Chemists Have Solutions I Solution Chemistry Chemists Have











![p. H – A Measure of Acidity p. H = -log [H+] Solution Is p. H – A Measure of Acidity p. H = -log [H+] Solution Is](https://slidetodoc.com/presentation_image/605441f923a44102e0d6311a18a040ff/image-31.jpg)
![p. OH = -log [OH-] [H+][OH-] = Kw = 1. 0 x 10 -14 p. OH = -log [OH-] [H+][OH-] = Kw = 1. 0 x 10 -14](https://slidetodoc.com/presentation_image/605441f923a44102e0d6311a18a040ff/image-32.jpg)







- Slides: 62

Chemists Have Solutions I Solution Chemistry

Chemists Have Solutions Solution Concentrations (Molarity, Molality, Percent) Acids and Bases 1. 2. n Theories ¨ n n Arrhenius, Brønsted-Lowry, Lewis Strong and Weak Acids and Bases The p. H scale Neutralization Reactions: Concepts and Problems Applications in Industry, Household, Health Basic Reduction and Oxidation 3. n n n Definitions: Oxidation, Reducing Agent, Oxidizing Agent Redox Reactions Applications and Examples: Batteries, Corrosion, Common oxidizing and reducing agents

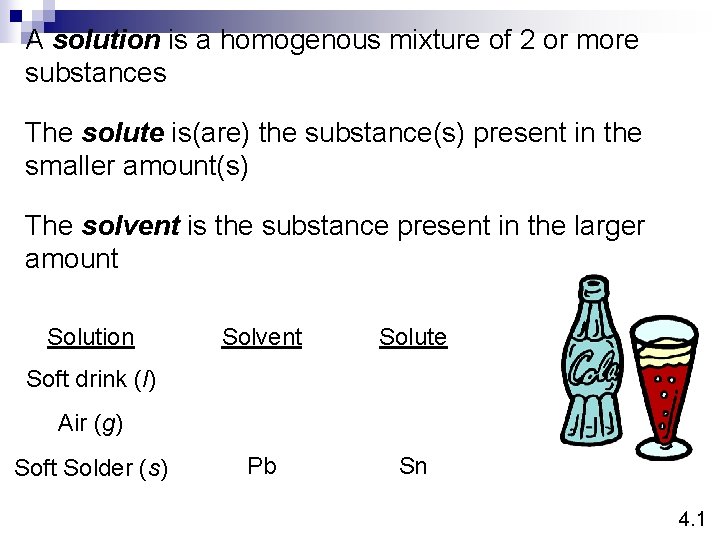
A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Solution Solvent Solute Pb Sn Soft drink (l) Air (g) Soft Solder (s) 4. 1

An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. nonelectrolyte weak electrolyte strong electrolyte 4. 1

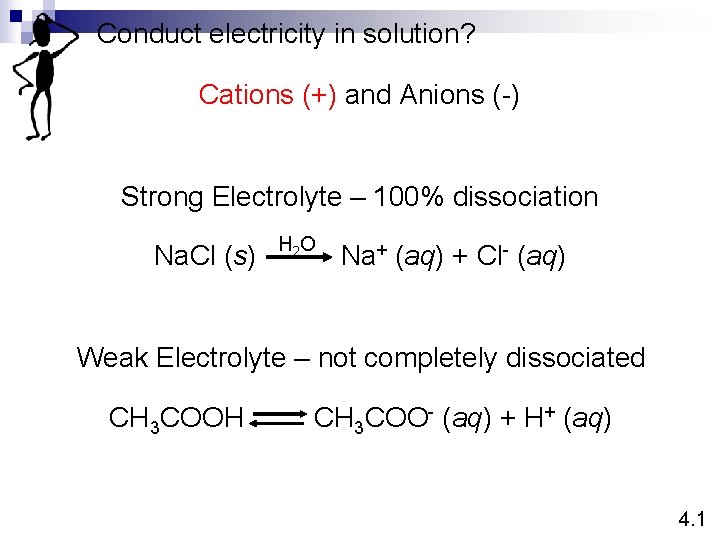
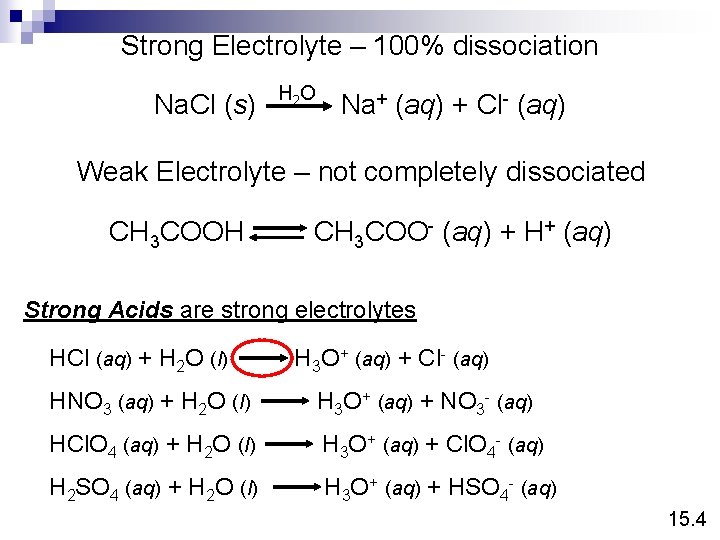
Conduct electricity in solution? Cations (+) and Anions (-) Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated CH 3 COOH CH 3 COO- (aq) + H+ (aq) 4. 1

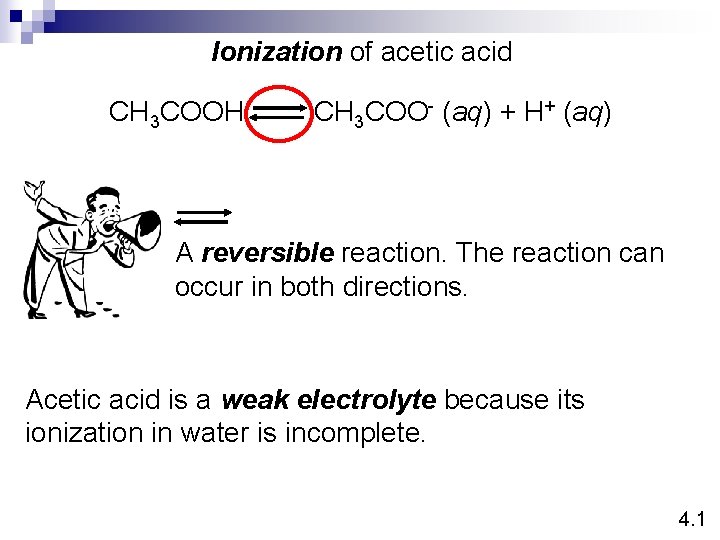
Ionization of acetic acid CH 3 COOH CH 3 COO- (aq) + H+ (aq) A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete. 4. 1

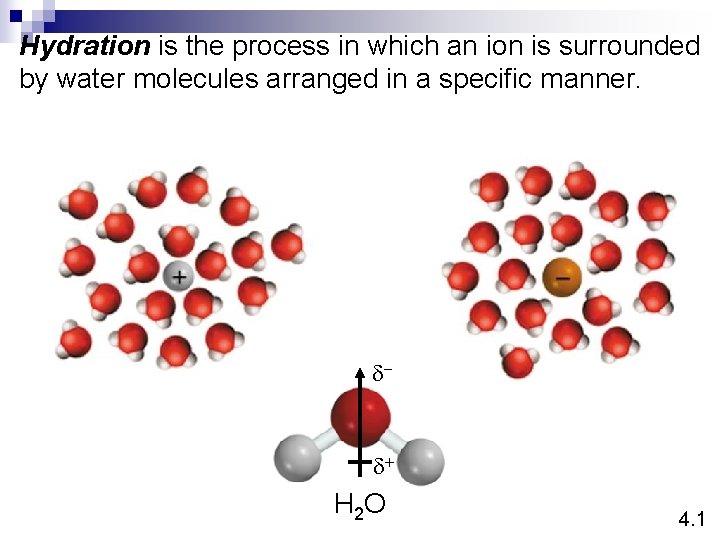
Hydration is the process in which an ion is surrounded by water molecules arranged in a specific manner. d- d+ H 2 O 4. 1

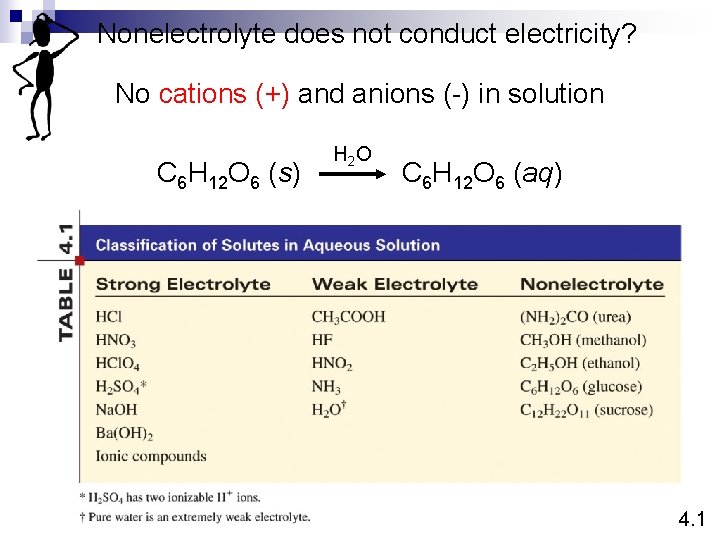
Nonelectrolyte does not conduct electricity? No cations (+) and anions (-) in solution C 6 H 12 O 6 (s) H 2 O C 6 H 12 O 6 (aq) 4. 1

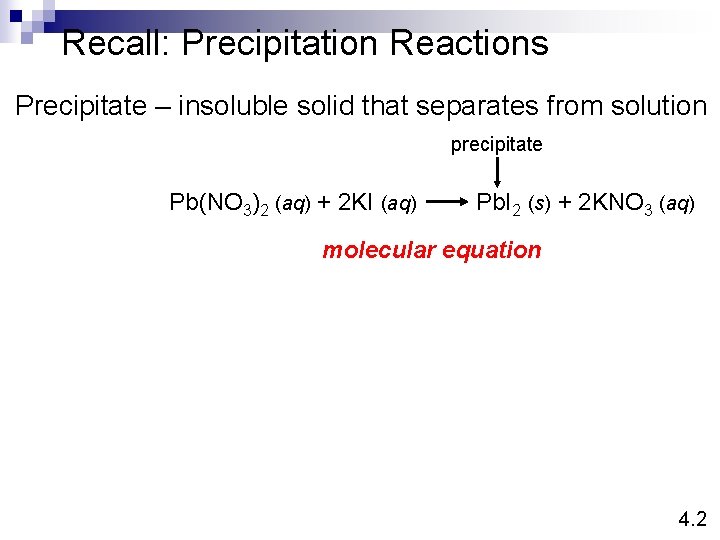
Recall: Precipitation Reactions Precipitate – insoluble solid that separates from solution precipitate Pb(NO 3)2 (aq) + 2 KI (aq) Pb. I 2 (s) + 2 KNO 3 (aq) molecular equation 4. 2

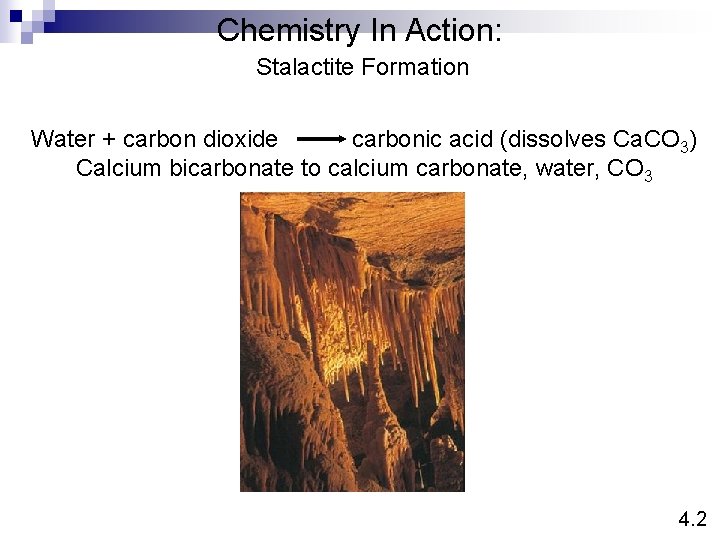
Chemistry In Action: Stalactite Formation Water + carbon dioxide carbonic acid (dissolves Ca. CO 3) Calcium bicarbonate to calcium carbonate, water, CO 3 4. 2

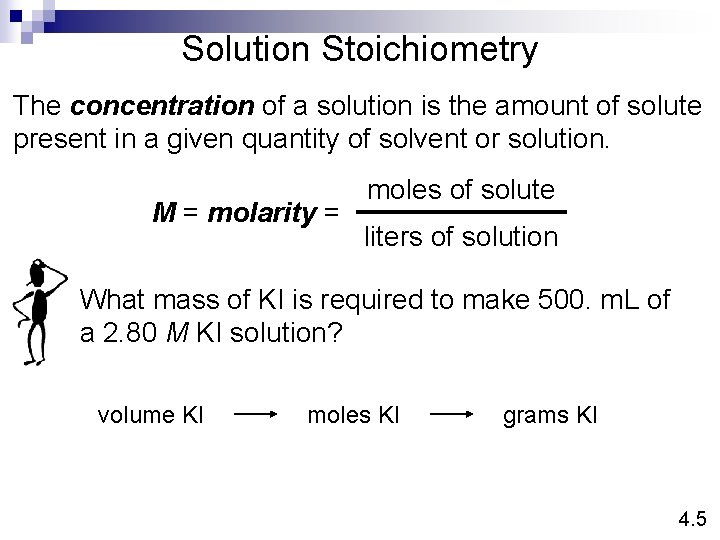
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? volume KI moles KI grams KI 4. 5

Solution Composition Molarity (M) Molarity: Moles of solute per liter of solution.

Test Yourself 1. 2. 3. 4. 5. How many moles of potassium bromide are in 200. 0 m. L of a 1. 5 M solution? What is the concentration, in M, of 20. 00 g Lead (II) Nitrate in 50. 0 L of solution? What is the concentration, in M, of 30. 0 g glucose (C 6 H 12 O 6), in 100. 0 m. L of water? Assume no volume changes. How much salt must I dissolve in 2. 00 L water to make a 5. 0 M solution? Assume no volume changes. What will be the mass of table salt I can recover from the complete drying of 50. 00 m. L 0. 50 M salt solution?

Solution Composition MOLALITY (m) Molality: Moles of solute per kilogram solvent.

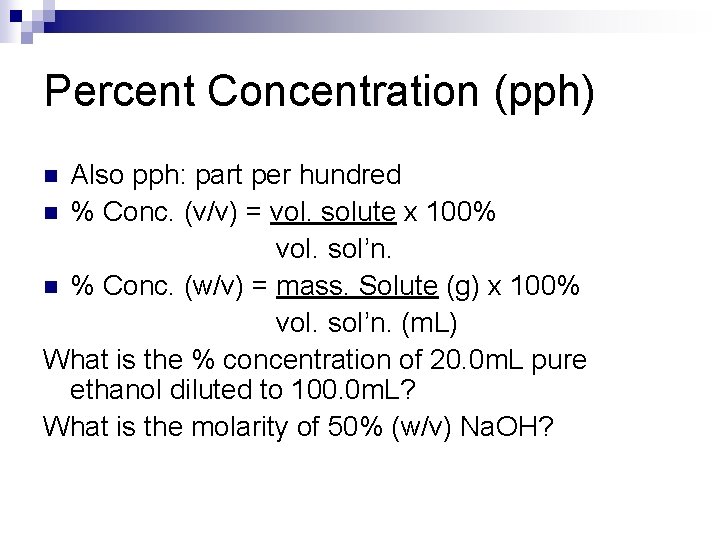
Percent Concentration (pph) Also pph: part per hundred n % Conc. (v/v) = vol. solute x 100% vol. sol’n. n % Conc. (w/v) = mass. Solute (g) x 100% vol. sol’n. (m. L) What is the % concentration of 20. 0 m. L pure ethanol diluted to 100. 0 m. L? What is the molarity of 50% (w/v) Na. OH? n

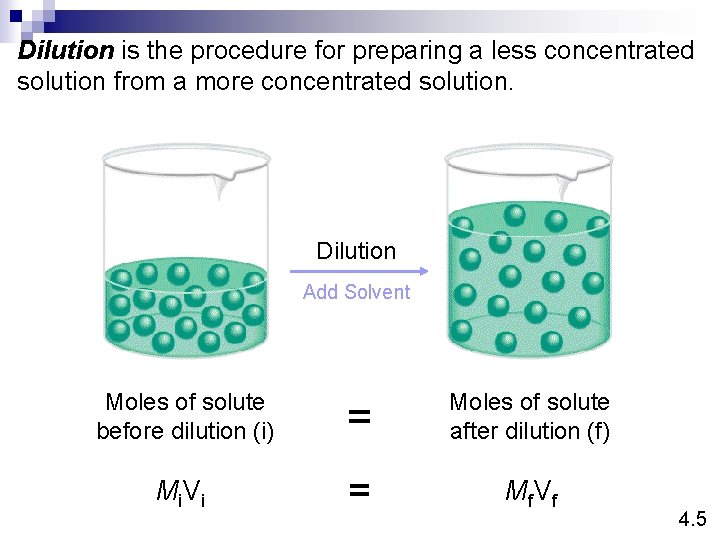
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f 4. 5

How would you prepare 60. 0 m. L of 0. 2 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi V i = Mf V f 4. 5

Test Yourself 1. 2. I have 20. 0 L of 1. 00 M HCl. If I need 0. 500 M HCl, to what final volume must I add water to the HCl I have? I need to make 200. 0 m. L of 0. 350 M Na. OH. I have an unlimited supply of water and stock 0. 50 M Na. OH. What must I do?

Test Yourself: Solution Stoichiometry 1. 2. Lead (II) nitrate and potassium iodide react in a methathesis reaction to form insoluble lead (II) iodide and aqueous potassium nitrate. If I combine 10. 0 m. L of 0. 1000 M lead (II) nitrate with 5. 00 m. L of 0. 2000 M potassium iodide, what will be the mass of lead iodide produced? I produced 1. 00 g of lead (II) iodide in the reaction above by combining 10. 0 m. L of lead (II) nitrate with an excess of potassium iodide. What was the concentration of lead (II) nitrate solution?

Chemists Have Solutions II Acids and Bases

Acids Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain citric acid. Cause color changes in plant dyes. React with certain metals to produce hydrogen gas. 2 HCl (aq) + Mg (s) Mg. Cl 2 (aq) + H 2 (g) React with carbonates and bicarbonates to produce carbon dioxide gas 2 HCl (aq) + Ca. CO 3 (s) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) Aqueous acid solutions conduct electricity. 4. 3

Bases Have a bitter taste. Feel slippery. Many soaps contain bases. Cause color changes in plant dyes. Aqueous base solutions conduct electricity. 4. 3

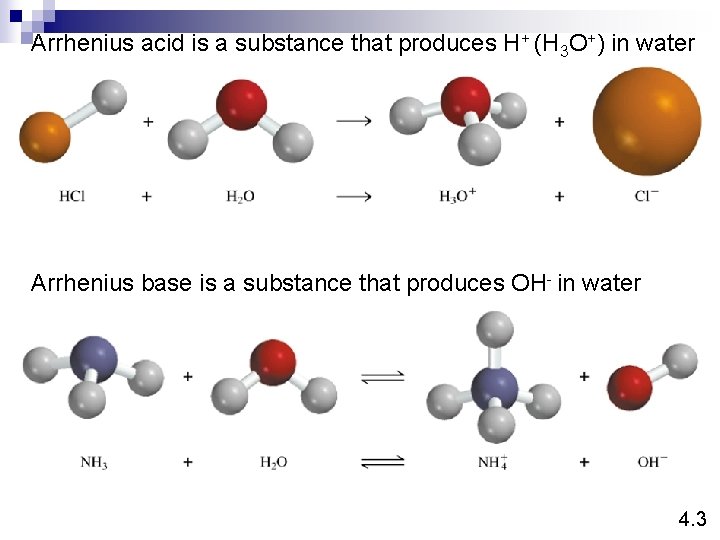
Arrhenius acid is a substance that produces H+ (H 3 O+) in water Arrhenius base is a substance that produces OH- in water 4. 3

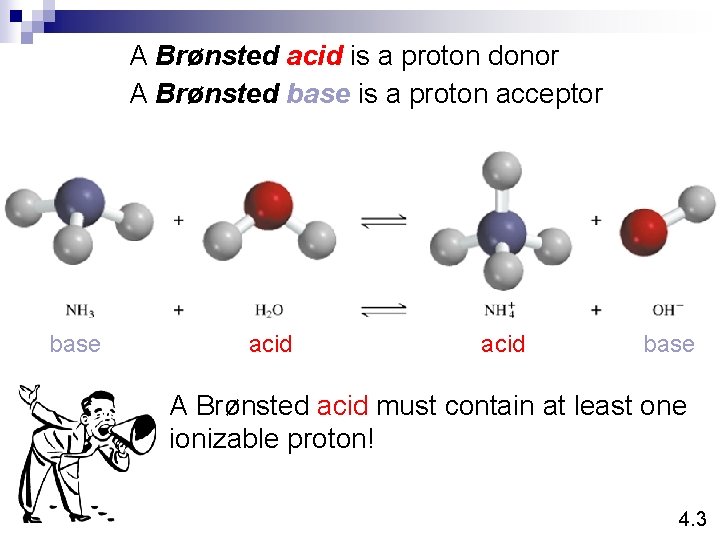
A Brønsted acid is a proton donor A Brønsted base is a proton acceptor base acid base A Brønsted acid must contain at least one ionizable proton! 4. 3

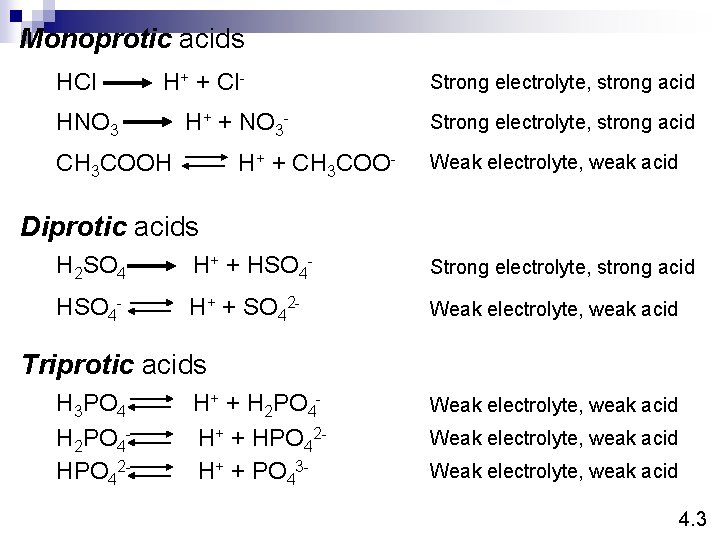
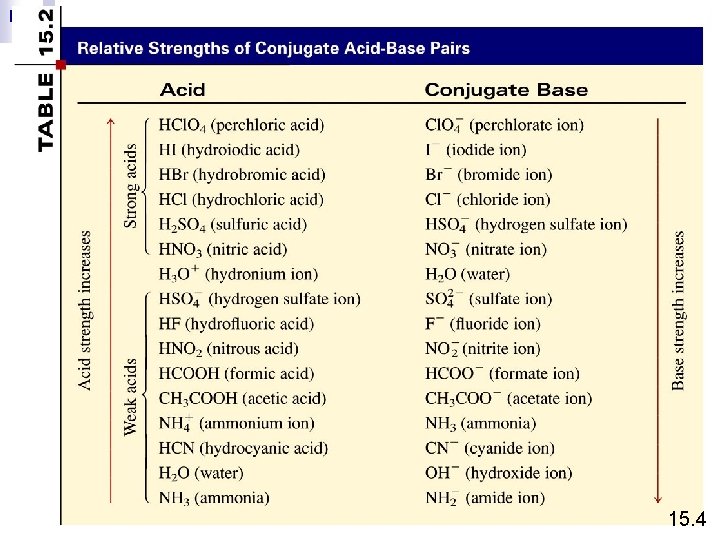
Monoprotic acids HCl H+ + Cl- HNO 3 H+ + NO 3 - CH 3 COOH H+ + CH 3 COO- Strong electrolyte, strong acid Weak electrolyte, weak acid Diprotic acids H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid 4. 3

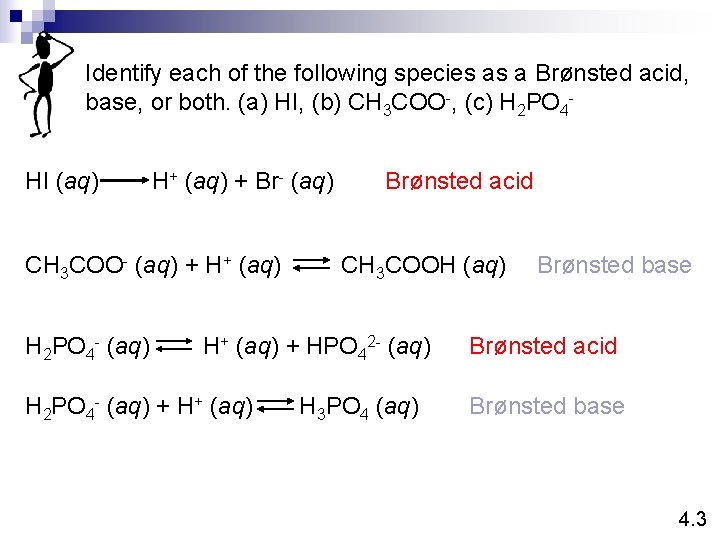
Identify each of the following species as a Brønsted acid, base, or both. (a) HI, (b) CH 3 COO-, (c) H 2 PO 4 HI (aq) H+ (aq) + Br- (aq) CH 3 COO- (aq) + H+ (aq) H 2 PO 4 - (aq) Brønsted acid CH 3 COOH (aq) H+ (aq) + HPO 42 - (aq) H 2 PO 4 - (aq) + H+ (aq) H 3 PO 4 (aq) Brønsted base Brønsted acid Brønsted base 4. 3

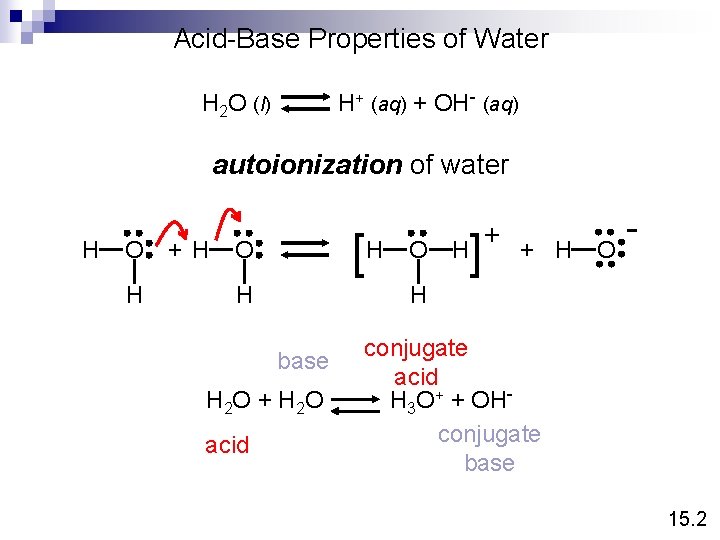
Acid-Base Properties of Water H+ (aq) + OH- (aq) H 2 O (l) autoionization of water H O H + H [H O H ] H + H O - H base H 2 O + H 2 O acid O + conjugate acid H 3 O+ + OHconjugate base 15. 2

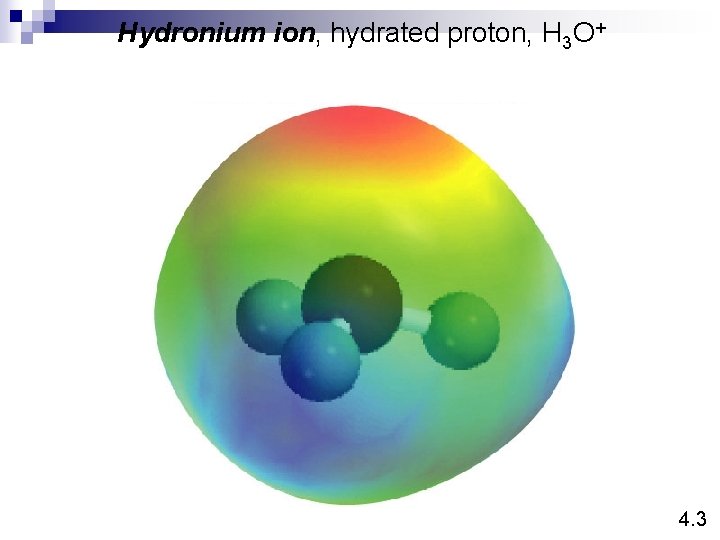
Hydronium ion, hydrated proton, H 3 O+ 4. 3

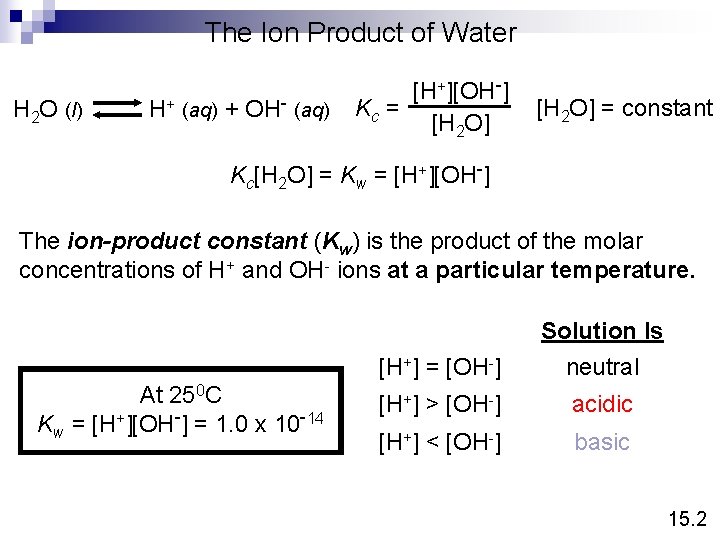
The Ion Product of Water H 2 O (l) H+ (aq) + OH- (aq) [H+][OH-] Kc = [H 2 O] = constant Kc[H 2 O] = Kw = [H+][OH-] The ion-product constant (Kw) is the product of the molar concentrations of H+ and OH- ions at a particular temperature. At 250 C Kw = [H+][OH-] = 1. 0 x 10 -14 [H+] = [OH-] Solution Is neutral [H+] > [OH-] acidic [H+] < [OH-] basic 15. 2

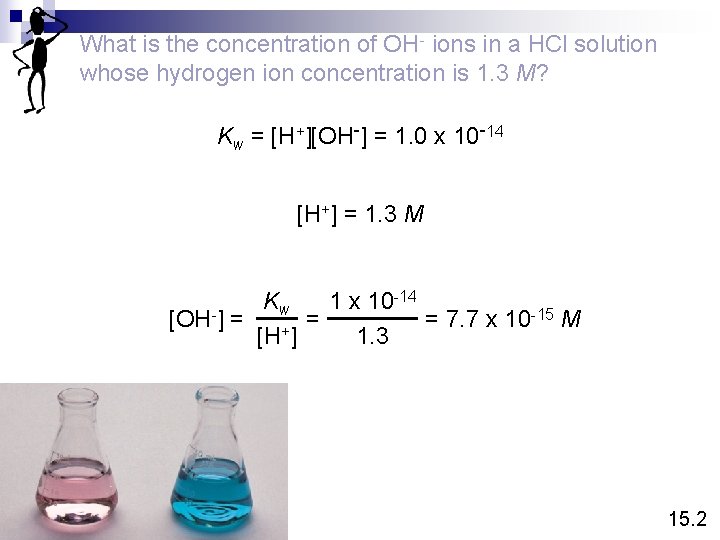
What is the concentration of OH- ions in a HCl solution whose hydrogen ion concentration is 1. 3 M? Kw = [H+][OH-] = 1. 0 x 10 -14 [H+] = 1. 3 M -14 K 1 x 10 w -15 M = = 7. 7 x 10 [OH-] = [H+] 1. 3 15. 2
![p H A Measure of Acidity p H log H Solution Is p. H – A Measure of Acidity p. H = -log [H+] Solution Is](https://slidetodoc.com/presentation_image/605441f923a44102e0d6311a18a040ff/image-31.jpg)
p. H – A Measure of Acidity p. H = -log [H+] Solution Is neutral [H+] = [OH-] At 250 C [H+] = 1 x 10 -7 p. H = 7 acidic [H+] > [OH-] [H+] > 1 x 10 -7 p. H < 7 basic [H+] < [OH-] [H+] < 1 x 10 -7 p. H > 7 p. H [H+] 15. 3
![p OH log OH HOH Kw 1 0 x 10 14 p. OH = -log [OH-] [H+][OH-] = Kw = 1. 0 x 10 -14](https://slidetodoc.com/presentation_image/605441f923a44102e0d6311a18a040ff/image-32.jpg)
p. OH = -log [OH-] [H+][OH-] = Kw = 1. 0 x 10 -14 -log [H+] – log [OH-] = 14. 00 p. H + p. OH = 14. 00 15. 3

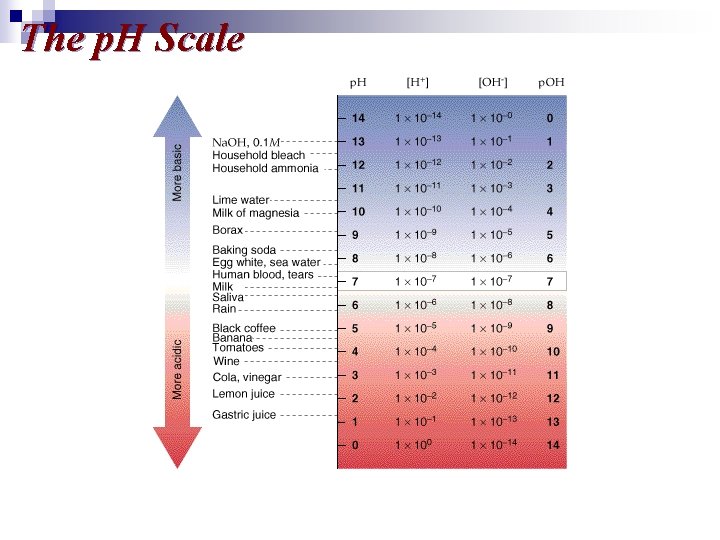
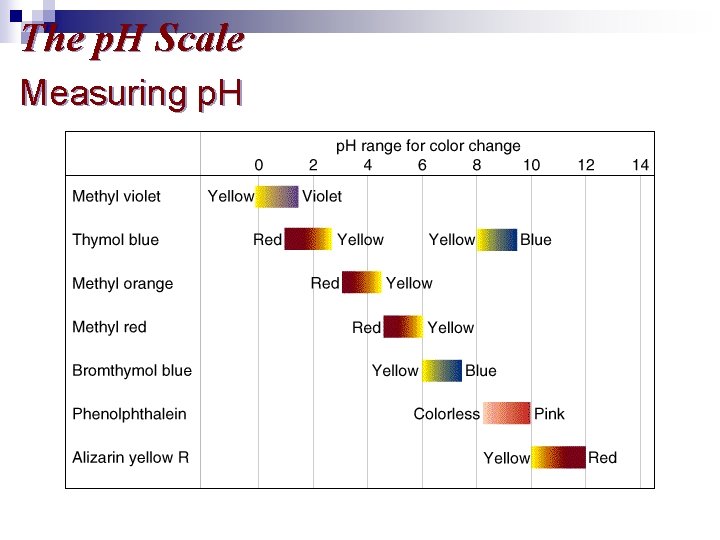
The p. H Scale

The p. H Scale Measuring p. H

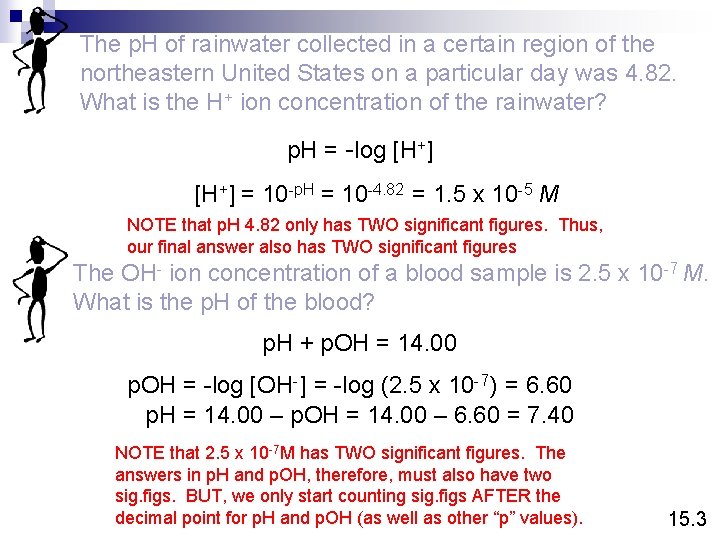
The p. H of rainwater collected in a certain region of the northeastern United States on a particular day was 4. 82. What is the H+ ion concentration of the rainwater? p. H = -log [H+] = 10 -p. H = 10 -4. 82 = 1. 5 x 10 -5 M NOTE that p. H 4. 82 only has TWO significant figures. Thus, our final answer also has TWO significant figures The OH- ion concentration of a blood sample is 2. 5 x 10 -7 M. What is the p. H of the blood? p. H + p. OH = 14. 00 p. OH = -log [OH-] = -log (2. 5 x 10 -7) = 6. 60 p. H = 14. 00 – p. OH = 14. 00 – 6. 60 = 7. 40 NOTE that 2. 5 x 10 -7 M has TWO significant figures. The answers in p. H and p. OH, therefore, must also have two sig. figs. BUT, we only start counting sig. figs AFTER the decimal point for p. H and p. OH (as well as other “p” values). 15. 3

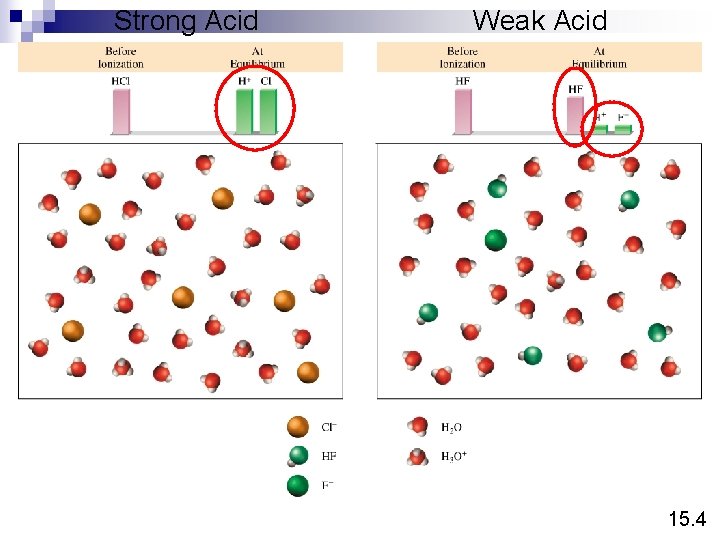
Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated CH 3 COOH CH 3 COO- (aq) + H+ (aq) Strong Acids are strong electrolytes HCl (aq) + H 2 O (l) H 3 O+ (aq) + Cl- (aq) HNO 3 (aq) + H 2 O (l) H 3 O+ (aq) + NO 3 - (aq) HCl. O 4 (aq) + H 2 O (l) H 3 O+ (aq) + Cl. O 4 - (aq) H 2 SO 4 (aq) + H 2 O (l) H 3 O+ (aq) + HSO 4 - (aq) 15. 4

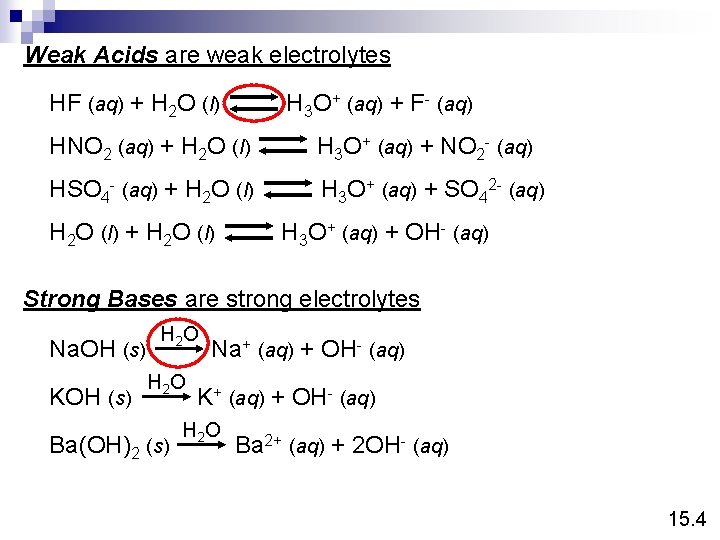
Weak Acids are weak electrolytes HF (aq) + H 2 O (l) H 3 O+ (aq) + F- (aq) HNO 2 (aq) + H 2 O (l) H 3 O+ (aq) + NO 2 - (aq) HSO 4 - (aq) + H 2 O (l) H 3 O+ (aq) + SO 42 - (aq) H 2 O (l) + H 2 O (l) H 3 O+ (aq) + OH- (aq) Strong Bases are strong electrolytes Na. OH (s) KOH (s) H 2 O Ba(OH)2 (s) Na+ (aq) + OH- (aq) K+ (aq) + OH- (aq) H 2 O Ba 2+ (aq) + 2 OH- (aq) 15. 4

Weak Bases are weak electrolytes F- (aq) + H 2 O (l) NO 2 - (aq) + H 2 O (l) OH- (aq) + HF (aq) OH- (aq) + HNO 2 (aq) Conjugate acid-base pairs: • The conjugate base of a strong acid has no measurable strength. • H 3 O+ is the strongest acid that can exist in aqueous solution. • The OH- ion is the strongest base that can exist in aqeous solution. 15. 4

15. 4

Strong Acid Weak Acid 15. 4

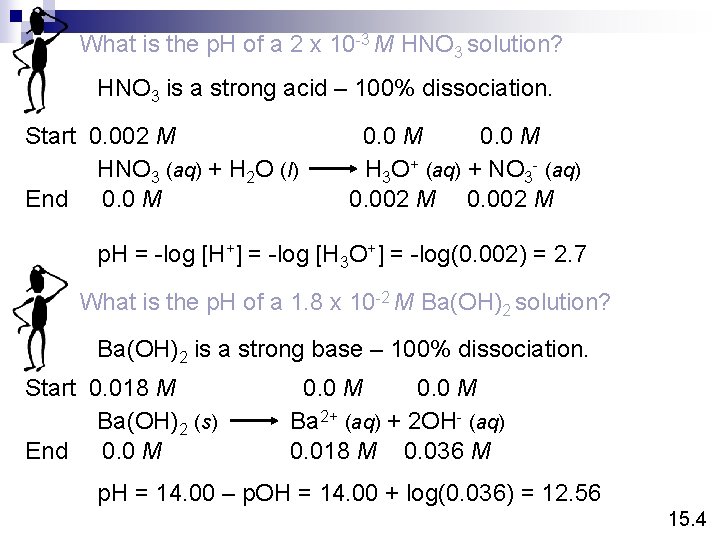
What is the p. H of a 2 x 10 -3 M HNO 3 solution? HNO 3 is a strong acid – 100% dissociation. Start 0. 002 M HNO 3 (aq) + H 2 O (l) End 0. 0 M H 3 O+ (aq) + NO 3 - (aq) 0. 002 M p. H = -log [H+] = -log [H 3 O+] = -log(0. 002) = 2. 7 What is the p. H of a 1. 8 x 10 -2 M Ba(OH)2 solution? Ba(OH)2 is a strong base – 100% dissociation. Start 0. 018 M Ba(OH)2 (s) End 0. 0 M Ba 2+ (aq) + 2 OH- (aq) 0. 018 M 0. 036 M p. H = 14. 00 – p. OH = 14. 00 + log(0. 036) = 12. 56 15. 4

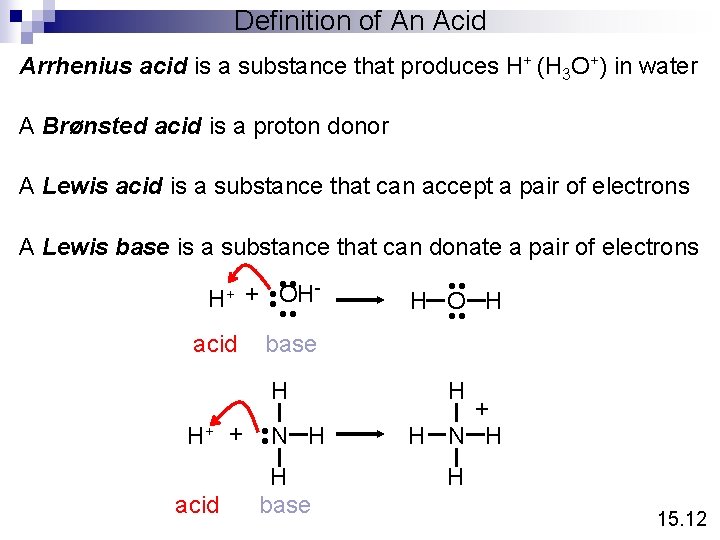
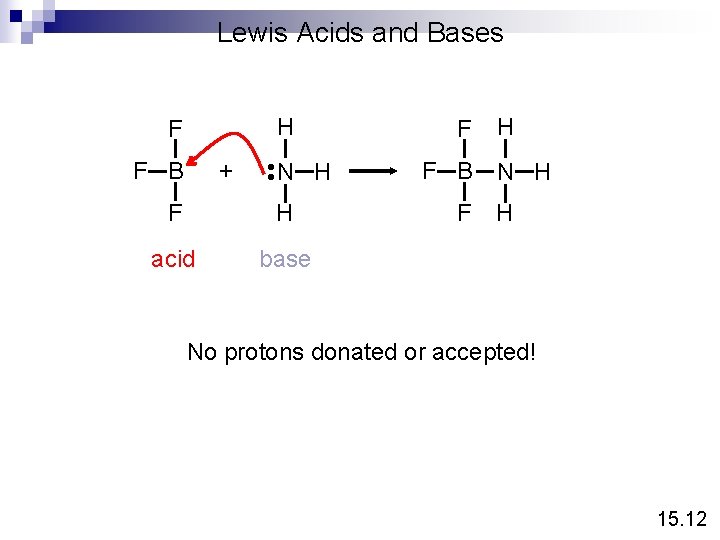
Definition of An Acid Arrhenius acid is a substance that produces H+ (H 3 O+) in water A Brønsted acid is a proton donor A Lewis acid is a substance that can accept a pair of electrons A Lewis base is a substance that can donate a pair of electrons • • H+ + OH • • acid base H acid N H • • H+ + H base • • H O H • • H + H N H H 15. 12

Lewis Acids and Bases H + F B N H • • F F H acid base F F B F H N H H No protons donated or accepted! 15. 12

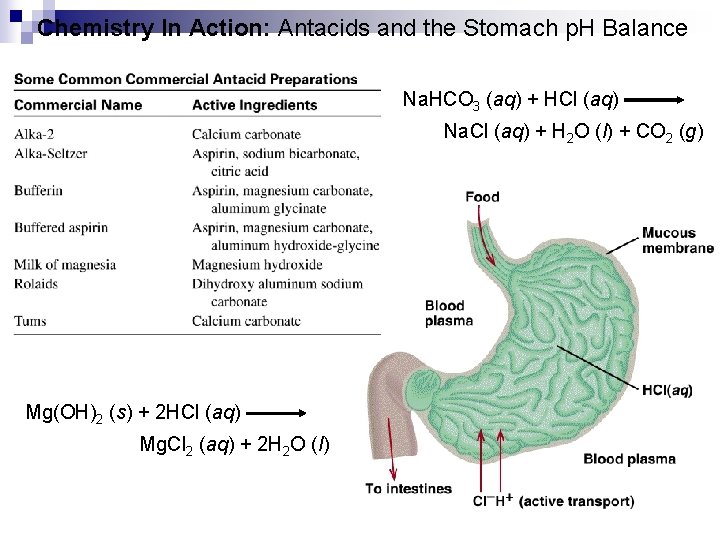
Chemistry In Action: Antacids and the Stomach p. H Balance Na. HCO 3 (aq) + HCl (aq) Na. Cl (aq) + H 2 O (l) + CO 2 (g) Mg(OH)2 (s) + 2 HCl (aq) Mg. Cl 2 (aq) + 2 H 2 O (l)

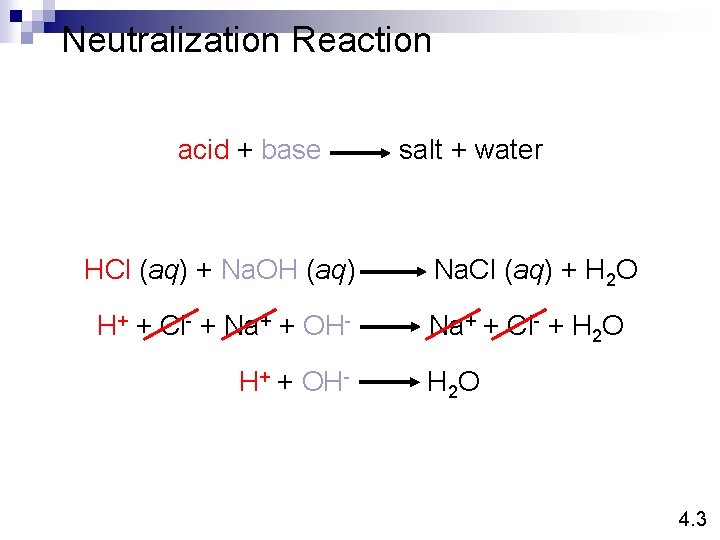
Neutralization Reaction acid + base HCl (aq) + Na. OH (aq) H+ + Cl- + Na+ + OHH+ + OH- salt + water Na. Cl (aq) + H 2 O Na+ + Cl- + H 2 O 4. 3

Sample Problem Sodium hydroxide reacts with HCl in a neutralization reaction. If I mix 30 m. L of 0. 2000 M Na. OH and 50 m. L of 0. 1000 M HCl, how many moles of Na. Cl will be produced? Follow-up question: What will be the final concentration of this salt? (Take note of the final volume of the combined solution. ) 1.

Test yourself 1. 2. 3. 4. What will be the p. H of a 0. 000211 M HCl solution? I dissolved 1. 00 g of Na. OH in 1. 000 L solution. What will be the final p. H? What volume of 0. 2000 M Na. OH is needed to completely neutralize 20. 00 m. L of 0. 1000 M HCl? If I evaporate the end product, what mass of salt will I obtain? I combined 10. 00 m. L 0. 2000 M Na. OH with 30. 00 m. L 0. 2000 M HCl. What will be the final p. H of the final solution created? (TIP: The final p. H will be based on any unreacted H+ or OH-. Assume all volumes are simply additive. )

Chemists Have Solutions III Redox Reactions

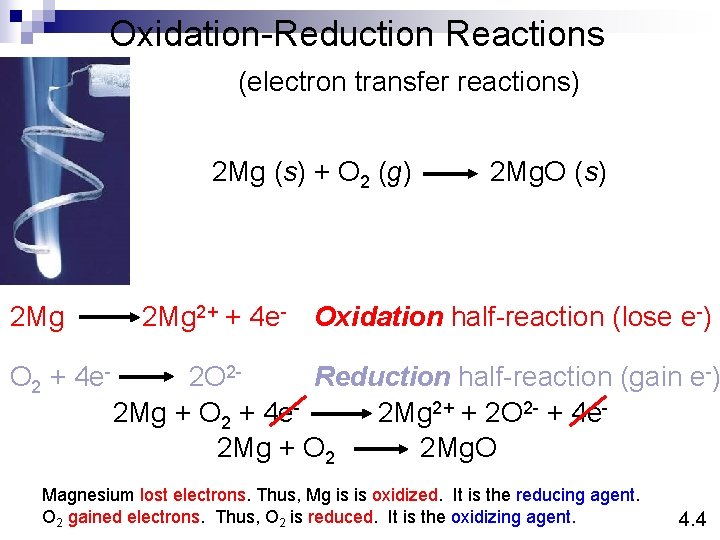
Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg (s) + O 2 (g) 2 Mg O 2 + 4 e- 2 Mg. O (s) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg + O 2 2 Mg. O Magnesium lost electrons. Thus, Mg is is oxidized. It is the reducing agent. O 2 gained electrons. Thus, O 2 is reduced. It is the oxidizing agent. 4. 4

Some Mnemonics n OIL RIG ¨ Oxidation electrons n Is Loss of electrons, Reduction Is Gain of LEO GER ¨ Loss of Electrons is Oxidation. Gain of Electrons is Reduction n n What is Oxidized is the reducing agent What is Reduced is the oxidizing agent

n The earlier definition involved electrons. Looking at simple reactions, we also find some trends. Usually: ¨ If a substance GAINS OXYGEN atoms, there is OXIDATION (naturally) ¨ If a substance GAINS HYDROGEN atoms, there is REDUCTION

4. 4

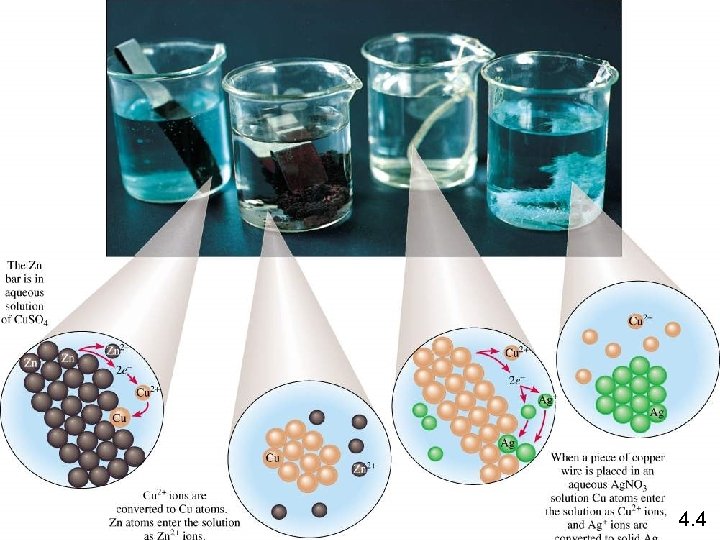
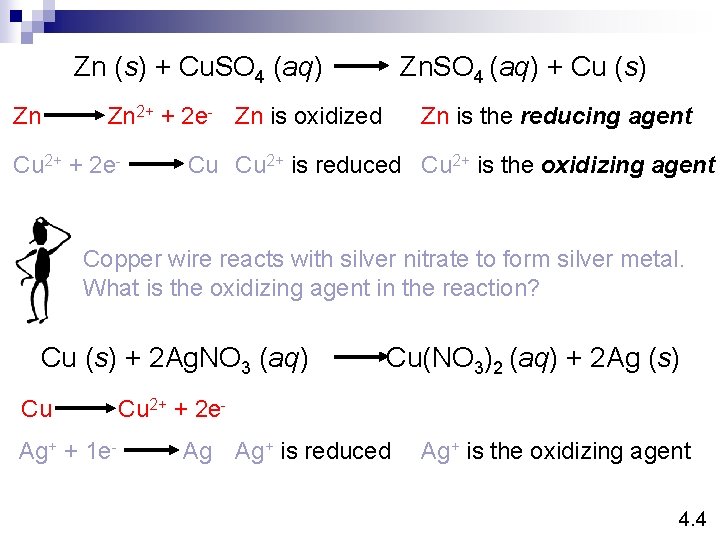
Zn (s) + Cu. SO 4 (aq) Zn Zn. SO 4 (aq) + Cu (s) Zn is the reducing agent Zn 2+ + 2 e- Zn is oxidized Cu 2+ + 2 e- Cu Cu 2+ is reduced Cu 2+ is the oxidizing agent Copper wire reacts with silver nitrate to form silver metal. What is the oxidizing agent in the reaction? Cu (s) + 2 Ag. NO 3 (aq) Cu Ag+ + 1 e- Cu(NO 3)2 (aq) + 2 Ag (s) Cu 2+ + 2 e. Ag Ag+ is reduced Ag+ is the oxidizing agent 4. 4

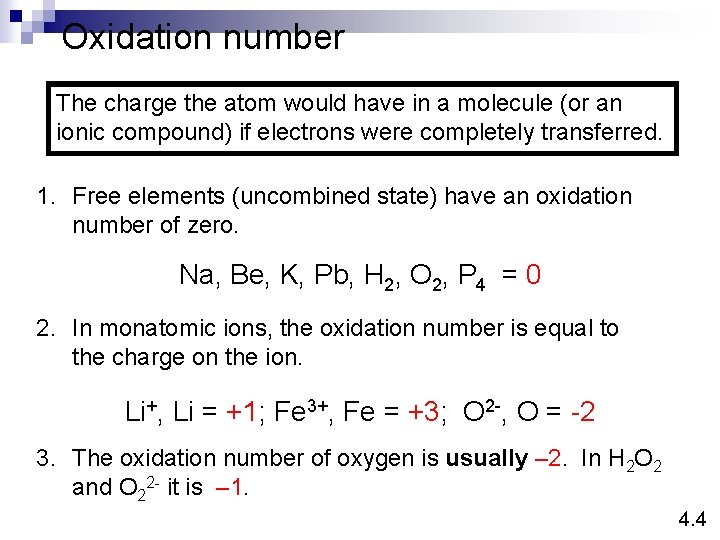
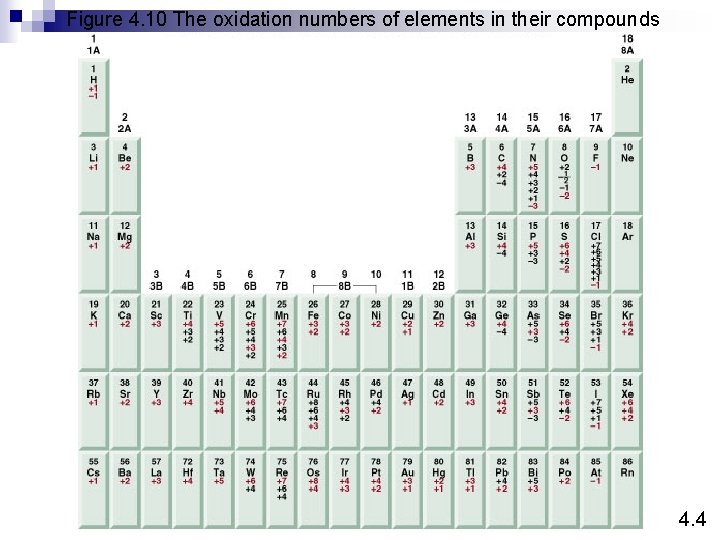
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1. 4. 4

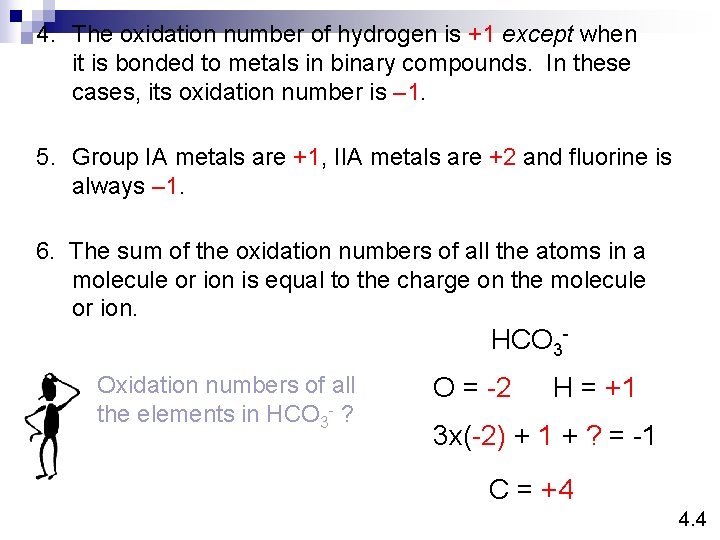
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. HCO 3 Oxidation numbers of all the elements in HCO 3 - ? O = -2 H = +1 3 x(-2) + 1 + ? = -1 C = +4 4. 4

Figure 4. 10 The oxidation numbers of elements in their compounds 4. 4

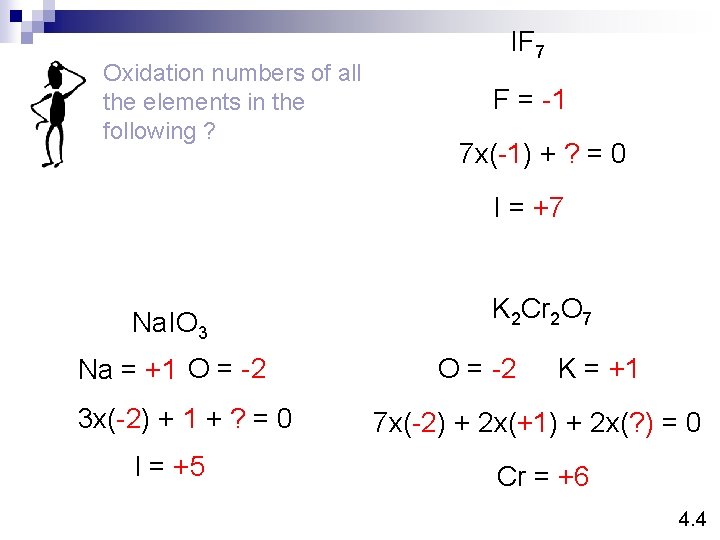
Oxidation numbers of all the elements in the following ? IF 7 F = -1 7 x(-1) + ? = 0 I = +7 Na. IO 3 Na = +1 O = -2 3 x(-2) + 1 + ? = 0 I = +5 K 2 Cr 2 O 7 O = -2 K = +1 7 x(-2) + 2 x(+1) + 2 x(? ) = 0 Cr = +6 4. 4

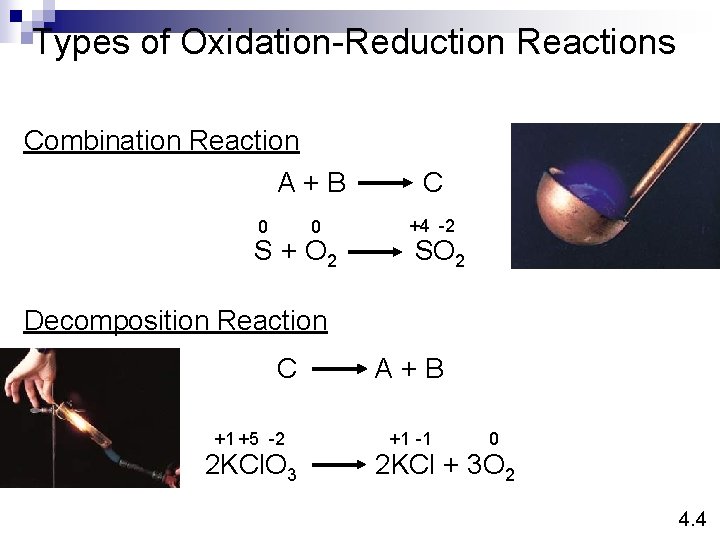
Types of Oxidation-Reduction Reactions Combination Reaction A+B C 0 +4 -2 0 S + O 2 SO 2 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2 4. 4

Types of Oxidation-Reduction Reactions Displacement Reaction (Examples here all single displacement rxns) A + BC 0 +1 Sr + 2 H 2 O +4 0 Ti. Cl 4 + 2 Mg 0 -1 Cl 2 + 2 KBr AC + B +2 0 Sr(OH)2 + H 2 Hydrogen Displacement 0 +2 Ti + 2 Mg. Cl 2 Metal Displacement -1 0 2 KCl + Br 2 Halogen Displacement 4. 4

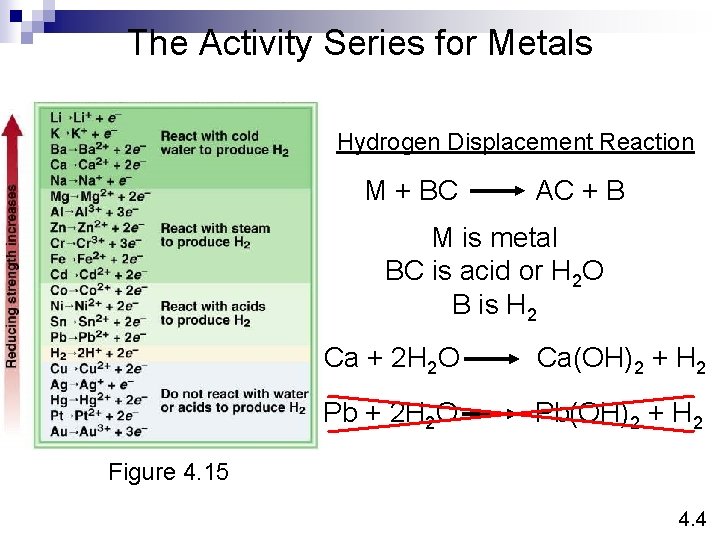
The Activity Series for Metals Hydrogen Displacement Reaction M + BC AC + B M is metal BC is acid or H 2 O B is H 2 Ca + 2 H 2 O Ca(OH)2 + H 2 Pb + 2 H 2 O Pb(OH)2 + H 2 Figure 4. 15 4. 4

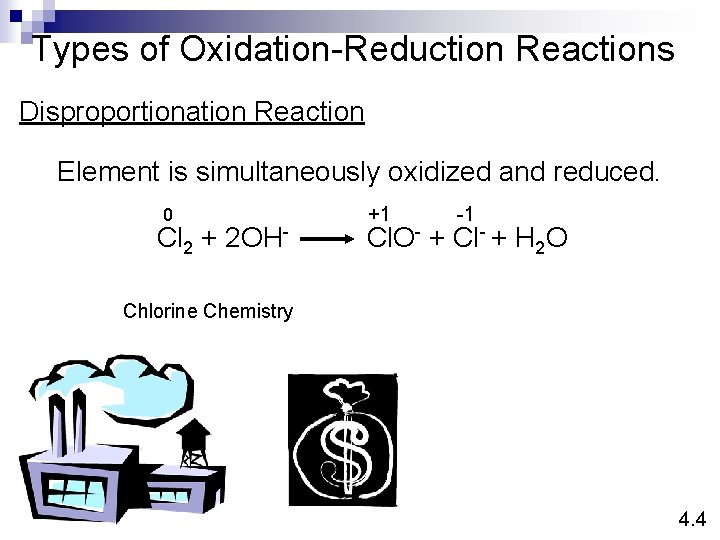
Types of Oxidation-Reduction Reactions Disproportionation Reaction Element is simultaneously oxidized and reduced. 0 Cl 2 + 2 OH- +1 -1 Cl. O- + Cl- + H 2 O Chlorine Chemistry 4. 4

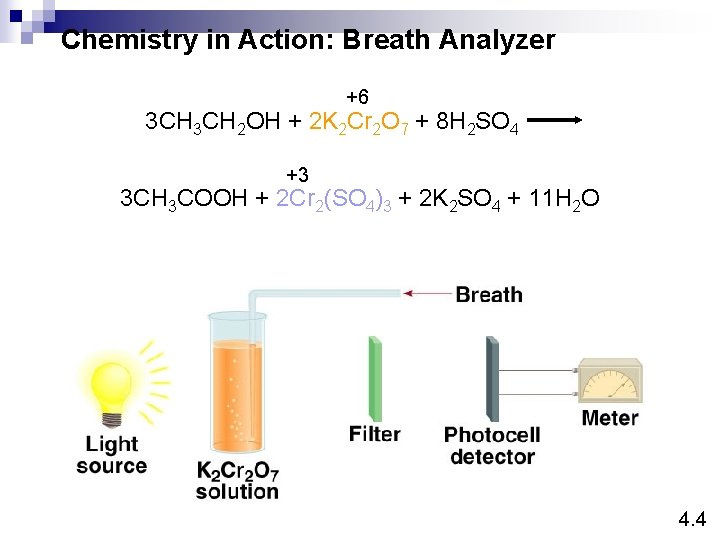
Chemistry in Action: Breath Analyzer +6 3 CH 2 OH + 2 K 2 Cr 2 O 7 + 8 H 2 SO 4 +3 3 CH 3 COOH + 2 Cr 2(SO 4)3 + 2 K 2 SO 4 + 11 H 2 O 4. 4
 A chemists shorthand way of representing chemical reaction.
A chemists shorthand way of representing chemical reaction. A chemistry shorthand way of representing chemical reaction
A chemistry shorthand way of representing chemical reaction The periodic table displays the symbols and
The periodic table displays the symbols and A chemist shorthand way
A chemist shorthand way Why do chemists use the mole
Why do chemists use the mole Chemists open
Chemists open How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Metallic bond formula
Metallic bond formula Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? Vapour pressure
Vapour pressure Molarity equation examples
Molarity equation examples Solution equation chemistry
Solution equation chemistry Solution chemistry definition
Solution chemistry definition Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry 5 taste
5 taste Regents chemistry solutions practice problems
Regents chemistry solutions practice problems Chapter 12 solutions chemistry
Chapter 12 solutions chemistry Chapter 13 solutions chemistry
Chapter 13 solutions chemistry Modern chemistry solutions
Modern chemistry solutions Ap chemistry solutions
Ap chemistry solutions Living by chemistry solutions
Living by chemistry solutions Dimensional analysis worksheet 2
Dimensional analysis worksheet 2 Dimensional analysis metric conversions worksheet
Dimensional analysis metric conversions worksheet What is catalystfive
What is catalystfive Chemistry in biology section 3 water and solutions
Chemistry in biology section 3 water and solutions Which shape has 6 faces 12 edges and 8 vertices
Which shape has 6 faces 12 edges and 8 vertices Words have meaning and names have power
Words have meaning and names have power Does congress have the power to stop mail on saturdays
Does congress have the power to stop mail on saturdays Judge past tense
Judge past tense Samuel they have rejected me
Samuel they have rejected me I have decided i have resolved
I have decided i have resolved Ideas have consequences bad ideas have victims
Ideas have consequences bad ideas have victims Presente simple en ingles
Presente simple en ingles I have four legs and a tail i have no teeth
I have four legs and a tail i have no teeth Past simple can't
Past simple can't What is an echinoderm
What is an echinoderm Wireless guest access solution
Wireless guest access solution Computer organization and architecture 10th solution
Computer organization and architecture 10th solution Solubility curve
Solubility curve How to make a standard solution
How to make a standard solution Specific heat of syrup
Specific heat of syrup Gaseous solution
Gaseous solution Spherical wave equation
Spherical wave equation Dilute and concentrated
Dilute and concentrated Warehouse inventory management solution (wims) nulled
Warehouse inventory management solution (wims) nulled Vendor signage
Vendor signage Inverse function in real life
Inverse function in real life Gamification solution dynamics 365
Gamification solution dynamics 365 What is volumetric titration
What is volumetric titration How to solve molarity
How to solve molarity Suspensions examples
Suspensions examples Is fizzy drink a solution colloid or suspension
Is fizzy drink a solution colloid or suspension Tonicity foldable
Tonicity foldable Transition words for text structures
Transition words for text structures Suspension vs solution
Suspension vs solution Market australia unfranchise review
Market australia unfranchise review Samsung confidential
Samsung confidential Problem and solution signal words
Problem and solution signal words Singular solution of ode
Singular solution of ode Problem solution paragraphs
Problem solution paragraphs What is coring in pharmacy
What is coring in pharmacy Locker problem solution
Locker problem solution