HOMOGENEOUS CATALYST CHANDER JASWAL ASSISTANT PROFESSOR CHEMISTRY Homogeneous














- Slides: 35

HOMOGENEOUS CATALYST CHANDER JASWAL ASSISTANT PROFESSOR CHEMISTRY

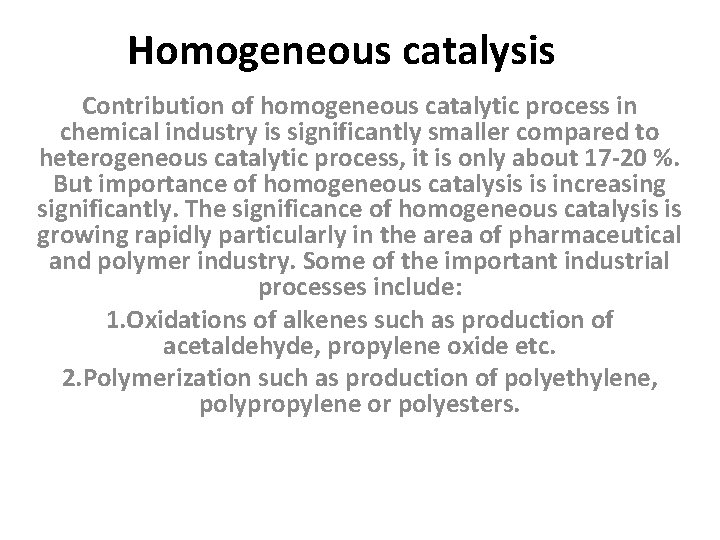
Homogeneous catalysis Contribution of homogeneous catalytic process in chemical industry is significantly smaller compared to heterogeneous catalytic process, it is only about 17 -20 %. But importance of homogeneous catalysis is increasing significantly. The significance of homogeneous catalysis is growing rapidly particularly in the area of pharmaceutical and polymer industry. Some of the important industrial processes include: 1. Oxidations of alkenes such as production of acetaldehyde, propylene oxide etc. 2. Polymerization such as production of polyethylene, polypropylene or polyesters.

• A new major development in homogeneous catalysis is the application of organometallic complexes as catalysts. The use of organometallic catalysts has revolutionized the homogeneous processes increasing economic viability. Another new area is biocatalysis involving enzymes catalysts. Enzyme catalysts are highly selective and active for production of fine chemicals, pharmaceuticals etc.

• In homogeneous catalysis, all the reactants and catalysts are present in a single fluid phase and usually in the liquid phase. Homogeneous catalysts are the simple molecules or ions such as HF, H 2 SO 4, Mn+2 as well as complex molecules such as organometallic complexes, macrocyclic compounds and large enzyme molecules.

• Advantages 1. In many reactions, homogeneous catalysts are more active and/or selective compared to heterogeneous catalysts. 2. In homogeneous catalysis, the catalysts are molecularly dispersed within the fluid. Hence, pore diffusion limitations are absent. However, bulk phase mass transfer limitation may occurs. 3. Catalytic chemistry and mechanism for homogeneous catalysis are better studied and understood. Therefore, it is easier to control and manipulate the process parameters

–. • Disadvantages • However, homogeneous processes are also associated with some major disadvantages which result in limited use of these processes. These disadvantages are summarized below: • Homogeneous catalysts are stable only in relatively mild conditions which limit their applicability. • Since the catalysts are molecularly dispersed in the phase as the reactant, products and solvents, the separation at end of the process is difficult and expensive. In many cases, it is not possible to recover the catalyst.

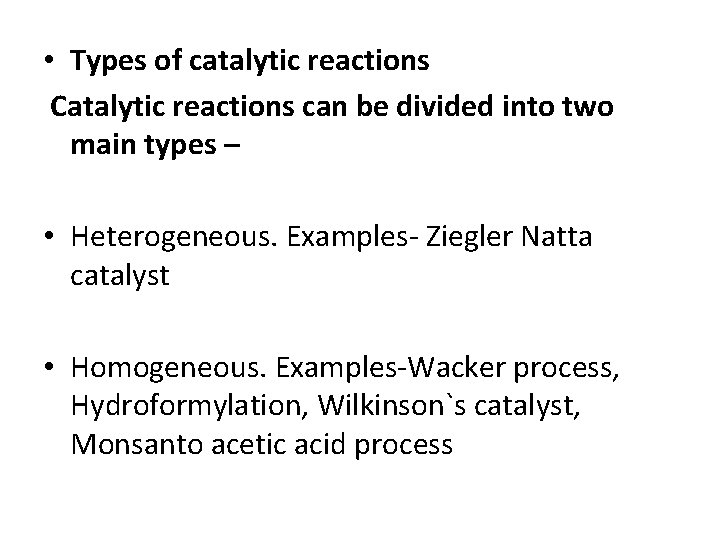
• Types of catalytic reactions Catalytic reactions can be divided into two main types – • Heterogeneous. Examples- Ziegler Natta catalyst • Homogeneous. Examples-Wacker process, Hydroformylation, Wilkinson`s catalyst, Monsanto acetic acid process

• Heterogeneous catalysis • In heterogeneous catalytic reaction, the catalyst and the reactants are in different phases. Reactions of liquid or gases in the presence of solid catalysts are the typical examples. • An example is the Contact Process for manufacturing sulphuric acid, in which the sulphur dioxide and oxygen are passed over a solid vanadium oxide catalyst producing sulphur trioxide. Several hydrocarbon transformation reactions such as cracking, reforming, dehydrogenation, isomerization also fall in this category.

• Homogeneous catalysis • In a homogeneous catalytic reaction, the catalyst is in the same phase as the reactants. Typically, all the reactants and catalysts are either in one single liquid phase or gas phase. Most industrial homogeneous catalytic processes are carried out in liquid phase. Ester hydrolysis involving general acid-base catalysts, polyethylene production with organometallic catalysts and enzyme catalyzed processes are some of the important examples of industrial homogeneous catalytic processes.

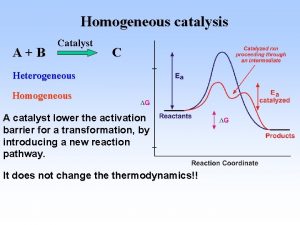
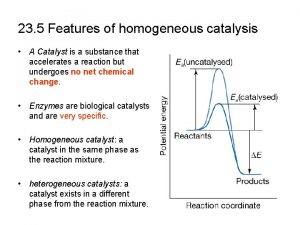
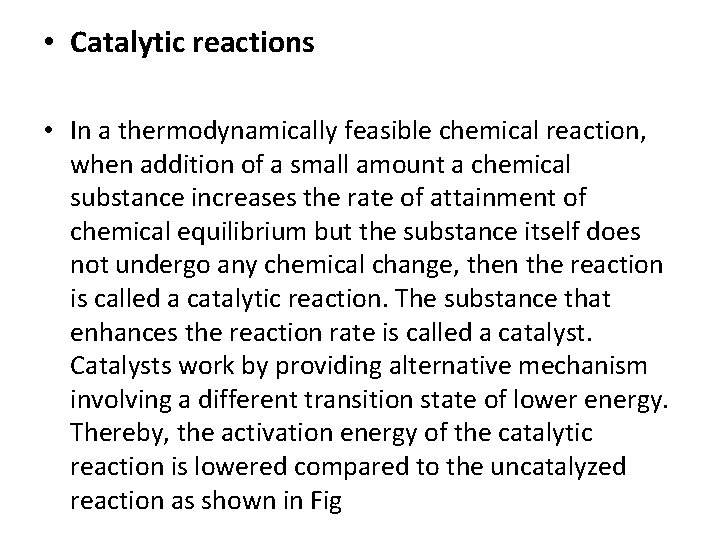
• Catalytic reactions • In a thermodynamically feasible chemical reaction, when addition of a small amount a chemical substance increases the rate of attainment of chemical equilibrium but the substance itself does not undergo any chemical change, then the reaction is called a catalytic reaction. The substance that enhances the reaction rate is called a catalyst. Catalysts work by providing alternative mechanism involving a different transition state of lower energy. Thereby, the activation energy of the catalytic reaction is lowered compared to the uncatalyzed reaction as shown in Fig


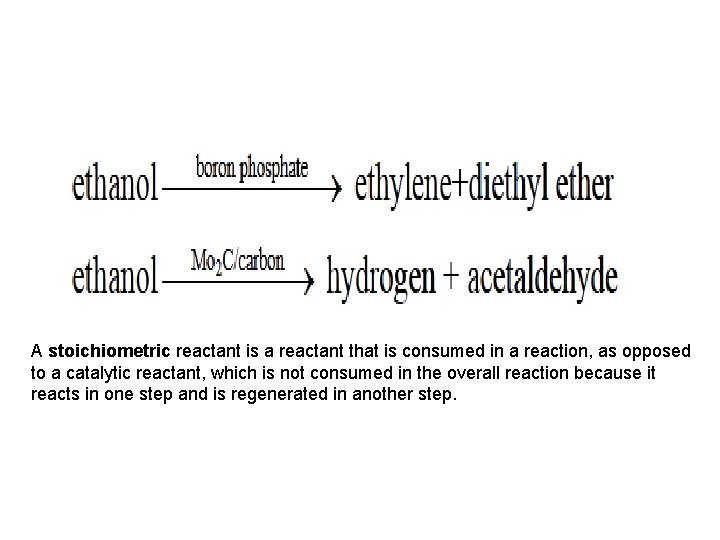
• A catalyst accelerates both the rates of the forward and reverse reaction. Equilibrium of a reversible reaction is not altered by the presence of the catalyst. For example, when oxidation of SO 2 is carried out in the presence of three different catalysts, namely Pt, Fe 2 O 3 and V 2 O 5 , the equilibrium composition is the same in all three cases. Another important characteristic of catalyst is its effect on selectivity. The presence of different catalysts can result in different product distribution from the same starting material. For example, decomposition of ethanol in the presence of different catalysts results in different products as shown • .

A stoichiometric reactant is a reactant that is consumed in a reaction, as opposed to a catalytic reactant, which is not consumed in the overall reaction because it reacts in one step and is regenerated in another step.

• Types of reactions • Several homogeneous catalytic systems are : 1. Acid base catalysis 2. Catalysis by metal ions 3. Catalysis by organometallic complexes 4. Catalysis by Lewis acids 5. Catalysis by enzymes

• Catalysis by acids or bases Acid –base catalysts are used in the following types of reactions: • Examples • Condensation • Dehydration • Hydrolysis • Halogenations

• Catalysis by Metal ions can act as catalysts. Metal ions function in different ways : – Metal ion can act as a “super acid”. It introduces positive charge into the substrate, making it more susceptible toward nucleophilic attack. – Metal ions can also act as templates. Metal ions are able to coordinate to more than 2 ligands and thereby bring the molecules together. • Metal ions can act as redox catalysts. Many metal ions can accept or donate electrons by changing their oxidation state and thereby participate in redox reactions.

• Catalysis by organometallic complexes • Presently, organometallic catalysts play major role in homogeneous catalysis. Organometallic complex consist of a central transition metal ion bonded to organic ligands such as R 2 C=CR 2, RCO, R 3 P, R 3 N, CO etc. Catalysis occurs through dissociationof ligands followed by co-ordination of reactant molecule to the metal ion. The transition metal ions react through exchange of d electrons. Organometallic complexes usually have octahedral or tetrahedral geometry. Reactions catalyzed by organometallic complexes include hydrogenation, hydroformylation, carbonylation and decarbonylation, hydrocarbon rearrangement, partial oxidations etc.

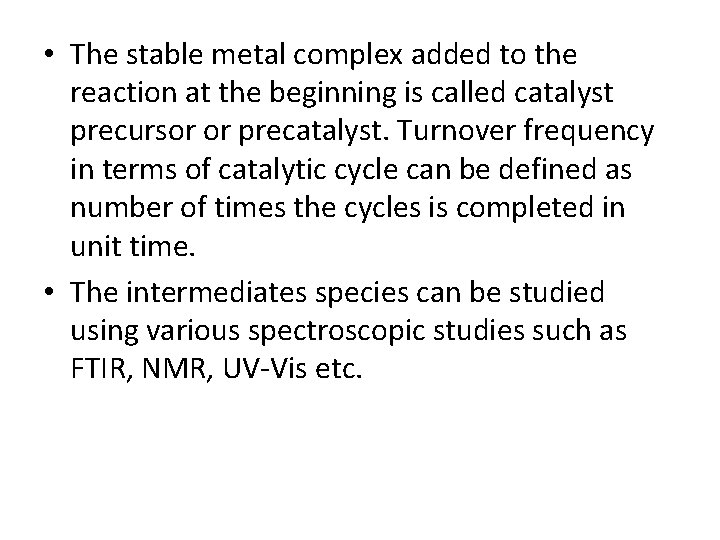
• Homogeneous catalysis mechanism is often represented by catalytic cycles. In catalytic cycles, usually catalysts are shown as member of cycle and all reactants and products are placed outside the cycle and connected to it by arrows.

For example, the cycle of a catalytic reaction having intermediates K and L can be represented as

• The stable metal complex added to the reaction at the beginning is called catalyst precursor or precatalyst. Turnover frequency in terms of catalytic cycle can be defined as number of times the cycles is completed in unit time. • The intermediates species can be studied using various spectroscopic studies such as FTIR, NMR, UV-Vis etc.

• Catalysis by Organometallic complexes • For heterogeneous catalysts the reaction starts with the adsorption of reactant on a vacant site on the catalyst surface. Similarly, for homogeneous organometallic complex catalysts, the reaction begins with the reactant molecule getting attached to a metal centre in the catalyst. Hence, the metallic centers must have vacant coordination sites. However, it is difficult to maintain vacant sites on the metallic center as the molecules are always in solvated conditions.

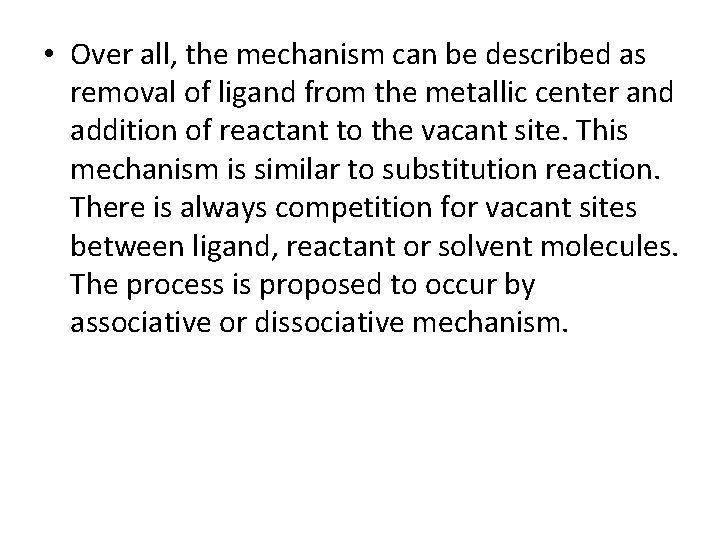
• Over all, the mechanism can be described as removal of ligand from the metallic center and addition of reactant to the vacant site. This mechanism is similar to substitution reaction. There is always competition for vacant sites between ligand, reactant or solvent molecules. The process is proposed to occur by associative or dissociative mechanism.

• Dissociative mechanism In dissociative ligand exchange mechanism, initially there is breaking of bond between the metal and leaving ligand. This is the slowest and rate controlling step. A solvent molecule occupies the open site. Subsequently, the solvent is replaced by reactant in a fast step.

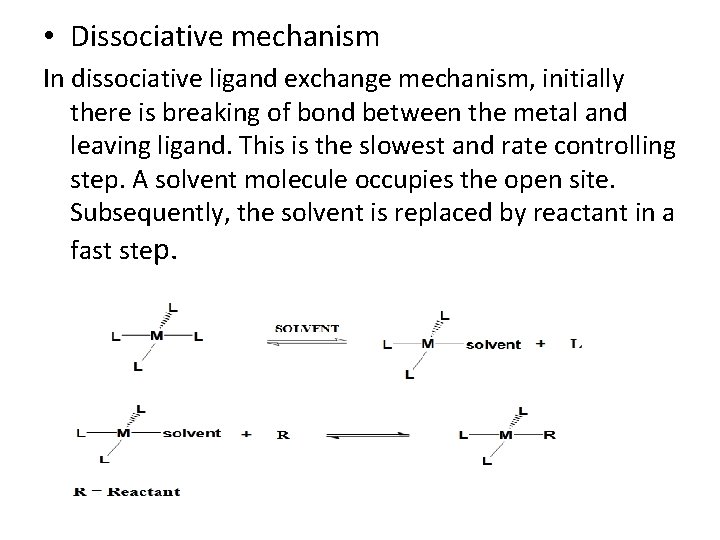
• Associative mechanism In associative ligand exchange process, bond breaking of ligand from metal and bond formation between metal and reactant occurs simultaneously as shown below.

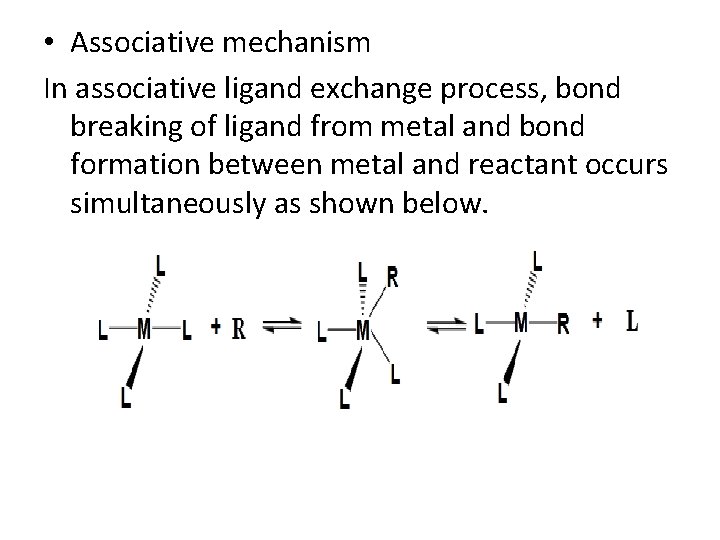
• Elementary steps The basic elementary steps that are involved in organometallic chemistry are discussed briefly. Insertion In insertion mechanism an unsaturated molecule inserts into a metal- anion bond. In the shown example, the CO inserts into the metal (Pt)-methyl bond acyl bond formed (R- CO) takes position of methyl group. The vacant position on metal is filled in solvent (S) molecule.

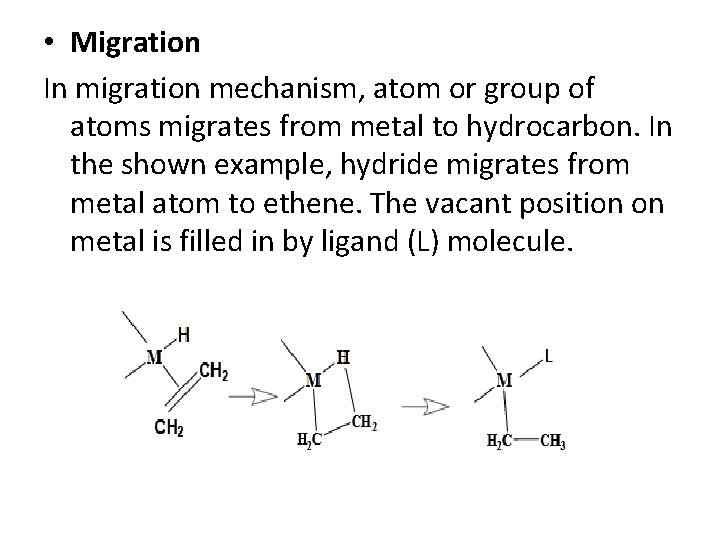
• Migration In migration mechanism, atom or group of atoms migrates from metal to hydrocarbon. In the shown example, hydride migrates from metal atom to ethene. The vacant position on metal is filled in by ligand (L) molecule.

• ß elimination This reaction is reverse of migration. The ßelimination requires a vacant site at the complex.

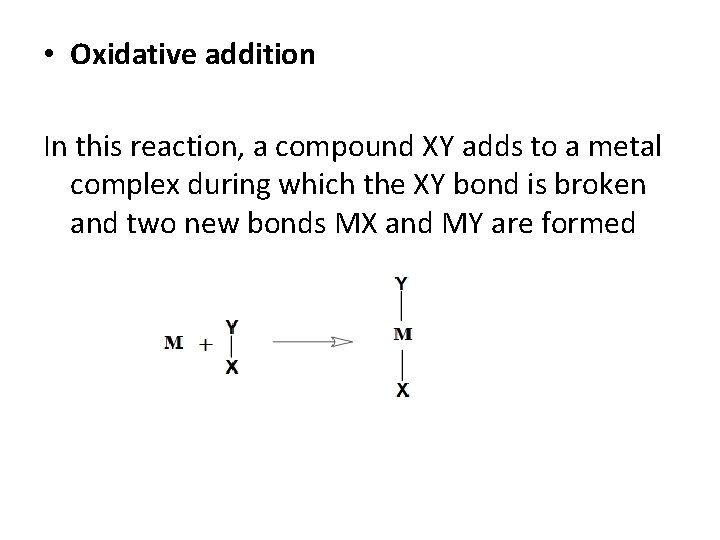
• Oxidative addition In this reaction, a compound XY adds to a metal complex during which the XY bond is broken and two new bonds MX and MY are formed

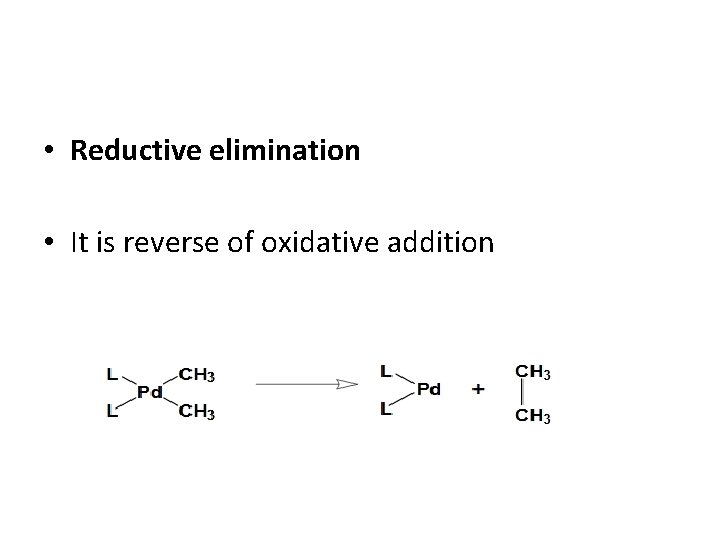
• Reductive elimination • It is reverse of oxidative addition

• Cycloaddition reaction involving metal • In presence of multivalent metal, alkene/alkynes form metallacyclic compounds.

• Activation of substrate toward nucleophilic attack • Co-ordination of alkene to electronegative metal (may carry a positive charge), activates the alkene toward attack of nucleophiles.

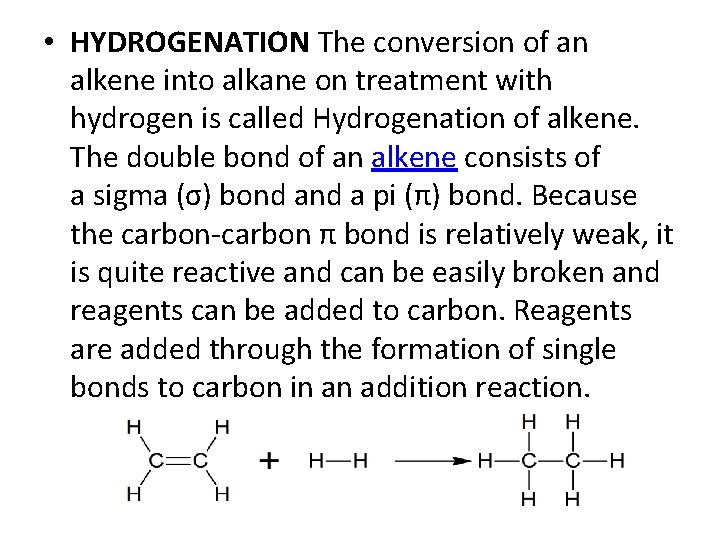
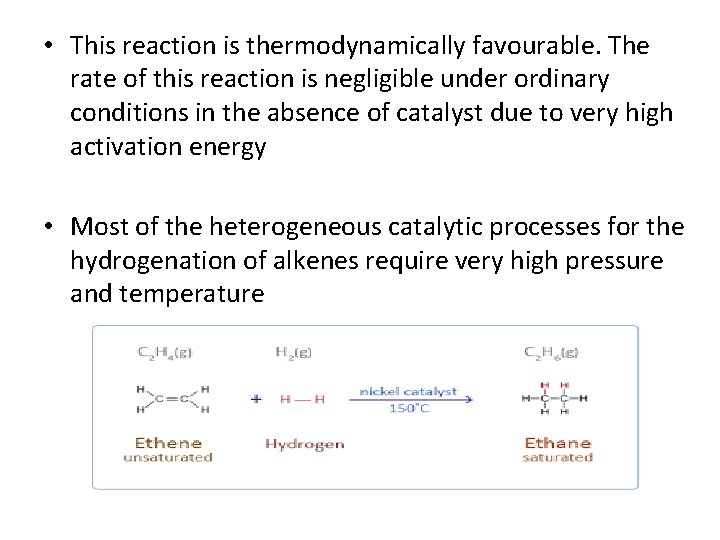
• HYDROGENATION The conversion of an alkene into alkane on treatment with hydrogen is called Hydrogenation of alkene. The double bond of an alkene consists of a sigma (σ) bond a pi (π) bond. Because the carbon-carbon π bond is relatively weak, it is quite reactive and can be easily broken and reagents can be added to carbon. Reagents are added through the formation of single bonds to carbon in an addition reaction.

• This reaction is thermodynamically favourable. The rate of this reaction is negligible under ordinary conditions in the absence of catalyst due to very high activation energy • Most of the heterogeneous catalytic processes for the hydrogenation of alkenes require very high pressure and temperature

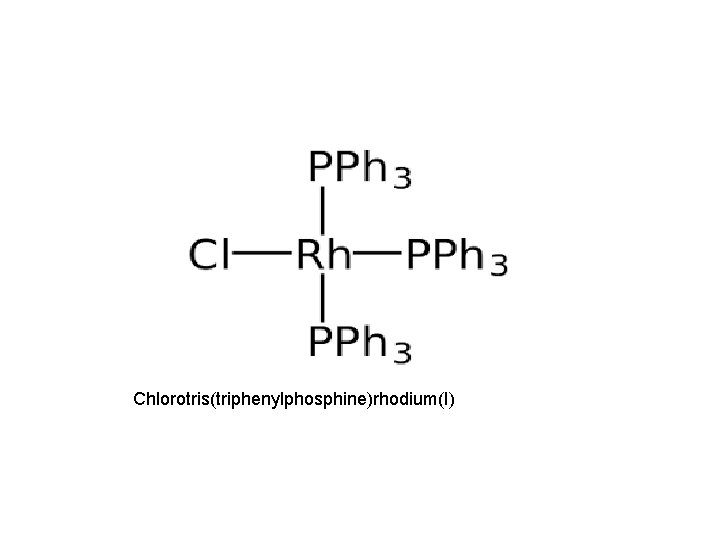
• However the homogenous catalytic process for hydrogenation of alkenes and alkynes in the presence of Wilkinson`s catalyst Rh. Cl(PPh 3)3, can be carried out at 1 atm pressure and room temperature(25 • )

Chlorotris(triphenylphosphine)rhodium(I)
 Faisal jaswal
Faisal jaswal Microsoft virtual academy
Microsoft virtual academy Anaerobes
Anaerobes Promotion from associate professor to professor
Promotion from associate professor to professor Cuhk salary scale 2020
Cuhk salary scale 2020 Homogeneous and non homogeneous differential equation
Homogeneous and non homogeneous differential equation Different types of mixtures
Different types of mixtures Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ziegler natta catalyst is
Ziegler natta catalyst is Policy catalyst first period 1780s to late 1800s
Policy catalyst first period 1780s to late 1800s Inorganic catalyst vs enzyme
Inorganic catalyst vs enzyme An example of prosthetic group
An example of prosthetic group Chapter 12 enzymes the protein catalyst
Chapter 12 enzymes the protein catalyst Speak book theme
Speak book theme Cisco loop detection
Cisco loop detection Xxxx websites
Xxxx websites How does temperature affect rate of reaction
How does temperature affect rate of reaction Alcohol covalent bond
Alcohol covalent bond Christ catalyst
Christ catalyst Catalyst corporate finance
Catalyst corporate finance Bio diesel
Bio diesel Heterogeneous catalyst
Heterogeneous catalyst Leadership agility definition
Leadership agility definition Rna catalyst
Rna catalyst Catalyst pharma consulting
Catalyst pharma consulting Protein catalyst
Protein catalyst Important properties of enzymes
Important properties of enzymes Wacker process
Wacker process Maxwell boltzmann distribution catalyst
Maxwell boltzmann distribution catalyst Oxymercuration of alkynes
Oxymercuration of alkynes Late binding data warehouse
Late binding data warehouse Catalyst literature
Catalyst literature Multi tubular reactor
Multi tubular reactor Does a catalyst affect enthalpy
Does a catalyst affect enthalpy Finely divided catalyst
Finely divided catalyst