Solution Chemistry Solutions A solution is a homogeneous































- Slides: 53

Solution Chemistry

Solutions • A solution is a homogeneous mixture of two or more substances in a single phase of matter. • Examples of solutions include salt water, air and alloys. • The dissolving medium (water) in a solution is called the solvent. • The substance dissolved (salt) in a solution is called the solute. • The solute is generally designated as that component of a solution that is of lesser quantity.

Examples • If we had a mixture of 25 m. L of ethanol and 75 m. L of water, the ethanol would be the solute and water would be the solvent. • PRACTICE: Identify the solute and solvent in a 1. 00 M Sr(NO 3)2(aq) solution.

Electrolytes • Solutions can be characterized by their electrical conductivity. • Solutions that contain strong electrolytes will conduct electricity, whereas solutions of weak or nonelectrolytes will conduct little or no electricity. • A strong electrolyte is a compound that dissociates completely in solution and produce mobile ions, such as Na. Cl. • A weak electrolyte is a compound that only partially dissociates (like vinegar, a weak acid), and a nonelectrolyte will not dissociate (an example is sugar). • http: //www. chem. iastate. edu/group/Greenbowe/sections/projectfolder/flashfiles/electro. Chem/conductivity. html

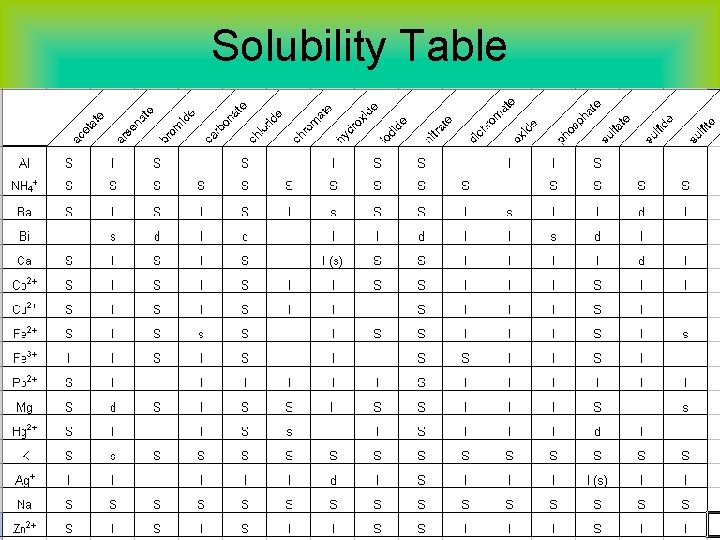
Solubility • Solubility is defined as the amount of a substance that can be dissolved in a given quantity of solvent. • Any substance whose solubility is less than 0. 01 mol/L will be referred to as insoluble. • We can predict whether a precipitate will form when solutions are mixed if we know the solubilities of different substances.

KISS (keep it simple solubility) Rules 1. All common compounds of Group I and ammonium ions are soluble. 2. All nitrates, acetates, and chlorates are soluble. 3. All halogen compounds (other than F) with metals are soluble, except those of Ag+, Hg 22+, and Pb 2+. (Pb 2+ halides are soluble in hot water. ) 4. All sulfates are soluble, except those of barium, strontium, calcium, lead, silver, and mercury (I). The latter three are slightly soluble. * Except for rules above, other ions are generally insoluble.

Solubility Table

What determines solubility? Three factors will affect solubility: #1: Nature of solute and solvent. Remember the rule: Like Dissolves Like Polar solvents (partial + or – charges) will easily dissolve charged particles or polar molecules. Nonpolar solvents (no charges, equal sharing of e-) will dissolve nonpolar solutes.

Factors Affecting Solubility #2: Temperature of solution. Generally warmer solutions will hold more solute (except for gases). #3: Pressure (gases only) on solution will increase solubility.

Sat. vs. Unsat. Solutions • A solution that cant dissolve any more solute is said to be saturated. • Additional solute will not dissolve if added to this solution. • An unsaturated solution can still hold more solute. It has not yet reached its capacity. • A supersaturated solution can be made by dissolving the solute under high temps and then carefully cooling them. These are unstable solutions and will suddenly precipitate if provoked.

The Dissolving Process

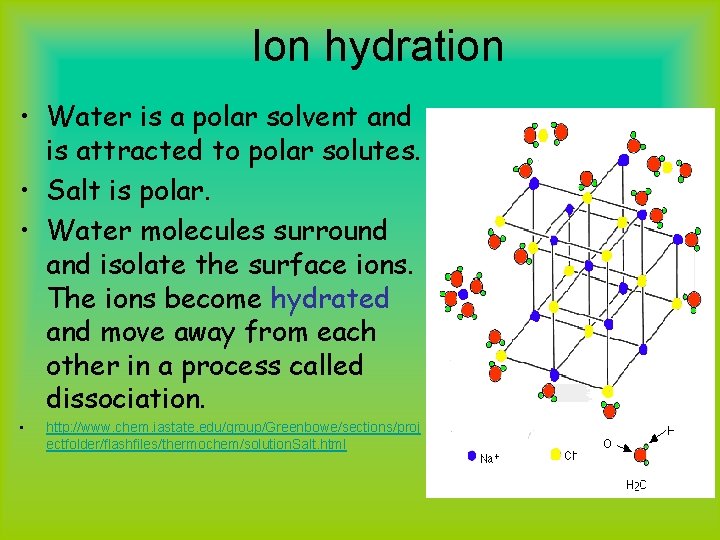
Ion hydration • Water is a polar solvent and is attracted to polar solutes. • Salt is polar. • Water molecules surround and isolate the surface ions. The ions become hydrated and move away from each other in a process called dissociation. • http: //www. chem. iastate. edu/group/Greenbowe/sections/proj ectfolder/flashfiles/thermochem/solution. Salt. html

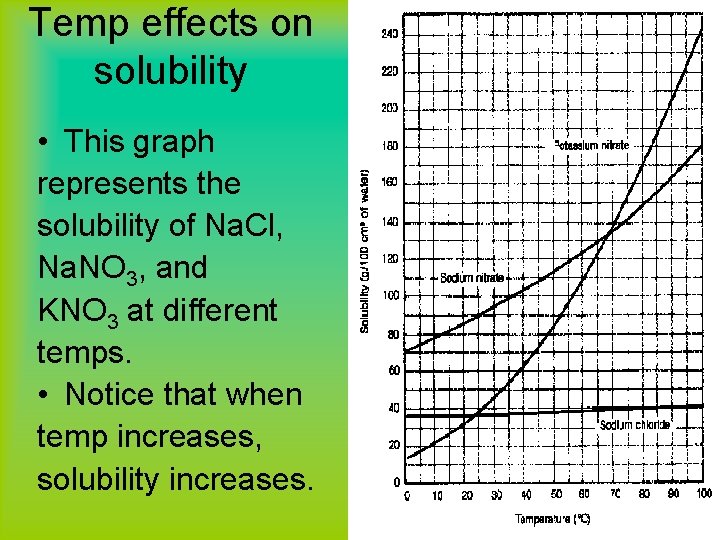
Temp effects on solubility • This graph represents the solubility of Na. Cl, Na. NO 3, and KNO 3 at different temps. • Notice that when temp increases, solubility increases.

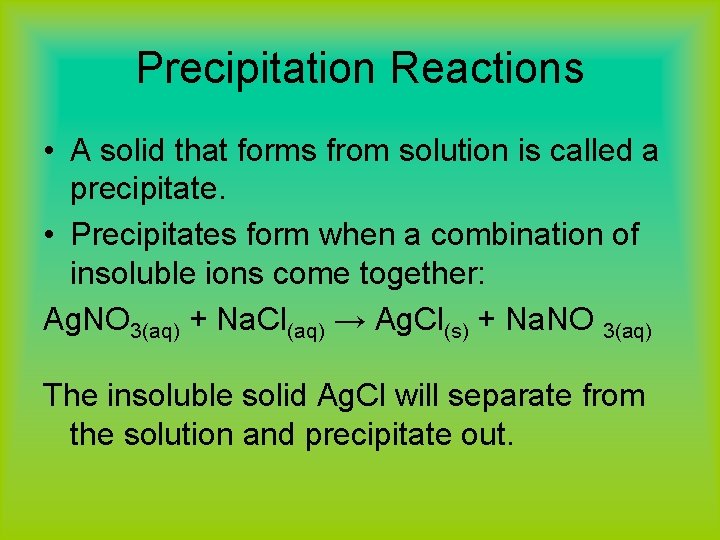
Precipitation Reactions • A solid that forms from solution is called a precipitate. • Precipitates form when a combination of insoluble ions come together: Ag. NO 3(aq) + Na. Cl(aq) → Ag. Cl(s) + Na. NO 3(aq) The insoluble solid Ag. Cl will separate from the solution and precipitate out.

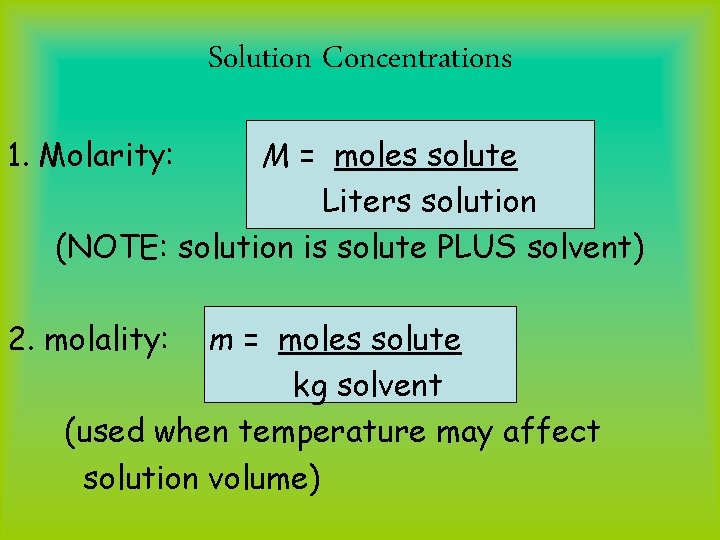
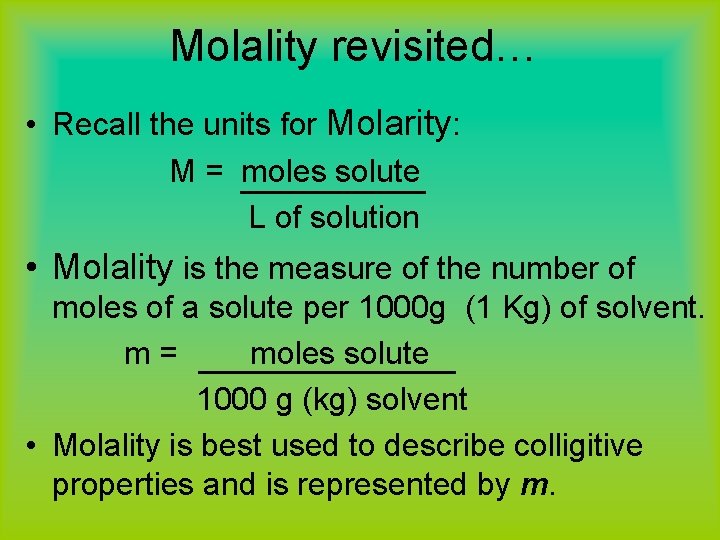
Solution Concentrations 1. Molarity: M = moles solute Liters solution (NOTE: solution is solute PLUS solvent) 2. molality: m = moles solute kg solvent (used when temperature may affect solution volume)

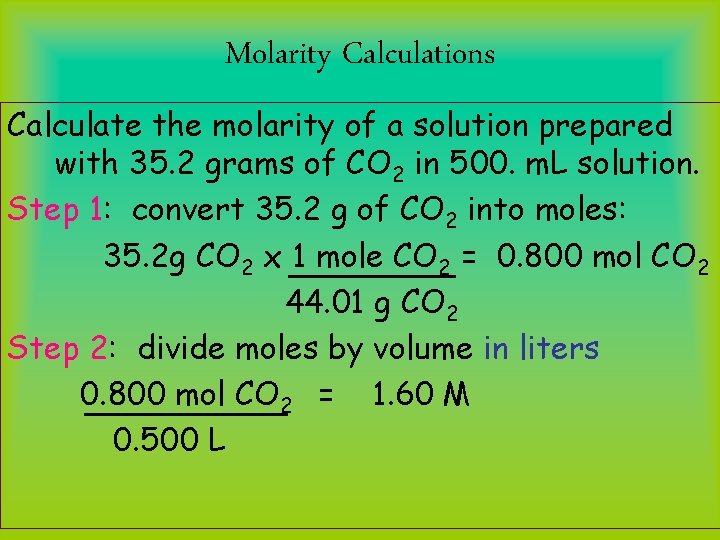
Molarity Calculations Calculate the molarity of a solution prepared with 35. 2 grams of CO 2 in 500. m. L solution. Step 1: convert 35. 2 g of CO 2 into moles: 35. 2 g CO 2 x 1 mole CO 2 = 0. 800 mol CO 2 44. 01 g CO 2 Step 2: divide moles by volume in liters 0. 800 mol CO 2 = 1. 60 M 0. 500 L

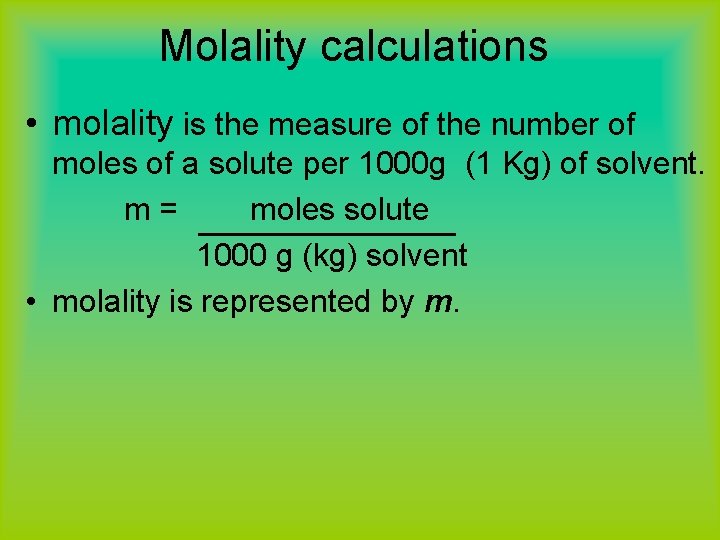
Molality calculations • molality is the measure of the number of moles of a solute per 1000 g (1 Kg) of solvent. m= moles solute 1000 g (kg) solvent • molality is represented by m.

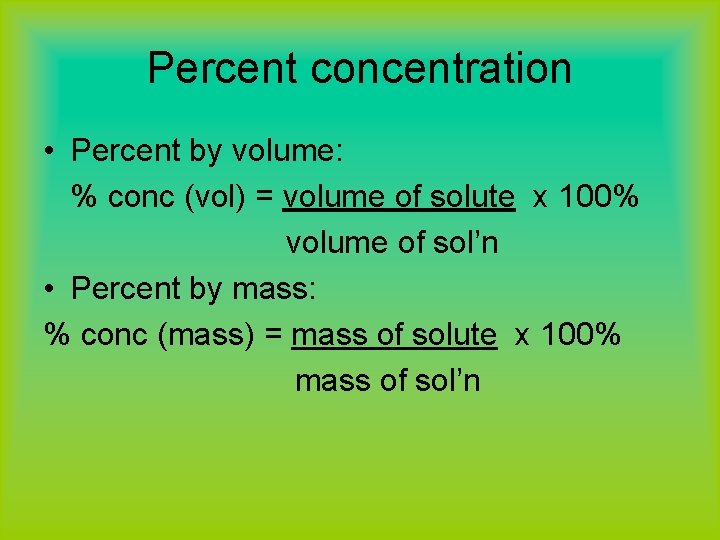
Percent concentration • Percent by volume: % conc (vol) = volume of solute x 100% volume of sol’n • Percent by mass: % conc (mass) = mass of solute x 100% mass of sol’n

Colligative Properties • Colligative comes from the Greek word kolligativ meaning glued together. • We use this term for the properties of substances (solutes and solvents) together. • Colligative properties of solutions depend only on the solvent and the concentration of the solute, not its identity.

There are 4 colligative properties: 1) 2) 3) 4) Vapor Pressure Lowering Boiling Point elevation Freezing Point Depression Osmotic Pressure Two of these properties will be investigated in the next few slides….

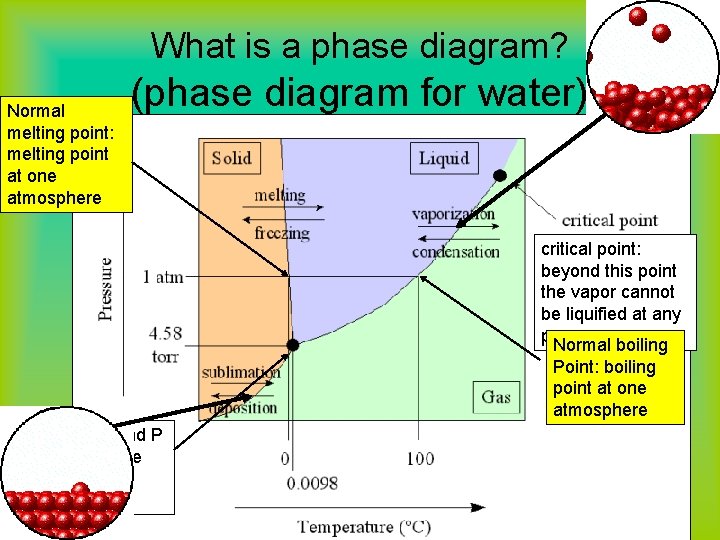
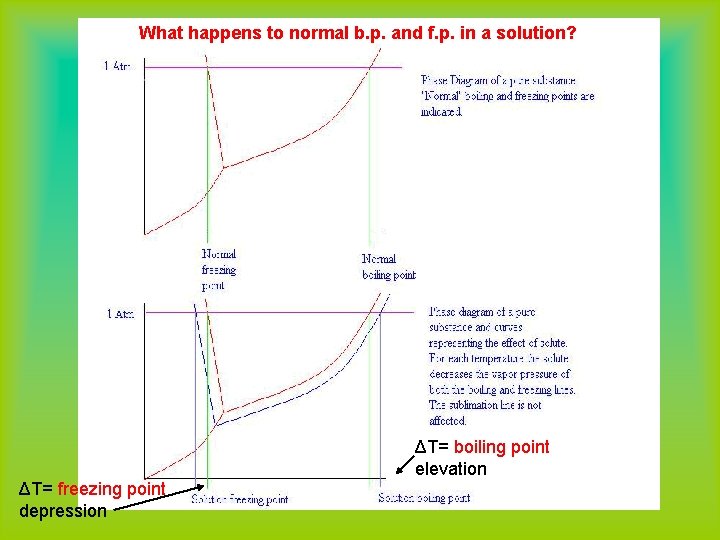
What is a phase diagram? Normal melting point: melting point at one atmosphere (phase diagram for water) critical point: beyond this point the vapor cannot be liquified at any pressure Normal boiling Point: boiling point at one atmosphere triple point: T and P at which all three states coexist in equilibrium

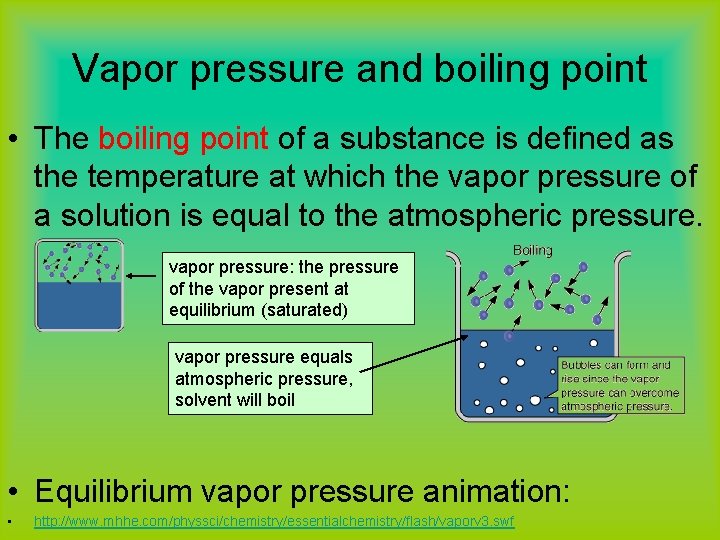
Vapor pressure and boiling point • The boiling point of a substance is defined as the temperature at which the vapor pressure of a solution is equal to the atmospheric pressure. vapor pressure: the pressure of the vapor present at equilibrium (saturated) vapor pressure equals atmospheric pressure, solvent will boil • Equilibrium vapor pressure animation: • http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/vaporv 3. swf

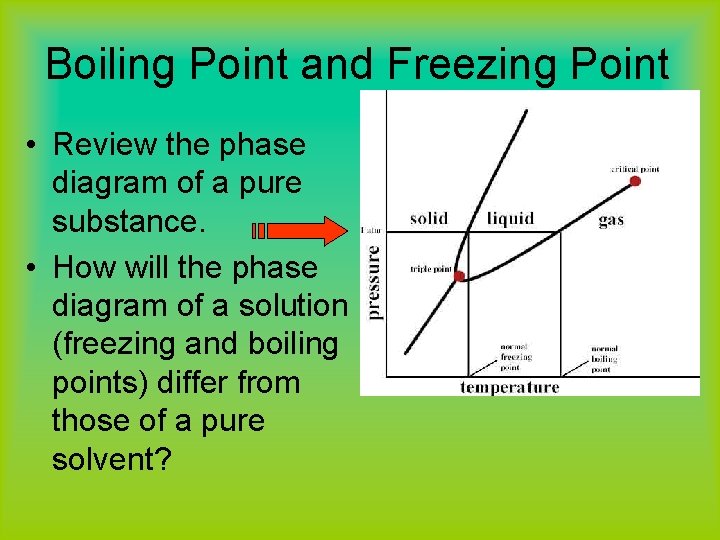
Boiling Point and Freezing Point • Review the phase diagram of a pure substance. • How will the phase diagram of a solution (freezing and boiling points) differ from those of a pure solvent?

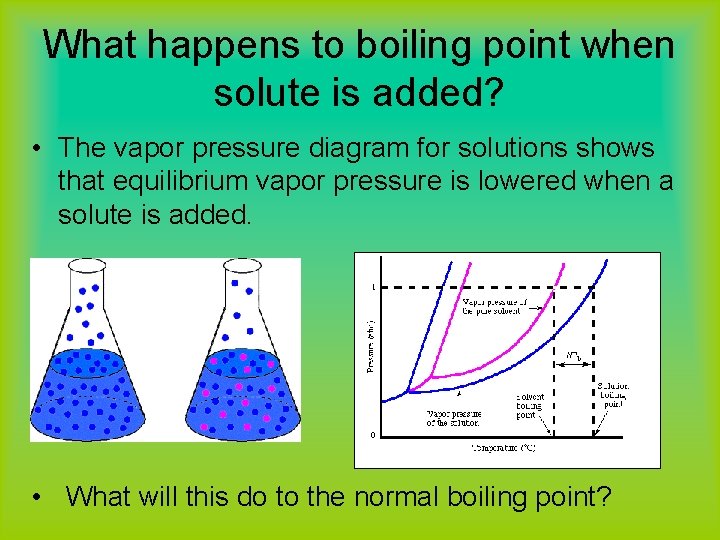
What happens to boiling point when solute is added? • The vapor pressure diagram for solutions shows that equilibrium vapor pressure is lowered when a solute is added. • What will this do to the normal boiling point?

What happens to normal b. p. and f. p. in a solution? ΔT= boiling point elevation ΔT= freezing point depression

Molality revisited… • Recall the units for Molarity: M = moles solute L of solution • Molality is the measure of the number of moles of a solute per 1000 g (1 Kg) of solvent. m= moles solute 1000 g (kg) solvent • Molality is best used to describe colligitive properties and is represented by m.

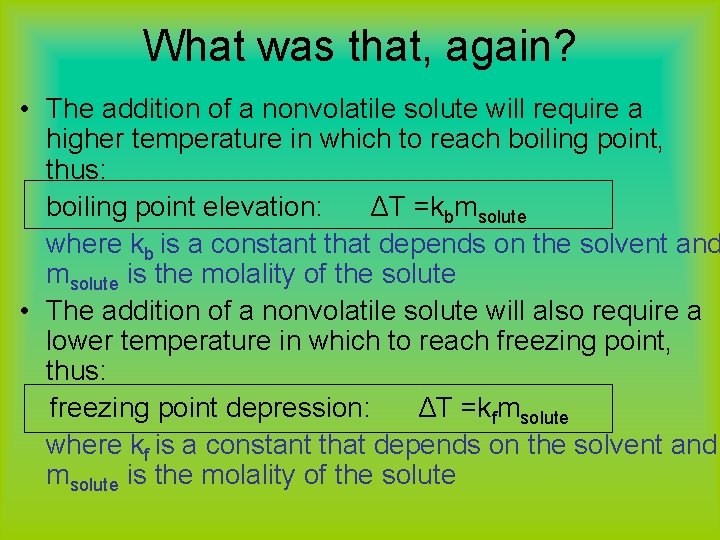
What was that, again? • The addition of a nonvolatile solute will require a higher temperature in which to reach boiling point, thus: boiling point elevation: ΔT =kbmsolute where kb is a constant that depends on the solvent and msolute is the molality of the solute • The addition of a nonvolatile solute will also require a lower temperature in which to reach freezing point, thus: freezing point depression: ΔT =kfmsolute where kf is a constant that depends on the solvent and msolute is the molality of the solute

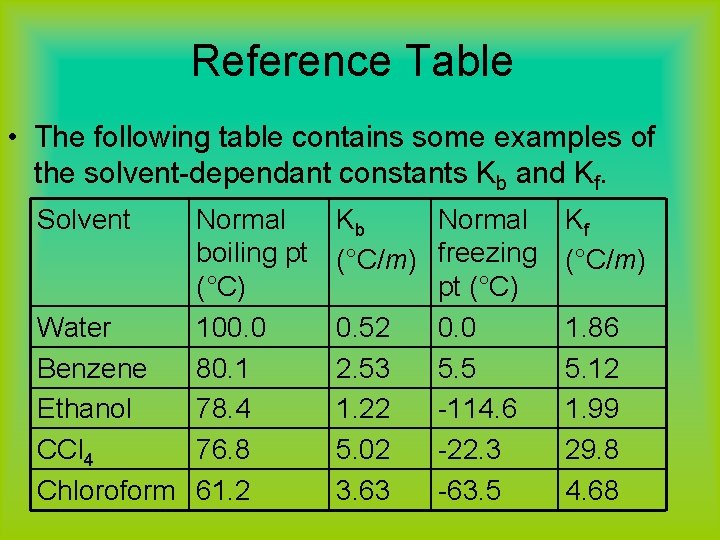
What are Kb and Kf for water? • It has been found experimentally that 1 mole of a nonvolatile solute particles will raise the boiling temperatures of 1 kg of water by 0. 52 C°. • The same concentration of solute will lower the freezing point of 1 kg of water by 1. 86 C°. • These two figures are the molal boiling point constant (Kb) and the molal freezing point constant (Kf) for dihydrogen oxide.

Reference Table • The following table contains some examples of the solvent-dependant constants Kb and Kf. Solvent Normal boiling pt (°C) Water 100. 0 Benzene 80. 1 Ethanol 78. 4 CCl 4 76. 8 Chloroform 61. 2 Kb Normal (°C/m) freezing pt (°C) 0. 52 0. 0 2. 53 5. 5 1. 22 -114. 6 5. 02 -22. 3 3. 63 -63. 5 Kf (°C/m) 1. 86 5. 12 1. 99 29. 8 4. 68

Ionic compounds and molality • A 1 m solution of sugar in water contains 1 mol of solute particles per 1 kg of solvent. It does NOT dissociate. • A 1 m solution of Na. Cl in water contains 2 mol of solute particles (because Na. Cl is completely soluble, it will dissociate in water into Na+ and Cl- ions) per 1 kg of solvent. • How many mol of solute would a 1 m calcium nitrate [Ca(NO 3)2] solution have per 1 kg of solvent? That’s right! 3 mol because of the Ca 2+ and the two NO 3 - ions.

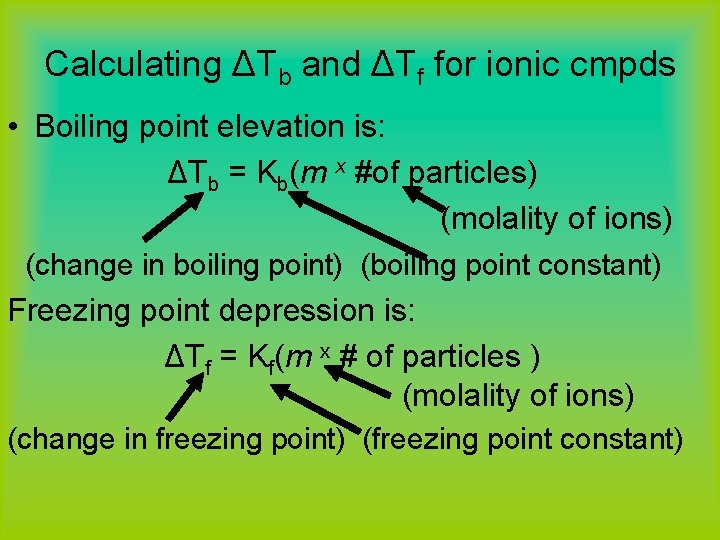
Calculating ΔTb and ΔTf for ionic cmpds • Boiling point elevation is: ΔTb = Kb(m x #of particles) (molality of ions) (change in boiling point) (boiling point constant) Freezing point depression is: ΔTf = Kf(m x # of particles ) (molality of ions) (change in freezing point) (freezing point constant)

Example Problem #1 If 55. 0 grams of glucose (C 6 H 12 O 6) are dissolved in 525 g of water, what will be the change in boiling and freezing points of the resulting solution? Step 1: Convert g of glucose to moles 55. 0 g x (1 mol) = 0. 305 mol (180. 18 g) Step 2: Convert g of water to kg 525 g 0. 525 kg Step 3: Calculate m 0. 305 mol /0. 525 kg = 0. 581 m

Continued… • Step 4: Obtain molal Kb and Kf from reference table. • Step 5: Place values into equation ΔTb = Kbm ΔTb = (0. 52°C/m)(0. 581 m) = 0. 30 ºC • This means that the boiling point will be elevated by 0. 30 °C. This solution will reach boiling point at 100. 30 °C. • But what about the change in freezing point? ?

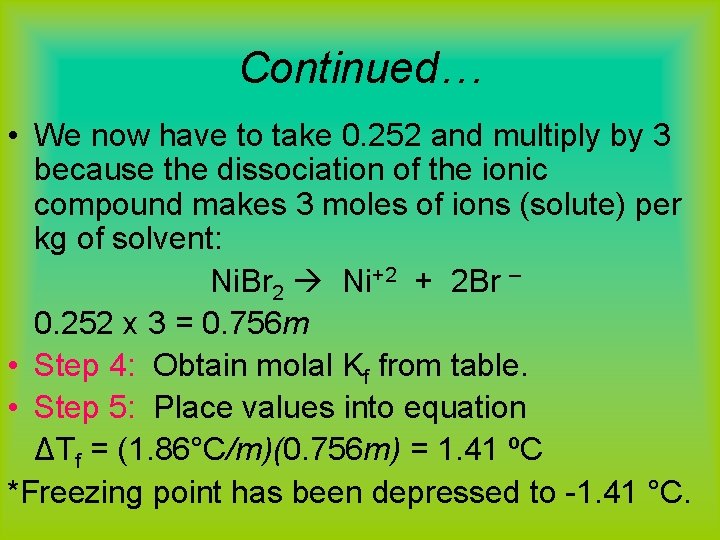
Example #2 (ionic cmpds) Calculate the change in freezing point of 24. 5 g nickel(II) bromide dissolved in 445 g of water. (assume 100% dissociation) Step 1: Convert g of Ni. Br 2 into moles: 24. 5 g x (1 mol) (218. 49 g) = 0. 112 mol Step 2: Convert solvent to kg 445 g 0. 445 kg Step 3: Calculate m 0. 112 mol/0. 445 kg = 0. 252 m

Continued… • We now have to take 0. 252 and multiply by 3 because the dissociation of the ionic compound makes 3 moles of ions (solute) per kg of solvent: Ni. Br 2 Ni+2 + 2 Br – 0. 252 x 3 = 0. 756 m • Step 4: Obtain molal Kf from table. • Step 5: Place values into equation ΔTf = (1. 86°C/m)(0. 756 m) = 1. 41 ºC *Freezing point has been depressed to -1. 41 °C.

• Coolants are used because it takes higher temperatures to reach boiling point. • Antifreeze causes fluids to need to reach lower temperatures in order to freeze. • This is also why salt is used on frozen roads and walkways. The salt dissolves in the snow and lowers the freezing point of the meltwater. It now takes colder temps to turn the water back into ice. • A 10% salt solution freezes at 20 ºF (-6 ºC), and a 20% solution freezes at 2 ºF (-16 ºC).

The End

Spectrophotometry: An Analytical Tool

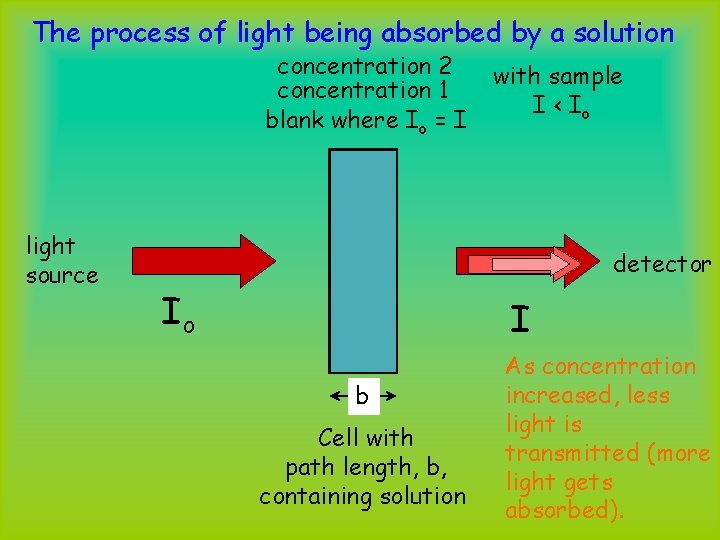
The process of light being absorbed by a solution concentration 2 concentration 1 blank where Io = I light source with sample I < Io detector Io I b Cell with path length, b, containing solution As concentration increased, less light is transmitted (more light gets absorbed).

Beer’s Law A = abc where a – molar absorptivity, b – path length, and c – molar concentration See the Beer’s Law Simulator

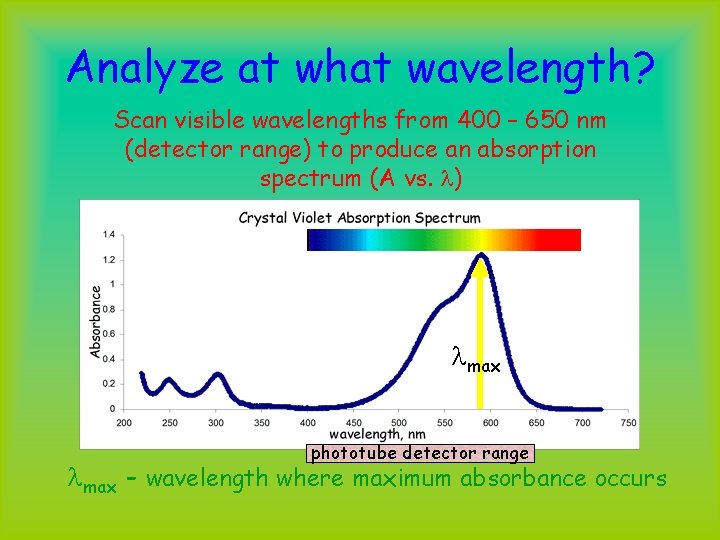
Analyze at what wavelength? Scan visible wavelengths from 400 – 650 nm (detector range) to produce an absorption spectrum (A vs. l) lmax phototube detector range lmax - wavelength where maximum absorbance occurs

The BLANK ü The blank contains all substances except the analyte. ü Is used to set the absorbance to zero: Ablank = 0 ü This removes any absorption of light due to these substances and the cell. ü Therefore, all measured absorbance is due to analyte.

The components of a Spec-20 D Light source - white light of constant intensity slits filter occluder Grating Phototube detects light & measures intensity slits Sample When blank is the sample Io is determined otherwise I is measured Separates white light into various colors Rotating the grating changes the wavelength going through the sample

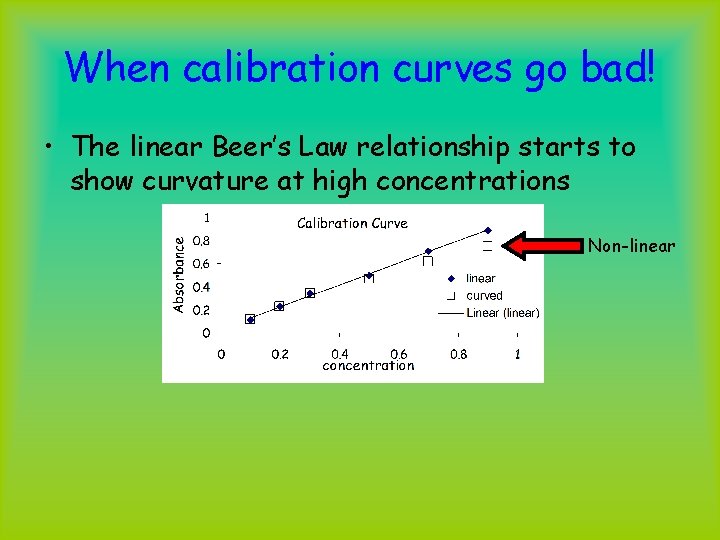
When calibration curves go bad! • The linear Beer’s Law relationship starts to show curvature at high concentrations Non-linear

Calibrating the Spec 20 • • • Plug in and turn on the Spec 20. It must warm up for 30 minutes before use. Set the instrument to the proper wavelength by turning the knob located on the right hand surface of the spectrophotometer. The wavelength setting can be seen through the window next to the knob. Obtain a properly cleaned cuvette and fill it about 3/4 full of the reference solution (water). With no cuvette in the sample holder, close the cover and rotate the zero light control knob(left front knob) to display a reading of 0. 0% transmittance. As long as this knob is not moved, no other adjustments to this control are needed. Place the reference solution cuvette in the sample holder, close the cover, and rotate the light control knob (front right knob) to display a reading of 100. 0% transmittance. This procedure must be repeated every time measurements are taken at a new wavelength.

Making Measurements • • Calibrate the instrument (see instructions on last slide) at the wavelength you wish to measure. You will use the solvent of your sample solution as your reference (this will usually be water). Fill a properly cleaned cuvette 3/4 full of you sample solution. Place your sample cuvette in the sample holder and close the cover. Read either the absorbance or percent transmittance as needed.

Chemistry of Ice Cream

When looking at ice cream at a microscopic level it is found that ice cream is made up of four phases: ice, air, fat and a concentrated aqueous solution. It is the relative amount of these phases and the interactions between them that determines the properties of the ice cream – whether soft and ‘whippy’ or hard. electron micrographs of ice cream a = air bubbles, c = ice crystals, f = fat and s = concentrated aqueous solution

ICE CREAM INGREDIENTS Today's ice cream has the following composition : a) At least 10% milk fat by legal definition, and usually between 10% and as high as 16% fat in some premium ice creams. b) between 9 and 12% milk solids (non-fat), the component which contains the proteins and carbohydrates (lactose) found in milk. c) 12% to 16% sweeteners, usually a combination of sucrose and glucose-based corn syrup sweeteners d) 0. 2% to 0. 5% added stabilizers and emulsifiers e) The rest, usually 55% to 64%, is water, which comes from the milk. Air is also whipped into ice cream to add volume and keep it from melting quickly.

ICE CREAM: FAT AND SUGAR • The fat component in ice cream adds richness of flavor and contributes to a smooth texture with creamy body. The milk solids-not-fat component also contributes to the flavor but more importantly improves the body and texture of the ice cream by offering some "chew resistance" and enhancing the ability of the ice cream to hold its air. • The sugars give ice cream its characteristic sweetness and enhance the perception of various fruit flavors. In addition, the sugars, including the lactose from the milk components, contribute to a depressed freezing point so that the ice cream has some unfrozen water associated with it at very low temperatures typical of their serving temperatures, -15 o to -18 o. C. Without this unfrozen water, the ice cream would be too hard to scoop.

ICE CREAM: STABILIZERS • The stabilizers are compounds (usually cellulose or bean gum) that are responsible for adding viscosity to the unfrozen portion of the water and thus holding this water so that it cannot migrate. This results in firmer ice cream. Without the stabilizers, the ice cream would become coarse with large ice crystals very quickly as water migrates and refreezes. • The smaller the ice crystals in the ice cream, the less detectable they are to the tongue. Especially in the distribution to supermarkets, the trunks of cars, and so on, ice cream has many opportunities to warm up, partially melt some of the ice, and then refreeze as the temperature is once again lowered. This process is known as heat shock and every time it happens, the ice cream becomes more icy tasting. Stabilizers help to prevent this.

ICE CREAM: EMULSIFIERS • Colloids are mixtures of two substances that are insoluble. Ice cream contains colloids since fat does not dissolve in water. The emulsifiers are a group of compounds in ice cream which aid in blending the fats and water. Emulsifiers are characterized by having a molecular structure which allows part of the molecule to be readily soluble in a polar compound such as water, and another part of the molecule to be more readily soluble in non-polar solvents such as fats. As a result, emulsifiers reside at the interface between fat and water.
