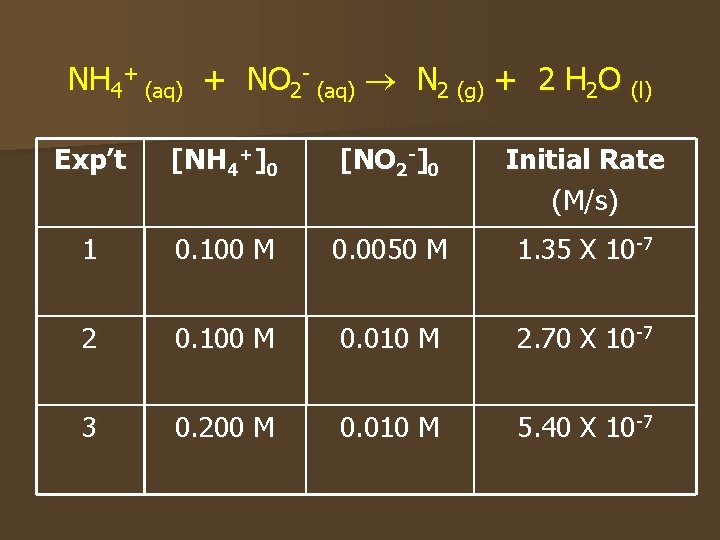
NH 4+ (aq) + NO 2 - (aq) N 2 (g) + 2 H 2 O (l) Exp’t [NH 4+]0 [NO 2 -]0 Initial Rate (M/s) 1 0. 100 M 0. 0050 M 1. 35 X 10 -7 2 0. 100 M 0. 010 M 2. 70 X 10 -7 3 0. 200 M 0. 010 M 5. 40 X 10 -7

Compare how the rate changes with concentration to find m, n n Compare expt’s 1 & 2 – [NH 4+] is constant, doubled [NO 2 -] – The rate doubled n Compare expt’s 2 & 3 – [NO 2 -] is constant, doubled [NH 4+] – The rate doubled
![Or take Ratio of Rates Rate 2 kNH 4nNO 2 m k0 Or, take Ratio of Rates Rate 2 = k[NH 4+]n[NO 2 -]m = k[0.](https://slidetodoc.com/presentation_image_h2/475e4c2dca55b8bbafb232c06f4f58e1/image-12.jpg)
Or, take Ratio of Rates Rate 2 = k[NH 4+]n[NO 2 -]m = k[0. 100]n[0. 010]m Rate 1 = k[NH 4+]n[NO 2 -]m = k[0. 100]n[0. 005]m 2. 70 X 10 -7 = [0. 010]m 1. 35 X 10 -7 = [0. 005]m 2. 00 = (2. 0)m m=1

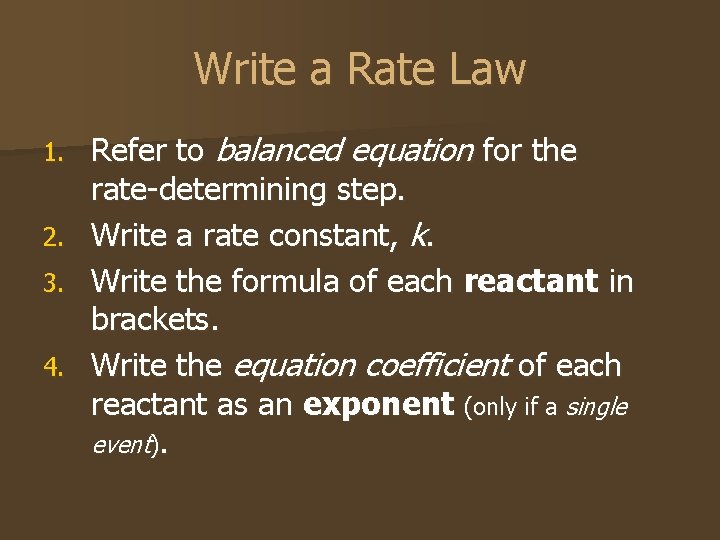
Write a Rate Law 1. 2. 3. 4. Refer to balanced equation for the rate-determining step. Write a rate constant, k. Write the formula of each reactant in brackets. Write the equation coefficient of each reactant as an exponent (only if a single event).

Example: Write a Rate Law Step 1: NO + Cl 2 NOCl 2 Step 2: NOCl 2 + NO 2 NOCl (fast) (slow) n Determine the overall balanced equation. n What is the intermediate? n Which step is rate-determining? n What is the rate law for the rate-determining step? n What is the order of the reaction with respect to NO and Cl 2?
 Regulate public conduct and set out duties owed to society
Regulate public conduct and set out duties owed to society Chemists open
Chemists open How did chemists begin the process of organizing elements
How did chemists begin the process of organizing elements How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? A chemists shorthand way of representing chemical reaction
A chemists shorthand way of representing chemical reaction Ionic and metallic bonding chapter 7 practice problems
Ionic and metallic bonding chapter 7 practice problems Chemist shorthand way of representing chemical reaction
Chemist shorthand way of representing chemical reaction Why do chemists use the mole
Why do chemists use the mole Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? A chemist shorthand way of presenting chemical reaction
A chemist shorthand way of presenting chemical reaction Facts about montesquieu
Facts about montesquieu Viking experiments
Viking experiments Doe in jmp
Doe in jmp Viking experiments
Viking experiments Eyewitness testimony video experiments
Eyewitness testimony video experiments