Section 1 Measuring Matter Chemists use the mole


- Slides: 10

Section 1: Measuring Matter Chemists use the mole to count atoms, molecules, ions, and formula units. K What I Know W What I Want to Find Out L What I Learned

• 8(A) Define and use the concept of a mole. • 8(B) Use the mole concept to calculate the number of atoms, ions, or molecules in a sample of material. • 2(G) Express and manipulate chemical quantities using scientific conventions and mathematical procedures, including dimensional analysis, scientific notation, and significant figures. Copyright © Mc. Graw-Hill Education Measuring Matter

Essential Questions • How is a mole used to indirectly count the number of particles of matter? • What is a common everyday counting unit to which the mole can be related? • How can moles be converted to number of representative particles and vice versa? Copyright © Mc. Graw-Hill Education Measuring Matter

Vocabulary Review New • molecule • mole • Avogadro’s number Copyright © Mc. Graw-Hill Education Measuring Matter

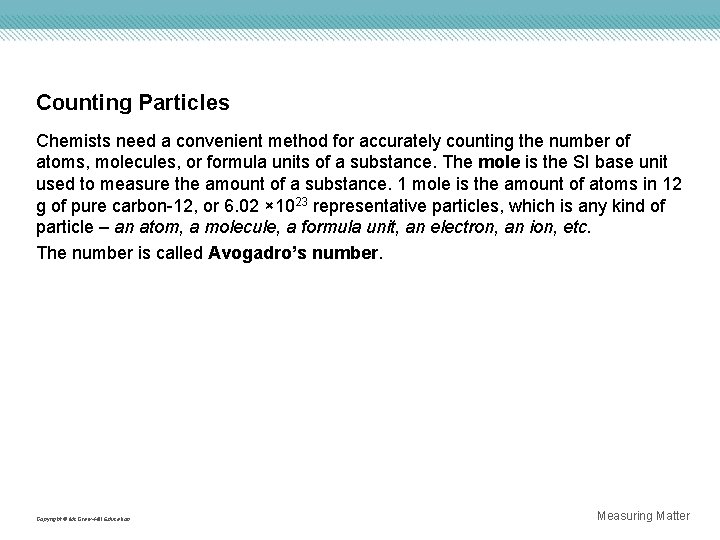
Counting Particles Chemists need a convenient method for accurately counting the number of atoms, molecules, or formula units of a substance. The mole is the SI base unit used to measure the amount of a substance. 1 mole is the amount of atoms in 12 g of pure carbon-12, or 6. 02 × 1023 representative particles, which is any kind of particle – an atom, a molecule, a formula unit, an electron, an ion, etc. The number is called Avogadro’s number. Copyright © Mc. Graw-Hill Education Measuring Matter

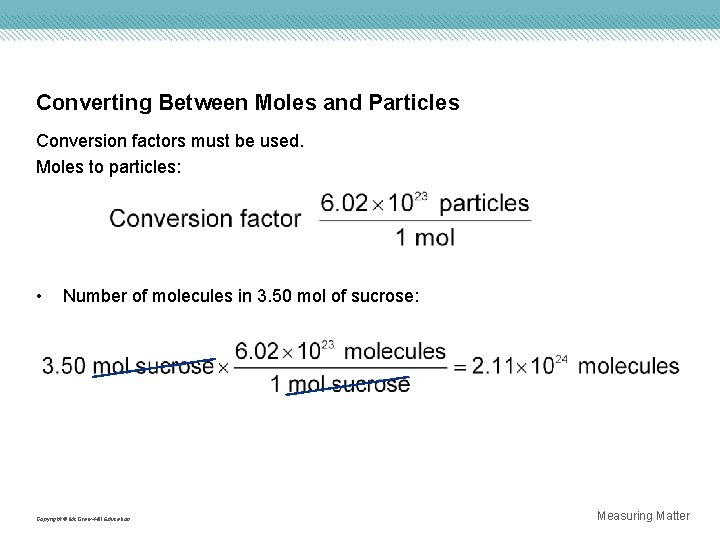
Converting Between Moles and Particles Conversion factors must be used. Moles to particles: • Number of molecules in 3. 50 mol of sucrose: Copyright © Mc. Graw-Hill Education Measuring Matter

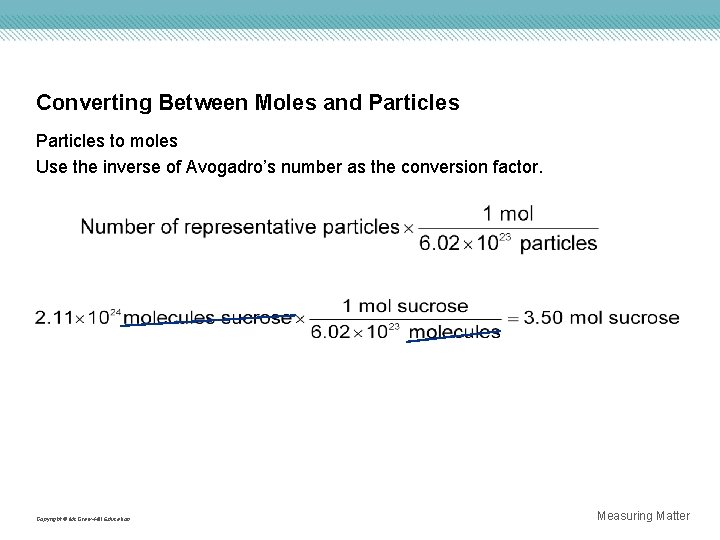
Converting Between Moles and Particles to moles Use the inverse of Avogadro’s number as the conversion factor. Copyright © Mc. Graw-Hill Education Measuring Matter

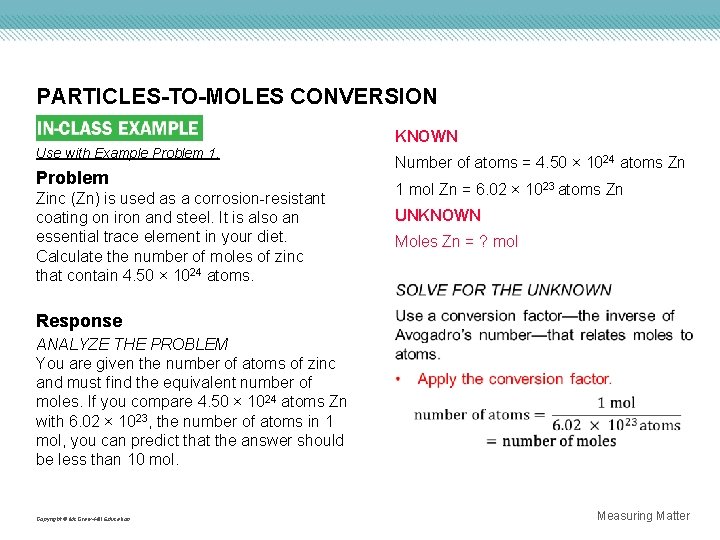
PARTICLES-TO-MOLES CONVERSION Use with Example Problem 1. Problem Zinc (Zn) is used as a corrosion-resistant coating on iron and steel. It is also an essential trace element in your diet. Calculate the number of moles of zinc that contain 4. 50 × 1024 atoms. KNOWN Number of atoms = 4. 50 × 1024 atoms Zn 1 mol Zn = 6. 02 × 1023 atoms Zn UNKNOWN Moles Zn = ? mol Response ANALYZE THE PROBLEM You are given the number of atoms of zinc and must find the equivalent number of moles. If you compare 4. 50 × 1024 atoms Zn with 6. 02 × 1023, the number of atoms in 1 mol, you can predict that the answer should be less than 10 mol. Copyright © Mc. Graw-Hill Education Measuring Matter

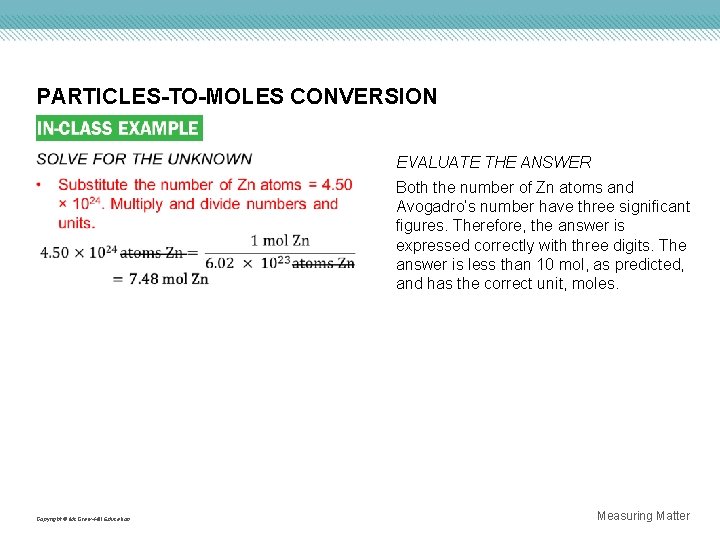
PARTICLES-TO-MOLES CONVERSION EVALUATE THE ANSWER Both the number of Zn atoms and Avogadro’s number have three significant figures. Therefore, the answer is expressed correctly with three digits. The answer is less than 10 mol, as predicted, and has the correct unit, moles. Copyright © Mc. Graw-Hill Education Measuring Matter

Review Essential Questions • How is a mole used to indirectly count the number of particles of matter? • What is a common everyday counting unit to which the mole can be related? • How can moles be converted to number of representative particles and vice versa? Vocabulary • mole • Avogadro’s number Copyright © Mc. Graw-Hill Education Measuring Matter