Classifying Chemical Reactions Chapter 9 Chemical Reaction n









- Slides: 18

Classifying Chemical Reactions Chapter 9

Chemical Reaction n- A process in which the physical and chemical properties of the original substances change as new substances with different physical and chemical properties are formed. n n Reactant(s) – starting substance(s) Product(s) – ending substance(s)

Reasons for chemical reactions: n atoms can obtain a complete set of valence electrons (by losing, gaining, or sharing) n atoms can become more stable

Evidence of a Chemical Reaction: n Precipitate – solid that falls out of a liquid n Bubbles (gas formed) n production of heat/light…ENERGY n color change

Chemical Equation n shorthand way to represent a chemical reaction n word equation – uses words (names) of reactants and products n Formula equation – uses symbols and formulas for reactants and products.

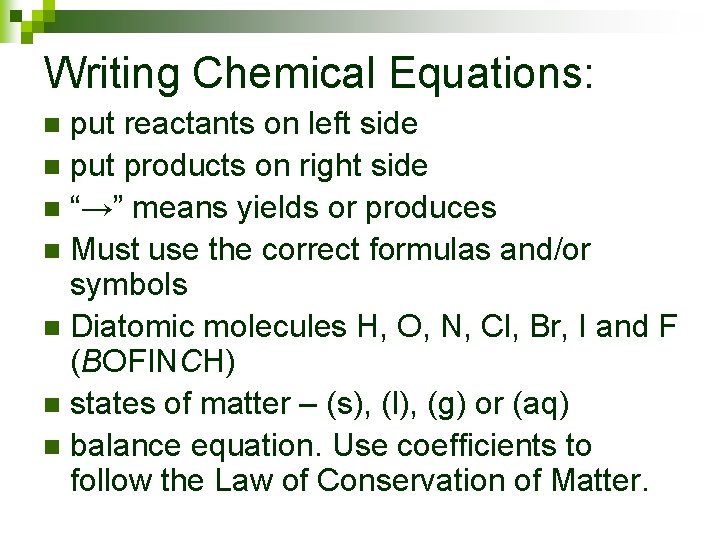
Writing Chemical Equations: put reactants on left side n put products on right side n “→” means yields or produces n Must use the correct formulas and/or symbols n Diatomic molecules H, O, N, Cl, Br, I and F (BOFINCH) n states of matter – (s), (l), (g) or (aq) n balance equation. Use coefficients to follow the Law of Conservation of Matter. n

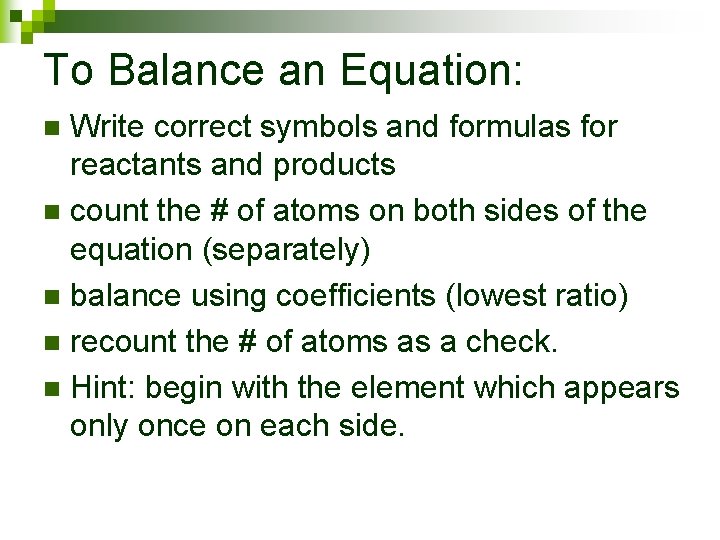
To Balance an Equation: Write correct symbols and formulas for reactants and products n count the # of atoms on both sides of the equation (separately) n balance using coefficients (lowest ratio) n recount the # of atoms as a check. n Hint: begin with the element which appears only once on each side. n

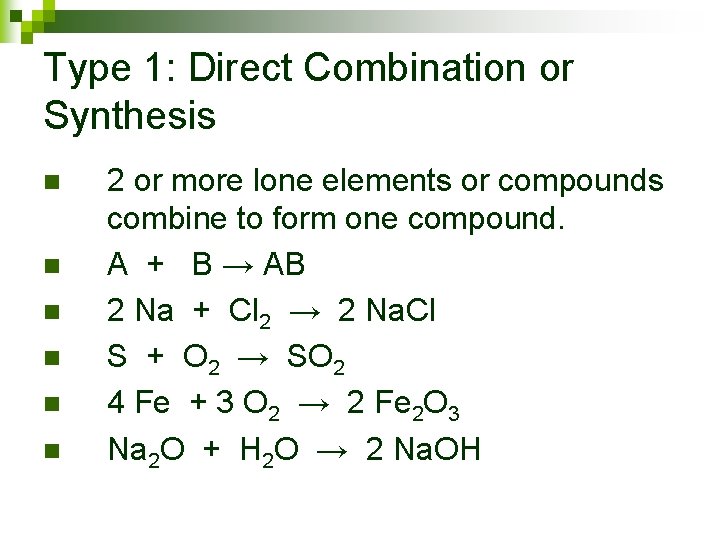
Type 1: Direct Combination or Synthesis

Type 1: Direct Combination or Synthesis n n n 2 or more lone elements or compounds combine to form one compound. A + B → AB 2 Na + Cl 2 → 2 Na. Cl S + O 2 → SO 2 4 Fe + 3 O 2 → 2 Fe 2 O 3 Na 2 O + H 2 O → 2 Na. OH

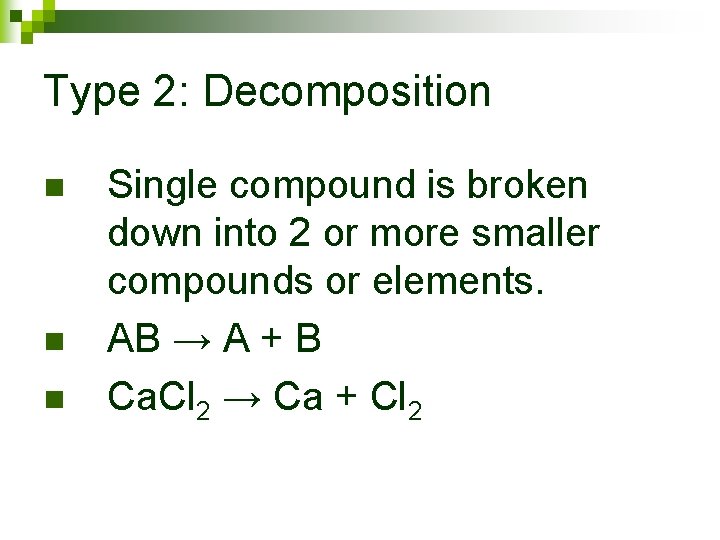
Type 2: Decomposition

Type 2: Decomposition n Single compound is broken down into 2 or more smaller compounds or elements. AB → A + B Ca. Cl 2 → Ca + Cl 2

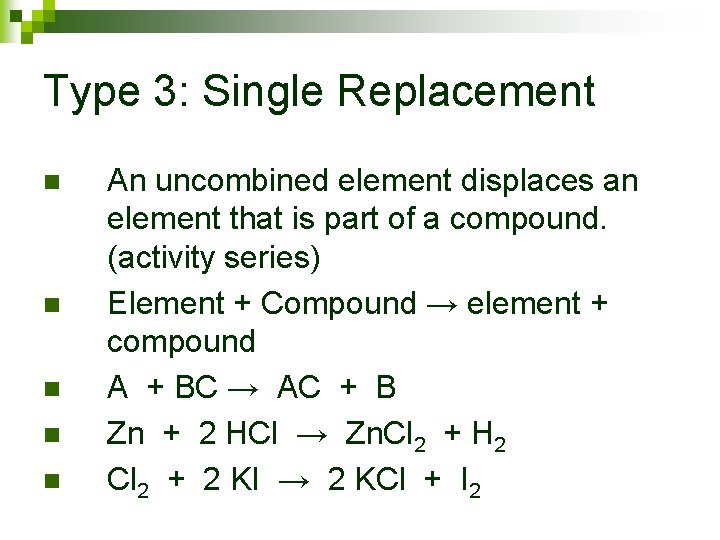
Type 3: Single Replacement

Type 3: Single Replacement n n n An uncombined element displaces an element that is part of a compound. (activity series) Element + Compound → element + compound A + BC → AC + B Zn + 2 HCl → Zn. Cl 2 + H 2 Cl 2 + 2 KI → 2 KCl + I 2

Type 3 continued Note: Metals replace metals, nonmetals replace nonmetals ¨ Note: In order to replace an element, the element must be more active than the one being replaced. ¨

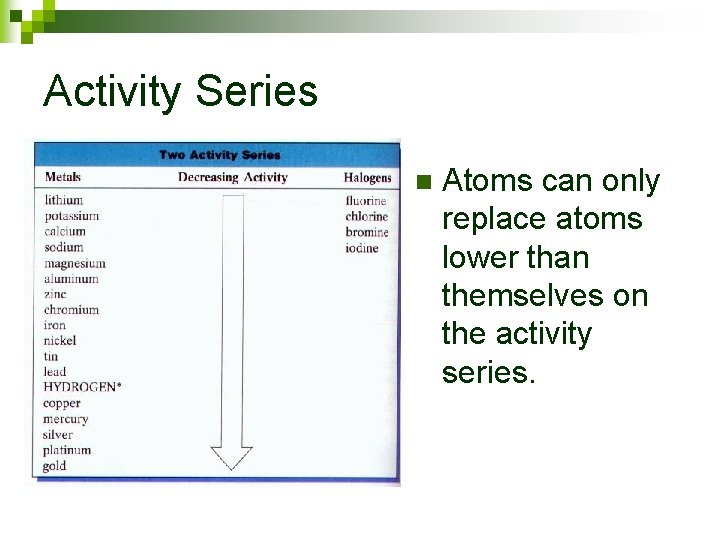
Activity Series n Atoms can only replace atoms lower than themselves on the activity series.

Type 4: Double Replacement

Type 4: Double Replacement n n n Compound + Compound → compound + compound AB + CD → AD + BC Factors for double replacement reactions: Most will only occur if reactants are in solution. Compounds separate into ions in solution. will likely proceed if: one of the products is a molecular compound

Other Special Types of Reactions n Complete Combustion of an Organic substance (Cx. Hy or Cx. Hy. Oz) ¨ Organic substance + O 2 → H 2 O + CO 2
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Section 1 chemical changes
Section 1 chemical changes Bomb power
Bomb power 20 examples of redox reaction
20 examples of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations 5 types of chemical reactions
5 types of chemical reactions