CHAPTER 13 Solutions Solution Solution A homogeneous mixture






































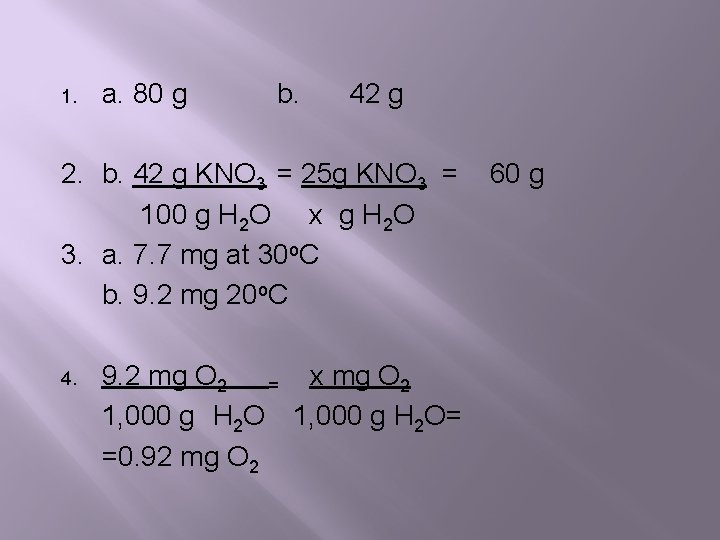

- Slides: 57

CHAPTER 13 Solutions

Solution � � Solution- A homogeneous mixture of two or more substances, the composition of which may vary. Homogenous- Composition is uniform, cannot distinguish the two parts

Components of solutions: � � Solute- The substance that is dissolved. Solvent- The substance doing the dissolving and is normally present in excess (bigger amount). � Water is the most common solvent. It is polar. � Example- in sugar water, sugar is the solute and water is the solvent

Properties of water � � Water is polar- caused by the positive and negative charges of the hydrogen and oxygen Water’s density is 1 g/m. L Universal solvent- multitude of things dissolve in water It’s the only substance that its solid form is less dense than it’s liquid form, that’s why ice floats. Molecules are further apart in ice than in water because of air.

Types of solutions � � Gaseous solutions- Two or more gases are mixed. Example: Air (oxygen, nitrogen, and carbon dioxide)

Liquid solutions � Liquid solutions- Solutions which have a liquid solvent and either a gas, liquid or solid as the solute. (Something dissolves in a liquid. ) Solute gas liquid solid � Solvent liquid Example Seltzer antifreeze salt water Aqueous solutions- water is the solvent

Types of Solutions � Solid solutions- Solutions which have a solid solvent and either a gas, liquid, or solid solute. Solute gas liquid solid Solvent solid Example charcoal filter dental filling copper in silver

Miscible vs. Immiscible � � � Miscible -pair of substances can be mixed together in any proportion to form a solution. Immiscible- pair of substances cannot be mixed together to form a solution. Example: Oil and water are immiscible. Like dissolves like- polar dissolves polar, nonpolar dissolves nonpolar

Solubility � Solubility- Amount of solute that will dissolve in a given amount of solvent

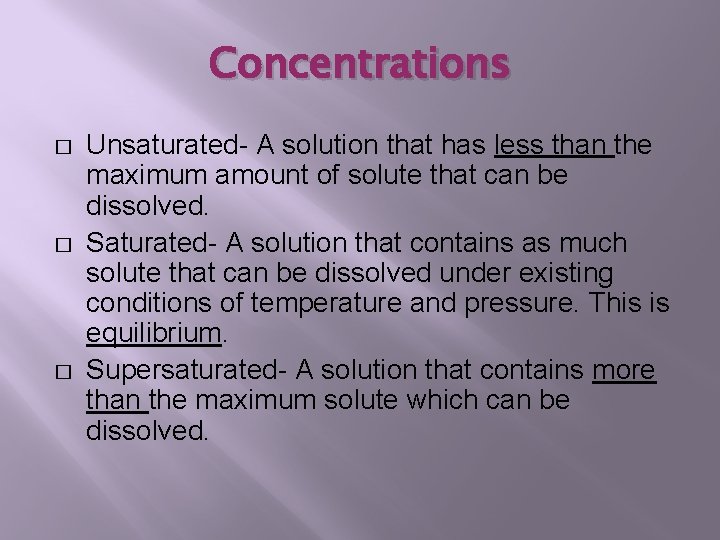
Concentrations � � � Unsaturated- A solution that has less than the maximum amount of solute that can be dissolved. Saturated- A solution that contains as much solute that can be dissolved under existing conditions of temperature and pressure. This is equilibrium. Supersaturated- A solution that contains more than the maximum solute which can be dissolved.

Solution equilibrium Eventually the water becomes so full of ions that it can no longer prevent some ions from re-attaching to the parent crystal. We call this a saturated solution and the solution is said to be in equilibrium. Equilibrium is very dependent on temperature for most solutions.

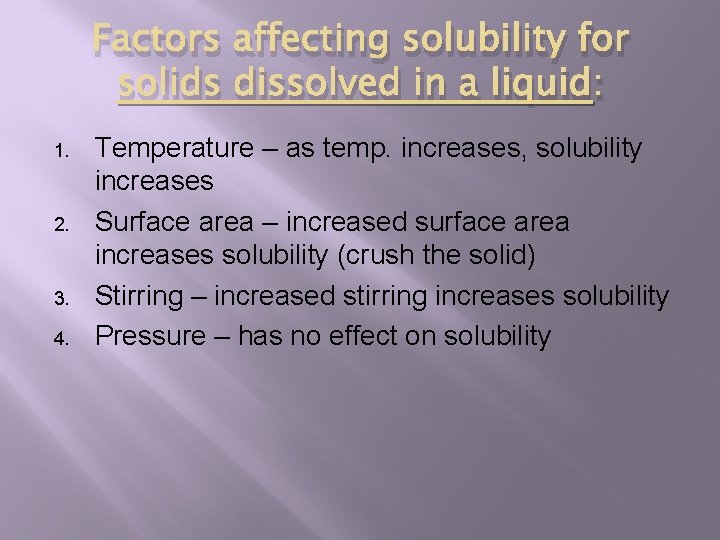
Factors affecting solubility for solids dissolved in a liquid: 1. 2. 3. 4. Temperature – as temp. increases, solubility increases Surface area – increased surface area increases solubility (crush the solid) Stirring – increased stirring increases solubility Pressure – has no effect on solubility

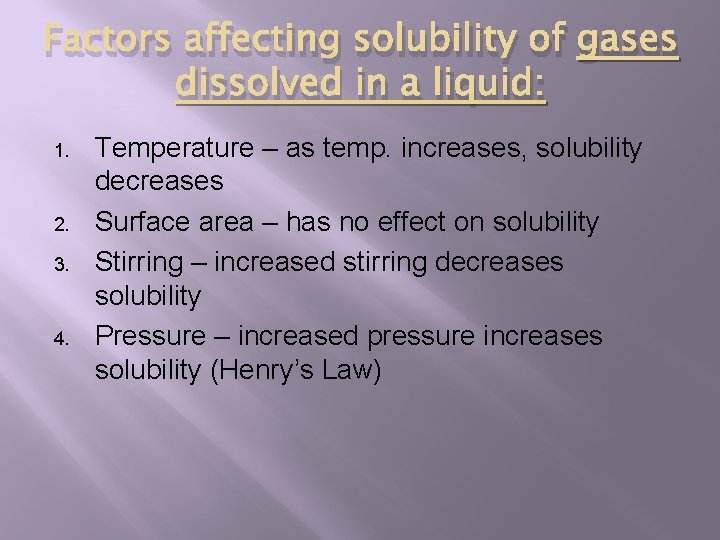
Factors affecting solubility of gases dissolved in a liquid: 1. 2. 3. 4. Temperature – as temp. increases, solubility decreases Surface area – has no effect on solubility Stirring – increased stirring decreases solubility Pressure – increased pressure increases solubility (Henry’s Law)

Henry’s Law � � The solubility of a gas in a liquid is also dependent on the pressure that the gas exerts on the surface of the liquid. This is Henry’s Law. Increase the pressure and solubility increases. Decrease the pressure and the solubility decreases. Did you ever notice that you soda goes flat once it is opened?

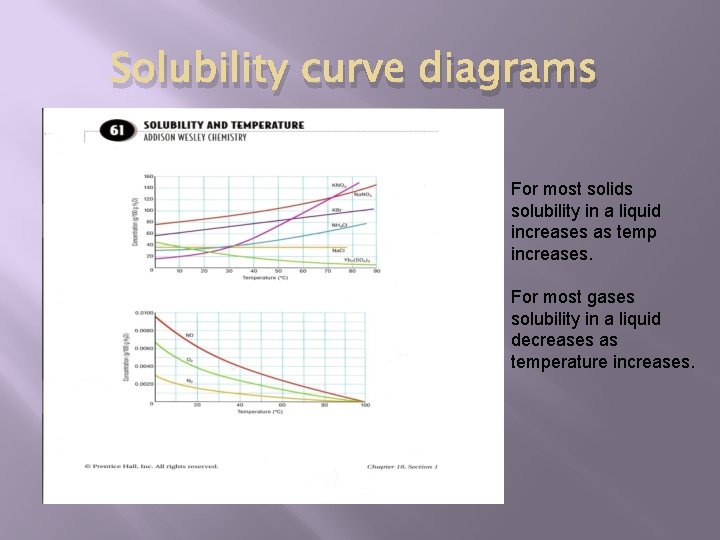
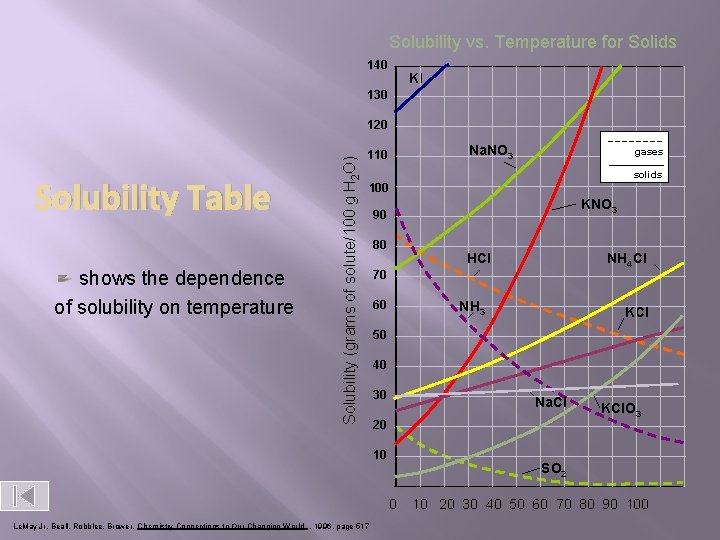
Solubility curve diagrams For most solids solubility in a liquid increases as temp increases. For most gases solubility in a liquid decreases as temperature increases.

Solubility curve diagrams � Solubility curve- graphs that show what mass of solute will dissolve in 100 m. L of solvent

Saturation � � � What is the solubility of Sodium Acetate at 60 o. C? What mass of sodium acetate will dissolve in 250 m. L of water at 60 o. C? Is 40 grams of sodium acetate in 50 m. L of water at 60 o. C saturated, unsaturated, or supersaturated?

CH 14 - SOLUTIONS

Mixtures � Mixture: blend of 2 or more kinds of matter physically, each retaining its own properties � Heterogeneous � Not uniform in composition � Ex) Vegetable Soup, chocolate � Homogeneous � Uniform in composition � Salt Water � Solutions chip cookies

Alloys � � Alloys are solutions of two or more metals mixing evenly (homogenous) Ex- steel, bronze, brass, sterling silver

Suspensions � � Suspension: Particles of a solvent are so large that they settle out unless they are mixed or agitated (heterogeneous) Example: Clay in Water, Muddy Water

Colloids � � Colloids: Mixtures you can’t see through Examples:

Tyndall Effect �Used to distinguish between solutions and colloids �Light is scattered when shone through a colloid

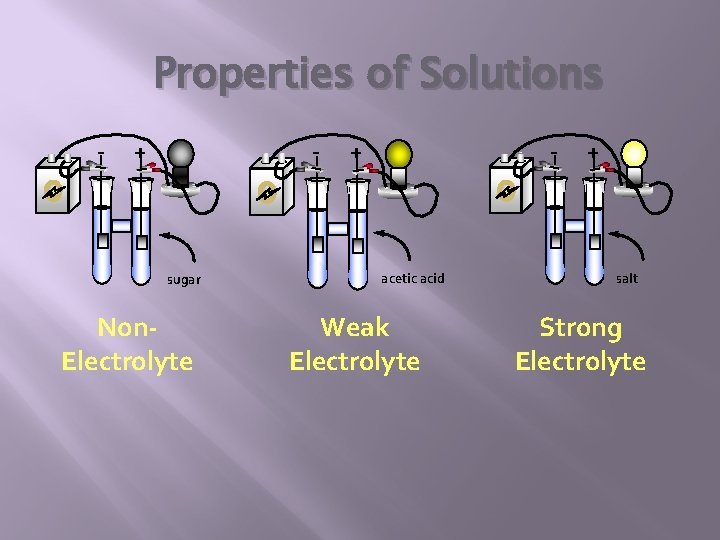
Electrolytes vs Non Electrolyte = a substance that dissolves in water to give an electrically conducting solution. (would make a light bulb light up) Ex) Ionic solids and acids like HCl or Na. Cl Nonelectrolyte = a substance that dissolves in water to give a nonconducting or very poorly conducting solution. Ex) Covalent compounds like sugar

Dissociation � � � When an ionic compound dissolves in water, the ions separate from each other (cations and anions) Ex: Na. Cl(s) Na+(aq) + Cl-(aq) Ex: Ca. Cl 2(s) Ca+2(aq) + 2 Cl-(aq)

Properties of Solutions - - + sugar Non. Electrolyte - + acetic acid Weak Electrolyte + salt Strong Electrolyte

Dissolution- the rate at which a substance dissolves

FACTORS AFFECTING DISSOLUTION: 1. Particle size – area of solute particles exposed to the action of the solvent particles. Increase in surface area of the solute particles , solubility increases Example: fine table salt dissolves faster than rock salt

2. Stirring or Agitation increases the solubility of solid solute particles in a solvent. Because it hastens the contact between the surface of the solute and the solvent particles

3. Application of heat - solvent molecules move faster and come in contact frequently with the solute particles, increasing solubility. Except for other solutes where solubility hastens with decrease in the temperature of the solvent. Example: sodium hydroxide pellets dissolves slowly in hot water than in cold water.

Solubility � Solids are more soluble at. . . � high temperatures. u Gases are more soluble at. . . • low temperatures & • high pressures (Henry’s Law). • EX: nitrogen narcosis, the “bends, ” soda

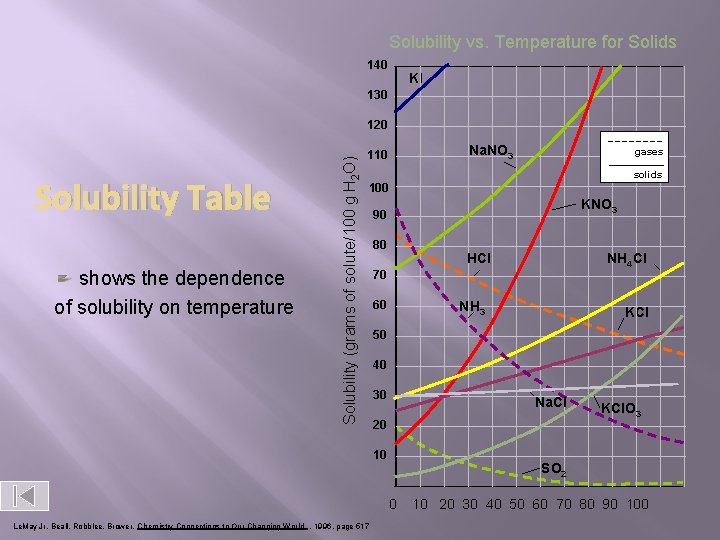
Solubility vs. Temperature for Solids 140 KI 130 Solubility Table shows the dependence of solubility on temperature Solubility (grams of solute/100 g H 2 O) 120 Na. NO 3 110 gases solids 100 KNO 3 90 80 HCl 70 60 NH 3 KCl 50 40 30 Na. Cl 20 10 KCl. O 3 SO 2 0 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 517 NH 4 Cl 10 20 30 40 50 60 70 80 90 100

How to determine the solubility of a given substance? � � Find out the mass of solute needed to make a saturated solution in 100 cm 3 of water for a specific temperature(referred to as the solubility). This is repeated for each of the temperatures from 0ºC to 100ºC. The data is then plotted on a temperature/solubility graph, and the points are connected. These connected points are called a solubility curve.


How to use a solubility graph? A. • IDENTIFYING A SUBSTANCE ( given the solubility in g/100 cm 3 of water and the temperature) Look for the intersection of the solubility and temperature.

Example: What substance has a solubility of 90 g/100 3 cm of water at a temperature of 25ºC ?


Example: What substance has a 3 solubility of 200 g/100 cm of water at a temperature of 90ºC ?


B. Look for the temperature or solubility • Locate the solubility curve needed and see for a given temperature, which solubility it lines up with and visa versa.

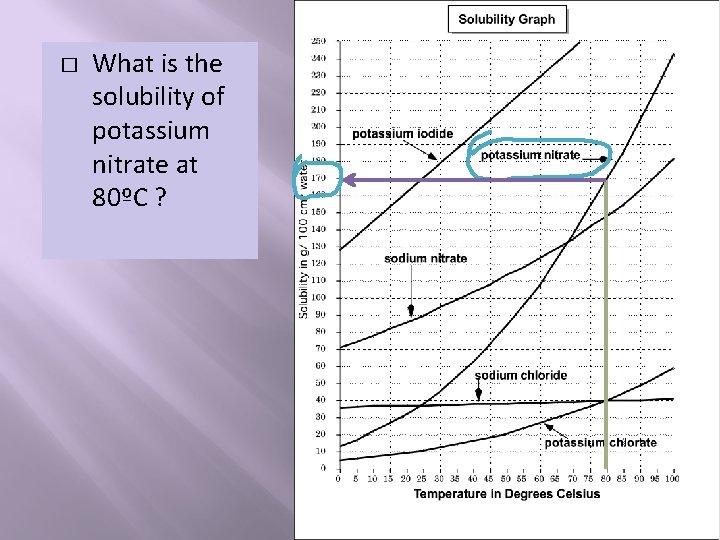
� What is the solubility of potassium nitrate at 80ºC ?

At what temperature will sodium nitrate have a solubility of 95 g/100 cm 3 ?

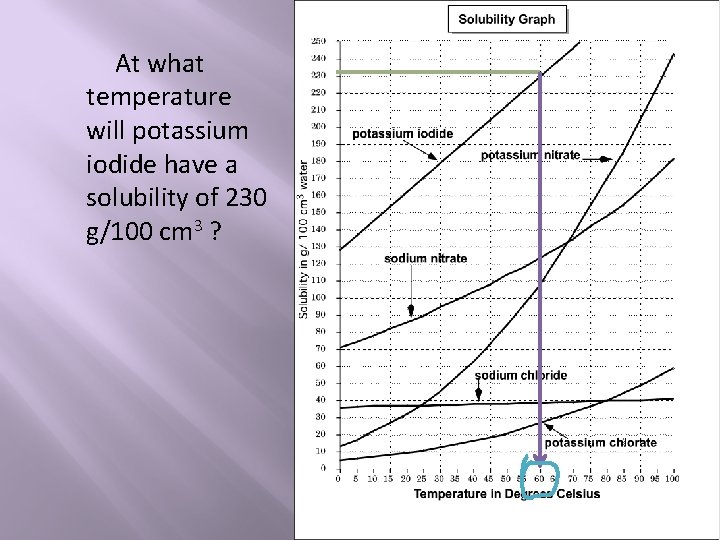
At what temperature will potassium iodide have a solubility of 230 g/100 cm 3 ?

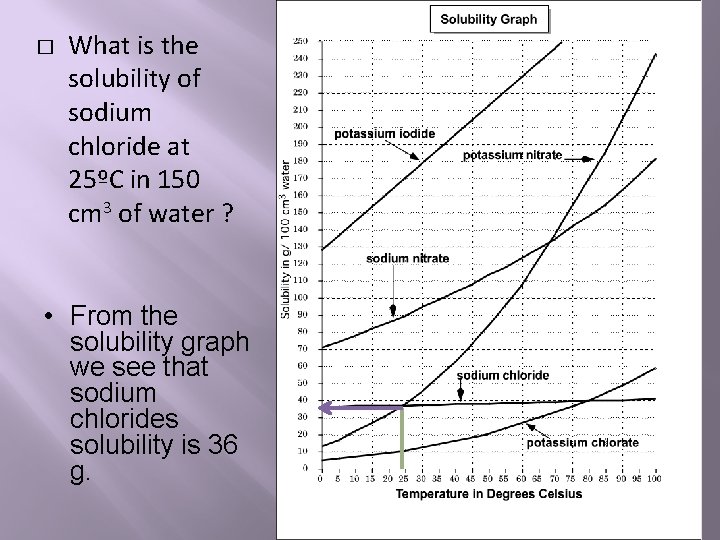
� What is the solubility of sodium chloride at 25ºC in 150 cm 3 of water ? • From the solubility graph we see that sodium chlorides solubility is 36 g.

Place this in the proportion below and solve for the unknown solubility. Solve for the unknown quantity by cross multiplying. Solubility in grams = unknown solubility in grams 100 cm 3 of water other volume of water ___36 grams____ = unknown solubility in grams 100 cm 3 of water 150 cm 3 water The unknown solubility is 54 grams. You can use this proportion to solve for the other volume of water if you're given the other solubility.

C. Determine if a solution is saturated, unsaturated, or supersaturated. • If the solubility for a given substance places it anywhere on it's solubility curve it is saturated. • If it lies above the solubility curve, then it's supersaturated, • If it lies below the solubility curve it's an unsaturated solution. Remember though, if the volume of water isn't 100 cm 3 to use a proportion first as shown above.

Solubility how much solute dissolves in a given amt. of solvent at a given temp. SOLUBILITY CURVE KNO 3 (s) KCl (s) Solubility (g/100 g H 2 O) HCl (g) Temp. (o. C) unsaturated: supersaturated: above the line solution could hold more solute; below line solution has “just right” amt. of solute; on line solution has “too much” solute dissolved in it;

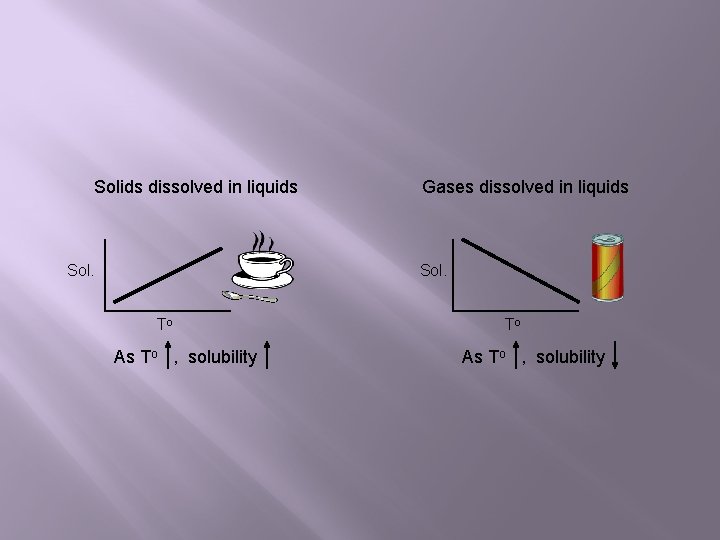
Solids dissolved in liquids Sol. Gases dissolved in liquids Sol. To As To , solubility

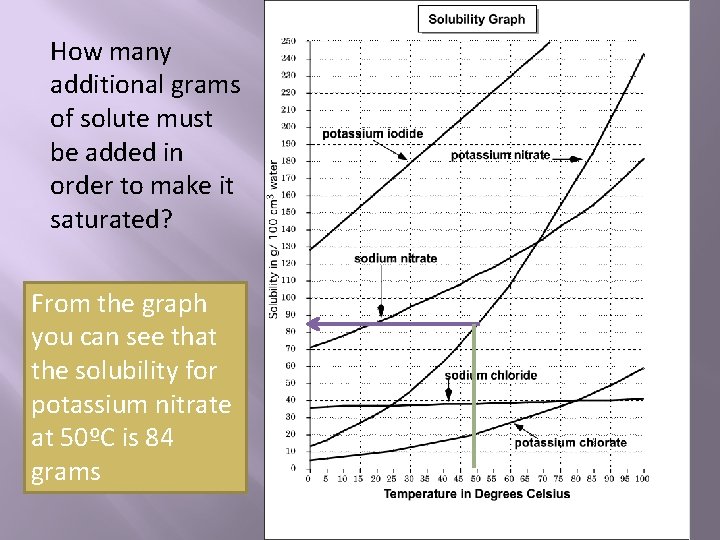
Sometimes you'll need to determine how much additional solute needs to be added to a unsaturated solution in order to make it saturated. For example, 30 grams of potassium nitrate has been added to 100 cm 3 of water at a temperature of 50ºC.

How many additional grams of solute must be added in order to make it saturated? From the graph you can see that the solubility for potassium nitrate at 50ºC is 84 grams

If there already 30 grams of solute in the solution, all you need to get to 84 grams is 54 more grams ( 84 g-30 g )

Solubility vs. Temperature for Solids 140 KI 130 Solubility Table shows the dependence of solubility on temperature Solubility (grams of solute/100 g H 2 O) 120 Na. NO 3 110 gases solids 100 KNO 3 90 80 HCl 70 60 NH 3 KCl 50 40 30 Na. Cl 20 10 KCl. O 3 SO 2 0 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 517 NH 4 Cl 10 20 30 40 50 60 70 80 90 100

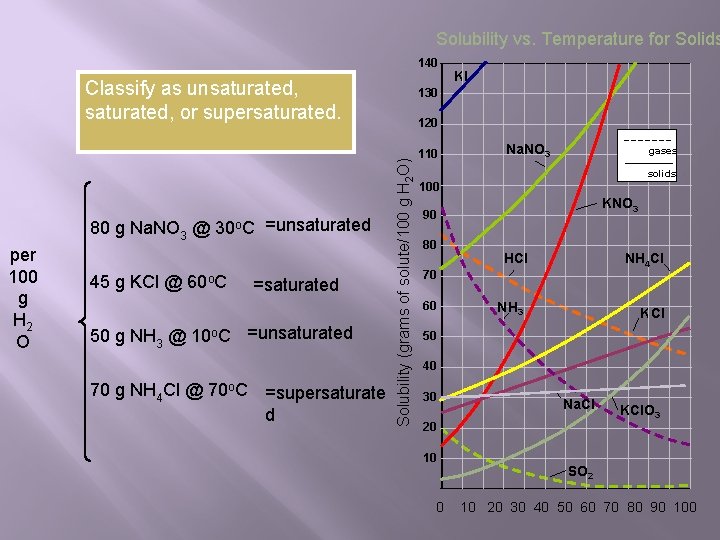
Solubility vs. Temperature for Solids 140 Classify as unsaturated, or supersaturated. per 100 g H 2 O 45 g KCl @ 60 o. C =saturated 50 g NH 3 @ 10 o. C =unsaturated 70 g NH 4 Cl @ 70 o. C =supersaturate d 130 120 Solubility (grams of solute/100 g H 2 O) 80 g Na. NO 3 @ 30 o. C =unsaturated KI 110 Na. NO 3 gases solids 100 KNO 3 90 80 HCl NH 4 Cl 70 60 NH 3 KCl 50 40 30 Na. Cl 20 10 KCl. O 3 SO 2 0 10 20 30 40 50 60 70 80 90 100

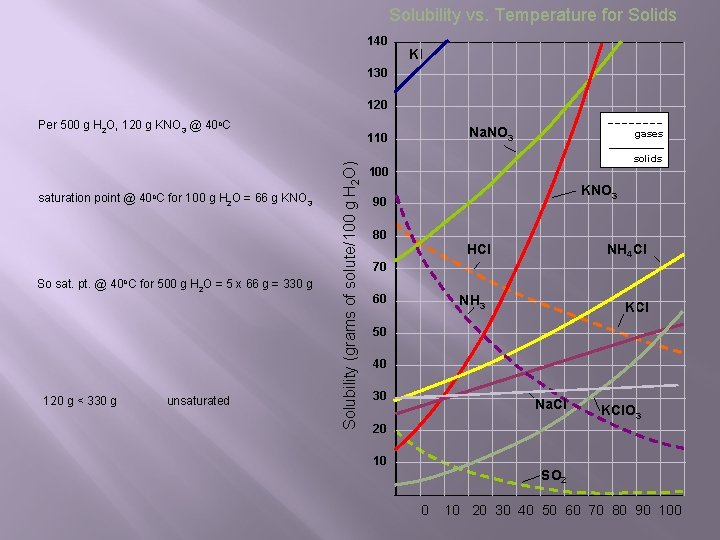
Solubility vs. Temperature for Solids 140 KI 130 120 Per 500 g H 2 O, 120 g KNO 3 @ 40 o. C So sat. pt. @ 40 o. C for 500 g H 2 O = 5 x 66 g = 330 g 120 g < 330 g unsaturated Solubility (grams of solute/100 g H 2 O) saturation point @ 40 o. C for 100 g H 2 O = 66 g KNO 3 Na. NO 3 110 gases solids 100 KNO 3 90 80 HCl NH 4 Cl 70 60 NH 3 KCl 50 40 30 Na. Cl KCl. O 3 20 10 SO 2 0 10 20 30 40 50 60 70 80 90 100

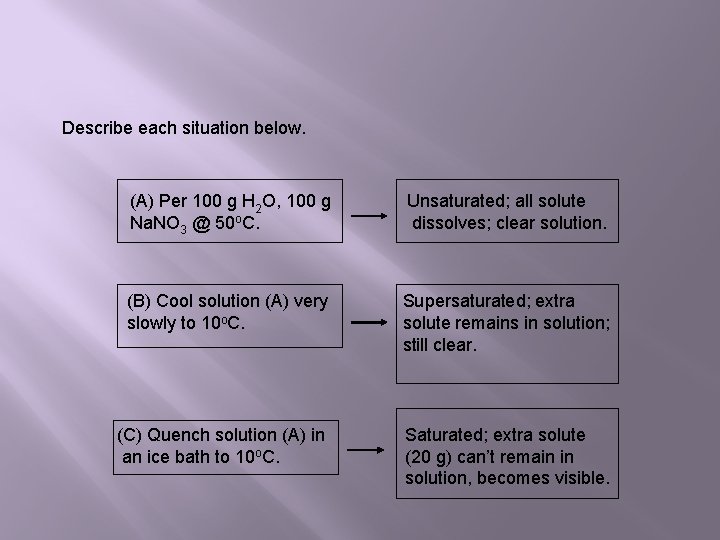
Describe each situation below. (A) Per 100 g H 2 O, 100 g Na. NO 3 @ 50 o. C. Unsaturated; all solute dissolves; clear solution. (B) Cool solution (A) very slowly to 10 o. C. Supersaturated; extra solute remains in solution; still clear. (C) Quench solution (A) in an ice bath to 10 o. C. Saturated; extra solute (20 g) can’t remain in solution, becomes visible.
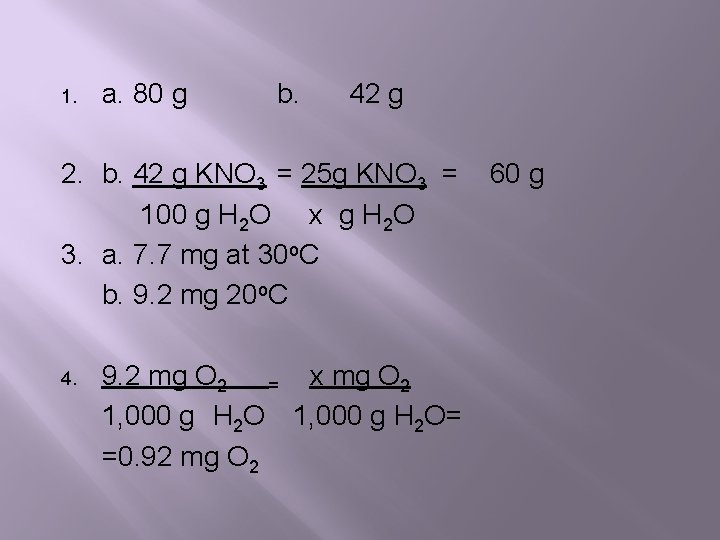
1. a. 80 g b. 42 g 2. b. 42 g KNO 3 = 25 g KNO 3 = 100 g H 2 O x g H 2 O 3. a. 7. 7 mg at 30 o. C b. 9. 2 mg 20 o. C 4. 9. 2 mg O 2 = x mg O 2 1, 000 g H 2 O= =0. 92 mg O 2 60 g

Nosce te ipsum