Solutions Chapter 13 Solution Process Solution homogeneous mixture








- Slides: 17

Solutions Chapter 13

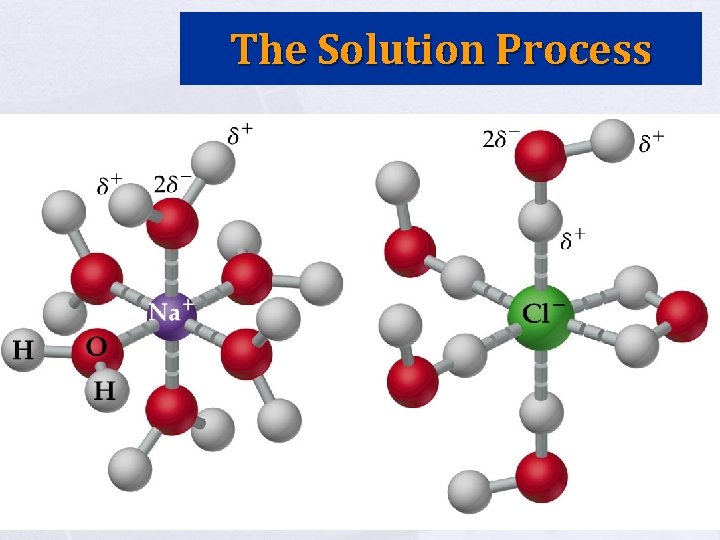
Solution Process Solution – homogeneous mixture of solute and solvent Ø Ø Can be gases, liquids, or solids Solvent is component present in the largest amount (rest are solutes) Intermolecular forces are rearranged with solutions of condensed phases. Ø Ex. Na. Cl in Water – Water molecules orient themselves on Na. Cl; H-bonds between waters have to be broken; Na. Cl dissociates; ion-dipole forces form; interaction is called solvation or hydration


The Solution Process

Solubility Dissolution – formation of a solution Crystallization – “destruction” of a solution If these processes are in equilibrium, the solution is saturated. Solubility is the amount of solute required to form a saturated solution. Ø Ø Unsaturated Supersaturated

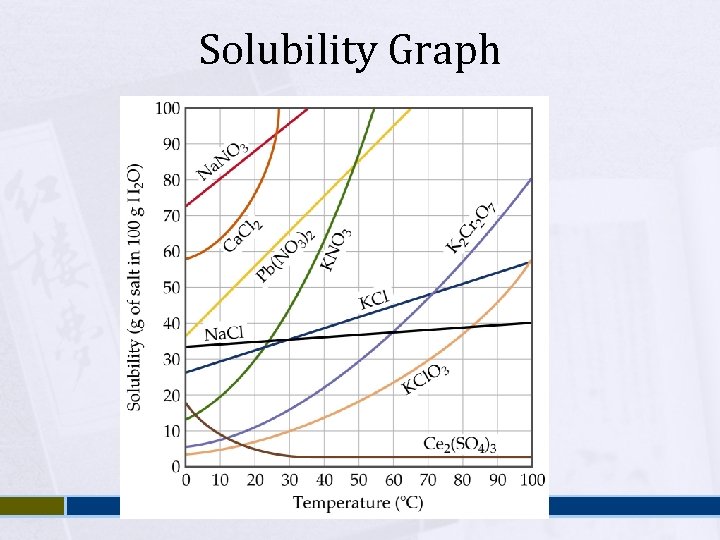
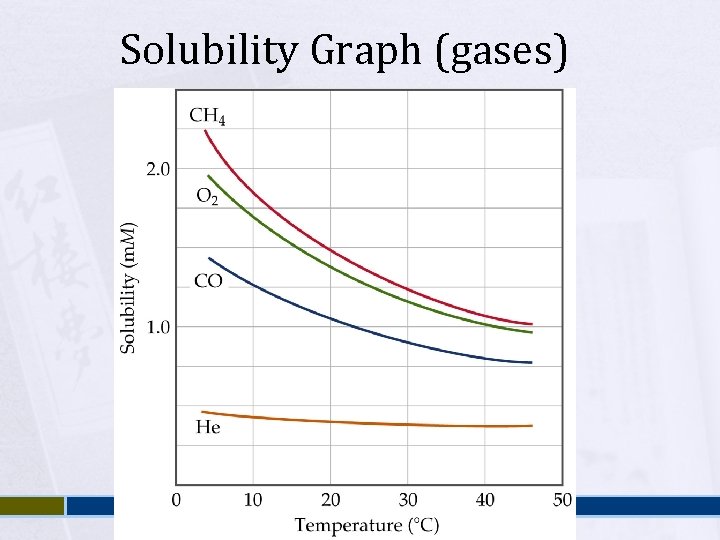
Factors affecting Solubility Nature of the solute and solvent Ø Ø Temperature Ø Ø Intermolecular forces – favorable? Miscible vs. immiscible / soluble vs. insoluble Generally, as temperature increases, solubility increases. Sometimes not the case – gases are less soluble at higher temperatures Pressure Ø Ø Solubility of a Gas is a function of the pressure of the gas over the solution. Increase pressure, increase solubility

Solubility: Pressure

Solubility Graph

Solubility Graph (gases)

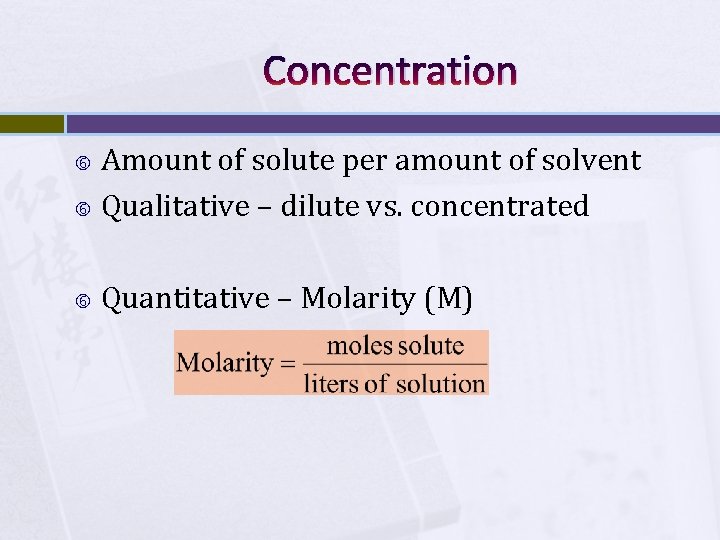
Concentration Amount of solute per amount of solvent Qualitative – dilute vs. concentrated Quantitative – Molarity (M)

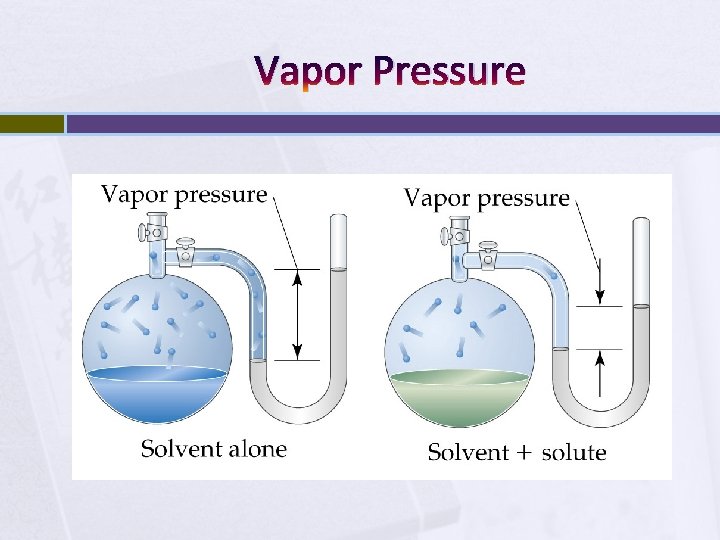
Colligative Properties Colligative properties depend on quantity of solute molecules. Ø (E. g. freezing point depression and melting point elevation. ) Lowering Vapor Pressure Ø Ø Ø Non-volatile solutes reduce the ability of the surface solvent molecules to escape the liquid. Therefore, vapor pressure is lowered. The amount of vapor pressure lowering depends on the amount of solute.

Vapor Pressure

Colligative Properties Boiling-Point Elevation Ø Ø Ø Non-volatile solute lowers the vapor pressure. Therefore the triple point - critical point curve is lowered At 1 atm (normal boiling point of pure liquid) there is a lower vapor pressure of the solution. Therefore, a higher temperature is required to reach a vapor pressure of 1 atm for the solution ( Tb).


Colligative Properties: Freezing Point Depression At 1 atm (normal boiling point of pure liquid) there is no depression by definition When a solution freezes, almost pure solvent is formed first. Ø Therefore, the sublimation curve for the pure solvent is the same as for the solution. Ø Therefore, the triple point occurs at a lower temperature because of the lower vapor pressure for the solution.

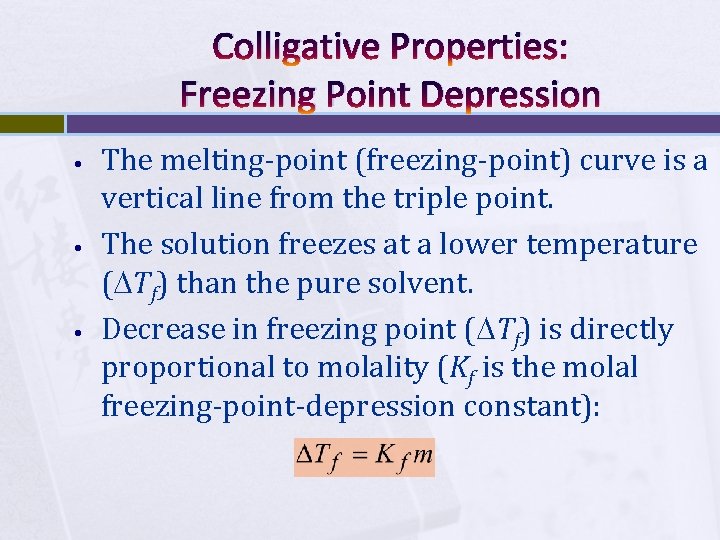
Colligative Properties: Freezing Point Depression • • • The melting-point (freezing-point) curve is a vertical line from the triple point. The solution freezes at a lower temperature ( Tf) than the pure solvent. Decrease in freezing point ( Tf) is directly proportional to molality (Kf is the molal freezing-point-depression constant):
